Abstract
Generation of effective immune responses against pathogenic microbes depends on a fine balance between pro- and anti-inflammatory responses. Interleukin-10 (IL-10) is essential in regulating this balance and has garnered renewed interest recently as a modulator of the response to infection at the JAK-STAT signaling axis of host responses. Here, we examine how IL-10 functions as the “master regulator” of immune responses through JAK-STAT, and provide a perspective from recent insights on bacterial, protozoan, and viral infection model systems. Pattern recognition and subsequent molecular events that drive activation of IL-10-associated JAK-STAT circuitry are reviewed and the implications for microbial pathogenesis are discussed.
Keywords: molecular and cellular biology, microbes, infection and immunity, microbial pathogenesis, host response, interleukin-10
Balanced Antimicrobial Defense Hinges on the IL-10-JAK-STAT Module
Innate immune activation in response to microbial pathogens occurs as a result of recognition of foreign microbes or their products by phagocytes via pattern recognition receptor (PRR)-dependent mechanisms. This leads to the activation of crucial phagocyte effector functions to combat microbes including the synthesis of reactive oxygen and nitrogen species, and generation of phagolysosomal proteases that mediate killing of invading microbes.1 Recognition of microbes by immune surveillance cells such as macrophages using PRRs initiates a signaling cascade that leads to the production of cytokines including interleukins (IL) and chemokines that drive antimicrobial mechanisms and regulate inflammatory responses to clear infection and achieve convalescence.
The transition of macrophages into effector cells for antimicrobial killing typically stems from the classical pathway of activation by T cell- or NK cell-derived interferon (IFN)-γ. This largely occurs via the family of signal transducers and activators of transcription known as the STAT proteins that relay activation messages from ligated cytokine receptors at the cell surface to the nucleus for transcriptional activation.2 STAT1, after its phosphorylation at the IFN-γ receptor, is a starting point for classical macrophage activation because it induces a broad transcriptional program that includes many antimicrobial effector mechanisms. STAT3, on the other hand, has been labeled “the anti-inflammatory STAT.”1 These two opposing STAT signaling mechanisms exist at the crossroads of immune activation and suppression, and it is here that STATs influence microbial disease pathogenesis. Interruption or interference to normal STAT signaling mechanisms can dramatically alter the host response to infection with various pathogens and predispose individuals to disease as recently reviewed elsewhere.3
In the last few years, IL-10 has garnered renewed interest as a key modulator of innate immune responses to pathogenic microbes because several studies have revealed novel functions of this cytokine in the control of infectious disease.4-8 An emerging theme is to resolve how PRRs such as Toll-like receptors (TLRs) coordinate their actions upon sensing foreign microbes with Janus kinase (JAK)-STAT circuitry to link IL-10 with diverse pathogen recognition events, microbial survival strategies, and downstream effector mechanisms for host defense. Host responses to microbes resulting from the ligation of TLRs such as TLR4 for LPS,9 TLR2 for lipoteichoic acid,10 TLR5 for flagellin monomers11 and TLR9 for CpG motifs12 are carefully regulated to control the degrees of immune activation and suppression during disease.13 The role of IL-10 as a major regulator that connects these recognition events with appropriately balanced pro- and anti-inflammatory responses is underscored by these recent studies, which illustrates the complexity of IL-10 actions in overall host defense at the axis of infection-immunity.4-8
Molecular pathways of IL-10 signaling through JAK-STAT are not only associated with antimicrobial defense against potential pathogens but evidence is accumulating that these pathways are also involved in tolerance to commensal flora. Lactobacillus rhamnosus for example, as a normal microbe inhabitant of the placental mucosa, triggers signaling pathways involving IL-10 and JAK-STAT to control tumor necrosis factor (TNF)-α production, which prevents pre-term birth.14 This example of moderation of local inflammatory conditions by lactobacilli during pregnancy is not the only commensal-host interaction that relies on the IL-10-JAK-STAT circuitry for good human health outcomes. The role of IL-10 and STAT3 in maintenance of tolerance and homeostasis in the gut, for example, is evident from seminal papers describing the development of chronic enterocolitis in gene-deficient mice.15,16 More recently, the identification of pediatric patients with mutations in the IL-10 receptor who develop enterocolitis shows the relevance of IL-10 for tolerance to gut commensals in the human system.17 These observations show that commensals interact with local immune surveillance mechanisms and IL-10 and its related JAK-STAT signaling module serves to safeguard against potentially tissue-damaging inflammation. Importantly, the underlying molecular mechanisms of immune signaling that occur subsequent to commensal or pathogen detection and IL-10 production, including how IL-10 affects JAK-STAT circuitry, how it deactivates pathogen-sensing cells and how this influences microbial clearance during infection is an area of intense current research. Here, we examine recent studies of IL-10 at the nexus of infection immunity in the context of immune suppression through JAK-STAT and consider the consequences of downstream signaling through this module for microbial pathogenesis.
Diverse Pathogens Induce IL-10 and Activate the IL-10 Receptor Complex
IL-10 is a prototypic anti-inflammatory cytokine that is produced in response to a multitude of pathogens18 and acts as the master regulator of immunity to infection as recently reviewed elsewhere.19 In acute infection, one of the central roles for IL-10 is to deactivate macrophages and terminate inflammatory responses in order to limit excessive release of tissue-damaging, pro-inflammatory mediators that are synthesized by cells such as macrophages to kill microbes. IL-10 is released from various cells including macrophages, dendritic cells, subsets of CD4+ and CD8+ T cells and B cells, and therefore functions as a vital immune modulator at various stages of infection.19 The role of IL-10 in limiting collateral tissue damage that arises from acute inflammation in both infectious and non-infectious disease has been increasingly characterized over the past 5 y.20-24 In addition to acute infectious conditions and the aforementioned effects mediated by commensal flora, the influence of IL-10 on microbial pathogenesis is nuanced in states of chronic infection such as with mycobacteria, for example, where the immune suppressive effects of IL-10 can promote the survival of microorganisms and contribute to persistent disease. In this regard, some pathogens appear to proactively induce IL-10 as a virulence strategy to interfere with inflammation and proactively abrogate antimicrobial effector functions. Mycobacterium avium, for example, is one of several pathogenic bacteria that induces IL-10, which, following ligation of the IL-10 receptor complex and the triggering of subsequent JAK-STAT signaling cascades, influences the progression of infection (Fig. 1).25 Similarly, M. avium-induced IL-10 prevents TNF-α production and macrophage apoptosis, and this may represent one possible mechanism for increased pathogen survival by providing an intracellular niche.25 Other examples of IL-10 induction by pathogens leading to chronic infection include Leishmania major26 and M. tuberculosis.27,28
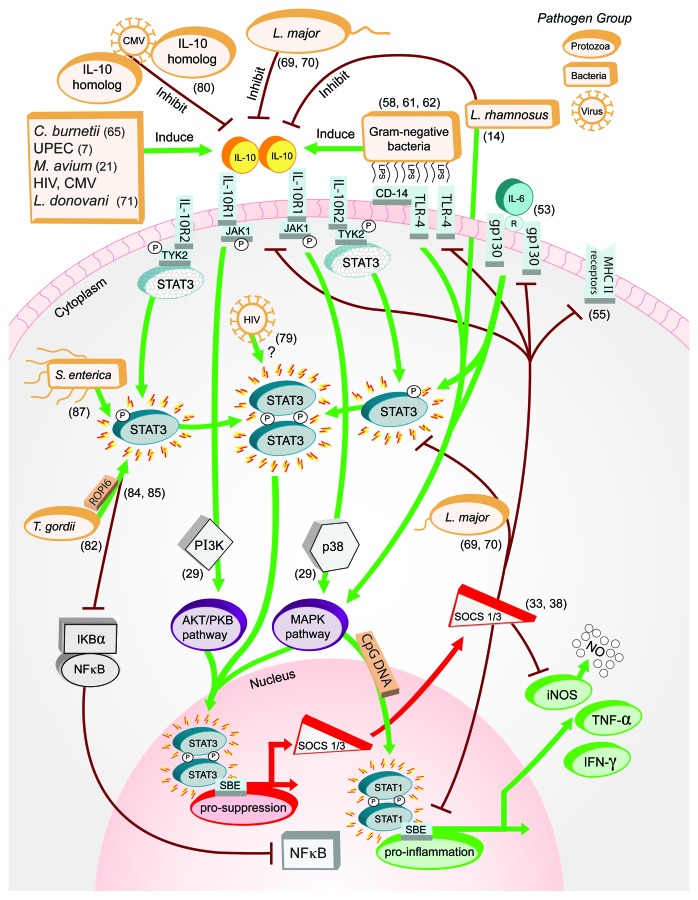
Figure 1. Interactions between microbial pathogens and IL-10-JAK-STAT signaling pathway elements. Recent studies (reference shown in parentheses) have shown C. burnetii, M. avium, S. agalactiae and other Gram-negative pathogens induce IL-10 synthesis in contrast to protozoa such as L. major and commensal L. rhamnosus that inhibit its production. STAT3, normally recruited and phosphorylated at the IL-10 receptor complex, are directly engaged by pathogens including S. enteria and T. gondii. Viruses such as HIV also affect STAT3 directly, or indirectly by producing homologs that compete with IL-10 for receptor complex docking sites, as shown for CMV. Nuclear translocation of active STAT3 for contact with STAT binding elements is a potential pathway element for pathogen-driven effects that has not yet been described. SOCS1 and SOCS3, induced as a result of IL-10 signaling through MAPK and AKT/PKB, suppress LPS/TLR4/CD14-induced IL-10, IL-6 biological activity from gp130 receptors, MHC class II, and STAT1-induced mediators including NO, TNF-α and IFN-γ. Microbes such as L. major hijack this element within the pathway to abrogate suppressive effects of SOCS toward active STAT3 as a mechanism of interfering with antimicrobial responses in macrophages.
Functionally, IL-10 exerts its immune suppressive and other effects by interacting with the IL-10-specific receptors, IL-10 receptor-α (IL-10R1) and -β (IL-10R2). These receptors partner as a complex and are expressed only on hematopoietic cells including B cells, T cells, NK cells, macrophages and monocytes.29 Both are members of the class II cytokine receptor family.30 IL-10R1 acts as the ligand binding chain while IL-10R2 functions as the accessory chain that recruits JAKs to the intracellular domain.29 Activation of the IL-10 receptor complex necessitates a tetramer consisting of two IL-10R1 and two IL-10R2 chains, which bind homodimeric IL-10 to the extracellular domains of IL-10R1 (Fig. 1).29 IL-10R2 does not bind to IL-10 directly31 and binding of IL-10 to IL-10R1 without the co-presence of IL-10R2 fails to initiate signal transduction and relay of the immune regulatory message from IL-10. Successful engagement of the IL-10 receptor complex subsequently activates distinct JAK-STAT pathways and downstream signaling events that converge through various mechanisms to influence nuclear transcriptional events such as those mediated by NFκB (Fig. 1).32
IL-10 Biological Effects during Infection Occur via JAK-STAT Signal Relay
Engagement of the JAK-STAT signaling circuit by ligation of the IL-10 receptor complex occurs principally through STAT3. This is most likely to involve mediator genes induced by STAT3 transcriptional activity because the inhibitory effects of IL-10 require new protein synthesis. Recently, activated STAT3 was shown to initiate the biological effects of IL-10 in a tristetraprolin (TTP)-dependent manner.33 TTP removes certain cis-acting A + U rich (ARE)-containing unstable mRNAs such as TNF-α but is not the sole major mediator of IL-10-inhibitory effects because of the emerging picture that there is no single master mediator of IL-10-induced deactivation. Rather, multiple IL-10-induced genes control certain aspects of macrophage activation/deactivation and mediate distinct parts of the anti-inflammatory effects of IL-10. These include, for example, Bcl-3, which controls TNF-α but not IL-6, Dusp1, which blocks p38 mitogen-activated protein kinase (MAPK) and inhibits IL-6 and several chemokines (but not IL-12p40)34 and Nfil3, which inhibits IL-12p40 expression.35 STAT3-induced genes appear to act as transcriptional suppressors, inhibiting the recruitment of activating factors to target promoters, and may even induce secondary mediators.1 Products from STAT3 target genes can impede signal transduction from cell surface receptors and activate NFκB and MAPK pathways that directly control pro-suppression and pro-inflammatory transcriptional events in the nucleus (Fig. 1). While some studies have shown that IL-10 can activate STAT1 directly36,37 this pathway of signaling has not been associated with the anti-inflammatory effects of IL-10, and the precise outcomes of IL-10 signal relay through STAT1 remain unclear. Moreover, while IL-10 mediates its suppressive effects via the activation of STAT3, it is not the only cytokine to do so. IL-6 family cytokines including IL-6 and IL-11 also activate STAT3 and, to a lesser extent, STAT1, as well as MAPK and phosphatidylinositol 3-kinase (PI3K) cascades through the gp130 receptor to bring about its pleiotropic actions including anti-inflammatory effects, as recently reviewed elsewhere.38,39
Upon binding of IL-10 to its receptor complex, phosphorylation of the receptor-associated protein tyrosine kinases, JAK1 and TYK2 occurs (Fig. 1). JAK1 is recruited to the intracellular domain by the IL-10R1 chain, while TYK2 is recruited to the receptor complex by IL-10R2.40 These kinases serve as a temporary docking site for inactive cytosolic STAT1 and/or STAT3,41 which are recruited by JAK1 and TYK2 to the site upon phosphorylation of the IL-10R1 chain at two tyrosine residues.29 The STATs bind to the IL-10R1 chain via the Src homology 2 (SH2) domain and are tyrosine-phosphorylated by the receptor-associated JAKs. Activation of STAT3 leads to its homodimerization (similar to STAT1) and, although STAT1:STAT3 heterodimers have been described, evidence for these in IL-10 signal transduction is lacking.37,41 Translocation of activated STATs to the nucleus enacts high affinity binding with STAT-binding elements (SBEs), which promotes transcription of IL-10 responsive genes, including, for example, transcriptional regulators (Bcl-3 and Nfil3), signaling modulators (Dusp1), and those that can contribute to alternative macrophage activation.42
Regulation of JAK-STAT signal transduction downstream of the IL-10 receptor complex occurs at both the extracellular and intracellular interface to prevent excessive immune suppression or alternative macrophage activation that can impede efficient antimicrobial activity. Several regulators antagonize the translocation of signal transducers that induce the transcription of various IL-10 responsive genes within the nucleus such as those aforementioned. The most studied regulators in this regard are the suppressors of cytokine signaling (SOCS) proteins. SOCS1 and SOCS3 are both produced in response to IL-10, and both function to suppress JAK-STAT signaling, but via different mechanisms.43 In this manner, SOCS1 and SOCS3 regulate pro-inflammatory responses including TNF-α, IFN-γ, IL-6 and nitric oxide production via negative feedback loops that operate alongside IL-10 signaling.43-48 Where SOCS1 suppresses JAK activity directly by binding of its SH2 domain to tyrosine-phosphorylated JAK, SOCS3 necessarily binds to the activated receptor to inhibit JAK activity.43 Both SOCS also indirectly affect IL-10-associated JAK-STAT signaling through the MAPK system.49,50 With regard to pro-IL-10 responses, SOCS3 is produced in an LPS-dependent manner in macrophages as a result of TLR4 signaling, which activates the MAPK signaling cascade of ERK1/2, p38 and JNK.51 This induces IL-10, which drives JAK-STAT by activating STAT3, and this triggers further SOCS3 production.51 IL-10 induces STAT3 activation in monocytes52 thereby suppressing pro-inflammatory responses including TNF-α and NO production.45,46 SOCS3 is also the main negative feedback regulator for IL-6-mediated activation of the JAK-STAT pathway.53 However, it is important to note that SOCS3 is not a mediator of macrophage deactivation per se, although it is induced strongly by IL-10. Instead, SOCS3 controls STAT activation by IL-6.54-56 In the absence of SOCS3, IL-6 causes persistent STAT3 activation that correlates with an anti-inflammatory effect similar to the one induced by IL-10. IL-10 signaling appears to be insensitive to SOCS3 as a feedback inhibitor probably because the IL-10 receptor complex has no phosphotyrosine motifs to act as a SOCS3 recruitment site.53 SOCS1, the main function of which is the negative control of IFN-γ signaling,57 is also produced in an IL-10-dependent manner, and is thought to be responsible for the negative feedback inhibition of IL-10.53 Thus, both SOCS are involved in negative feedback inhibition of JAK-STAT signaling through distinct mechanisms, which enables them to contribute to fine-tuning IL-10 signaling and aid the balance of pro-inflammatory responses during infection.
Another regulatory mechanism in place to control the IL-10-JAK-STAT circuit is post-translational modification of STATs (including STAT3) by acetylation and serine phosphorylation. These effects provide some late-stage signal control to modulate the circuit after initiation of signaling, but also, some early control since, for example, STAT3 serine727 phosphorylation occurs rapidly after IL-10 or IL-6 stimulation and can influence STAT activity and cell differentiation.58 Other late-stage regulatory mechanisms include the protein inhibitors of activated STATs (PIAS), such as STAT3-specific PIAS3.59 The extent to which these late-stage mechanisms exert control over IL-10 signaling subsequent to STAT3 activation, however, remains unclear.60 One other class of negative regulators of cytokine secretion also impacts IL-10 activity: the so-called SH2-containing protein tyrosine phosphatases (SHPs).61 While PIAS proteins inhibit STAT dimerization and prevent STATs interacting with DNA, which restricts their availability,62 SHPs, in contrast, inhibit signaling by recruitment to phosphorylated tyrosine residues after JAK activation and dephosphorylate signaling components essential to kinase activation.61,62 Together, these composites of late-stage regulatory mechanisms illustrate the balance of IL-10-JAK-STAT signaling that is required to manage successful antimicrobial responses.
Finally, several accessory molecules influence the modulation of antimicrobial function in response to IL-10 through STAT3. One pathway that influences the IL-10 JAK-STAT circuit is through the kinases PI3K-Akt-glycogen synthase kinase 3 (GSK3) (PI3K-Akt-GSK3). This module was recently shown to positively regulate STAT3 signaling in macrophages.63 SOCS1 and SOCS3 positively regulate κB-containing promoter activity in a NFκB subunit p65/RelA-dependent manner in macrophages, leading to modulation of NFκB signaling and transcriptional responses.47,64 The exact mechanism behind this is not yet clear however it may be that the SOCS proteins inhibit the activation of STATs, which, in turn alleviates the competition between STATs and RelA for binding p300 in the nucleus, leading to activation of RelA-dependent transcription.65 IL-10 also induces Bcl-3,66,67 which interacts with the p50 subunit of NFκB, to bind to the TNF-α promoter and inhibits its production in response to LPS. Bcl-3 can boost NFκB-dependent gene transcriptional activation, so it may contribute to the inhibition of cytokines by upregulating IL-10-induced genes.1
Microbes Influence Pathogenesis Directly through the IL-10-JAK-STAT Circuit
The best-studied microbial product in relation to IL-10 synthesis and JAK-STAT signal transduction is LPS. Overall, IL-10 causes the downregulation of a number of LPS-inducible genes that encode pro-inflammatory mediators including IL-1 and IL-6,67,68 IFN-γ-inducible genes including the nitric oxide synthase gene,69 and IL-4-inducible genes including those encoding MHC class II.70 The products of these genes contribute to effective antimicrobial responses against several pathogens, which helps to explain the deleterious effects of IL-10 on immune control of these infections.71 In contrast, IL-10 is essential to the control of fatal hyperactive immune stimulation caused by the over-production of these mediators during systemic LPS challenge.72 This is illustrated in Figure 1 where systemic LPS drives excessive STAT1-triggered pro-inflammatory responses after initial signaling through TLR4, resulting in responses that cannot be controlled by SOCS-regulated immune suppression. Here, IL-10 determines the transition from reversible sepsis to irreversible shock by counterbalancing pro-inflammation with pro-suppression.73
For some pathogens, the LPS effects on IL-10 responses play an important role in pathogenesis. In Bordetella pertussis infection, for example, innate resistance hinges on TLR4 recognition of LPS and subsequent synthesis of IL-10, which inhibits inflammatory pathology that contributes to disease.74 Additional protective roles for IL-10 in reversing the lethal effects of LPS were recently shown in a lung injury model that examined IL-18 supplementation and the requirement of IL-10 in immune protection.75 Further evidence of the beneficial biological impact of IL-10 in specific infection involving LPS comes from studies in conditional STAT3−/− mice, where mice develop endotoxemia from excessive TNF-α, IL-1β and IFN-γ and succumb to septic peritonitis and multiple organ failure during systemic infection.16,76 These observations demonstrate the essential link between IL-10 and STAT3 for moderation of immune hyperactivity in response to Gram-negative pathogens and control of acute disease. Finally, a recent study showed that mice are protected from lethal endotoxic shock by liposomal delivery of SOCS3 plasmid DNA, which inhibits the development of macrophage LPS tolerance, although these observations need to be confirmed.77 Such endotoxin tolerance and refractory phenotypes in monocytes, which are associated with systemic Gram-negative infection and the progression of sepsis, are associated with a failure to upregulate inflammatory cytokines as a result of IL-10 synthesis.78 Thus, liposomal delivery of SOCS3 might represent an attractive means of controlling hyperactive immune responses at the level of systemic infection. In models of immune protection, prevention of LPS-induced TNF-α through IL-10-mediated JAK-STAT signaling via STAT3 probably represents the major mechanism of counter-acting brutal inflammatory responses that mediate collateral tissue pathology.32 Separate from TLR4, however, there are numerous other bacterial factors that influence JAK-STAT signaling events associated with IL-10 following activation of TLRs. For example lipoteichoic acid, which signals through TLR2, and CpG-ODN that signals through TLR9, are both able to inhibit the ability of IL-10 to induce the phosphorylation of STAT3 in macrophages through suppression of IL-10R function.79 There are also reports of other specific virulence-associated bacterial molecules effecting IL-10 activities following TLR ligation, separate from TLR4 engagement by LPS. For example, LcrV protein from Yersinia pestis specifically hijacks the TLR2/6 pathway to stimulate IL-10 production, and this impedes host protective inflammatory responses.80-82 The role of defined JAK-STAT signaling mechanisms in these IL-10 responses however, including those responses triggered by LcrV remains largely uncharacterized.
The relevance of observations of the effects of bacterial molecules beyond LPS on the signaling activity of the IL-10-JAK-STAT module is exemplified in infections involving strict intracellular pathogens such as Coxiella burnetii and leishmania. Here, the pathogenesis of infection is dramatically influenced by the dynamics of IL-10 and downstream JAK-STAT signaling through STAT3. In Q fever, for example, the genes for both IL-10 and STAT3 are highly upregulated by C. burnetii in males.83 This response has been correlated with IL-10-associated bacterial survival in monocytes where the immune suppressive effects of STAT3 activation may dampen cellular antimicrobial effector mechanisms and lead to poor microbe killing.84 In macrophages engineered to overexpress IL-10, C. burnetii survival has been associated with a non-microbicidal transcriptional program consisting of increased expression of arginase-1, mannose receptor and Ym1/2. This contrasts with a phenotype of inducible NO synthase and inflammatory cytokines needed to kill the bacteria, which occurs in the absence of high level IL-10 synthesis.85 These findings illustrate the importance of the level of activation of the IL-10-JAK-STAT circuitry based on engineered cell lines, which are impossible to gauge in IL-10-deficient or STAT3−/− mice. Similarly, transgenic mice overexpressing IL-10 can be good models with which to study the effects of IL-10 in chronic infections.42,86-88 Analogous to the pathogenesis of C. burnetii infection, IL-10 blocks bacterial killing in Mycobacterium tuberculosis-infected human macrophages by inhibiting phagosome maturation. In this case, the effect of IL-10 was shown to be STAT3-dependent, but independent of MAPKp38 and ERK1/2 activity in a recent study.89 In contrast to the pathogenesis of Q-fever, the production of SOCS3 in certain protozoan diseases appears to represent an essential negative feedback mechanism for driving immunity against intracellular parasites. For example, during Leishmania major infection, the production of SOCS3 diminishes IL-10 synthesis, which contributes to effective protozoan clearance (Fig. 1).90,91 Leishmania donovani, on the other hand, induces IL-10, and the subsequent STAT3 activation that drives the expression of IL-4Rα and arginase 1 enables these intracellular pathogens to circumvent NO-dependent killing by macrophages.92 The differential effects of IL-10 during infection with intracellular pathogens such as these may depend on the cellular source of IL-10 during infection and its interaction with infected vs. non-infected cells, as recently reviewed.49
Recent studies on classical extracellular pathogens that display intracellular lifestyle traits such as E. coli93-95 and streptococci96,97 have also revealed roles for IL-10-JAK-STAT signaling in disease pathogenesis. Uropathogenic E. coli (UPEC) are a primary cause of urinary tract infections whereas Streptococcus agalactiae mediates infections during pregnancy, and in neonates and elderly individuals.98 Observations that UPEC can invade and replicate within epithelial cells suggest that this organism may occupy an intracellular niche within the host. UPEC is also able to survive within primary mouse bone marrow-derived macrophages and a recent study suggested that some UPEC might subvert macrophage antimicrobial pathways similar to intracellular pathogens.93 S. agalactiae also displays some intracellular lifestyle straits such as survival in macrophages and induction of apoptosis in host cells.99 Both of these microbes trigger upregulation of IL-10 during infection of the urinary tract, and for UPEC this is associated with JAK-STAT signaling and SOCS3.7 In fact, JAK-STAT signaling was among the most highly activated canonical pathways triggered by UPEC during cystitis.7 Thus, IL-10 and related JAK-STAT signaling appears to be important in early immune responses in the bladder to these pathogens that display some traits of intracellular lifestyles within the host.
Viral-host pathogen studies have also provided important insights into the role of IL-10 in antimicrobial responses at the JAK-STAT axis. In co-infection models of HIV, for example, the virus stimulates infected cells to produce IL-10, which activates STAT3 to impede autophagy of bystander macrophages and monocytes (Fig. 1). The inhibition of phagocytic cell death has a direct impact of the pathogenesis of co-infection with other intracellular pathogens, whereby macrophages are prevented from normal killing of co-infecting M. tuberculosis and Toxoplasma gondii.100 Cytomegalovirus (CMV), which is typically associated with disease in the immune-compromised such as HIV-infected persons, synthesizes its own IL-10 homologs. These viral-derived IL-10 homologs interact with the IL-10 receptor complex on human cells, and thereby, directly compete with human IL-10 for receptor binding. As a result, CMV IL-10 homologs interfere with downstream signaling events stemming from ligation of the IL-10 receptor complex during immune responses to the virus (Fig. 1), which circumvents elimination of the virus and leads to chronic infection.101 In the pathogenesis of acute CMV infection, however, IL-10R signaling has been implicated in promoting the survival of NK cells, which contribute to innate responses and effective clearance of the virus.102 Together, the nuanced effects of IL-10 signaling through JAK-STAT during HIV and CMV infection emphasize the varied outcomes that can result from viral interference with this circuit in acute and chronic disease depending on the virus and circumstances of infection.
Some Pathogens Can Activate STAT3 Independent of IL-10
Many immune suppression signals in response to microbes occur as above through engagement of the IL-10 receptor complex and activation of the cognate downstream JAK-STAT module. However, not all mechanisms of immune suppression during infection are absolutely dependent on these signaling pathways through the IL-10-JAK-STAT module. For example, the intracellular pathogen T. gondii activates JAK-STAT signaling including anti-inflammatory STAT3 in an IL-10-independent manner103 to bring about suppression of TNF-α synthesis during infection and macrophage apoptosis (Fig. 1). Interference with host cell death is important in pathogenesis since enhanced microbe clearance related to the induction of apoptosis is a known effector mechanism in combating some intracellular pathogens.104 The suppressive effects of Toxoplasma on LPS-induced cytokine synthesis and IFN-γ-induced nitric oxide are mediated by the microbes’ rhoptry kinase, ROP16, which is injected into the host cell. Here, the enzyme activates STAT3, as well as STAT6 to bring about immune suppression that includes direct effects on arginase-1.105,106 Mice harboring a deletion of SOCS3 in macrophages succumb to toxoplasmosis, but their resistance is restored by anti-IL-6 administration, suggesting that in the absence of SOCS3, macrophages are hypersensitive to the anti-inflammatory properties of IL-6.107 These signaling events involving STAT3 and SOCS3 during T. gondii infection directly impact the pathogenesis of disease but do so independently of IL-10. Similar to T. gondii, Salmonella enterica induces IL-10-independent STAT3 activation in macrophages via an unknown mechanism. Immune suppression prevents severe inflammatory consequences at the gut epithelium associated with this infection.108 Thus, both of these organisms provide examples of microbes that induce immune suppression via STAT3 activation in an IL-10-independent manner, which influences microbe survival and pathogenesis. It will be important to determine the mechanisms of how these pathogens induce STAT3-driven immune suppressive effects independent of IL-10. For example, are there homologs of ROP16 that can activate STAT3 as observed for the protozoan T. gondii in other bacterial pathogens? What role, if any, do other cytokines such as IL-6 have in IL-10-independent activation of STAT3 during infection for immune suppression in response to pathogens like T. gondii and S. enterica?
Conclusions
The interplay that occurs between signaling pathways in response to IL-10 and antimicrobial outputs has emerged as a complex series of activating and inhibitory regulatory molecules that function through JAK-STAT. How pathogens hijack the JAK-STAT module through IL-10-dependent and -independent mechanisms in a manner that benefits their survival within the host is an intriguing area of current research. There are many other questions in addition to those above related to what gene targets are activated by pathogens that utilize the IL-10-JAK-STAT module for subversion of host immune responses. How such targets might be exploited for therapeutic benefit will be an important area for future exploration. In light of the recent studies these areas of research are primed for investigation.
Acknowledgments
This work was supported by a grant from the Australian National Health and Medical Research Council (569674). G.C.U. is supported by a Future Fellowship (FT110101048) from the Australian Research Council. C.K.T. is supported by a Prime Ministers Endeavor Fellowship from the Australian Government. We thank Roland Lang for critical review of the manuscript.
Glossary
Abbreviations:
- IL-10R
IL-10 receptor
- JAK1
Janus kinase 1
- TYK2
tyrosine kinase 2
- MHC II
major histocompatibility complex class II
- STAT
signal transducer and activator of transcription
- PI3K
phosphatidyl inositol 3-kinase
- AKT/PKB
AKT/protein kinase B
- MAPK
mitogen-activated protein kinase
- SOCS
suppressor of cytokine signaling
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- TNF-α
tumor necrosis factor-α
- IFN-γ
interferon-γ
- NFκB
nuclear factor κ-light-chain-enhancer of activated B cells
- IκB-α
inhibitory subunit of NFκB-α
- P
phosphorylation
- R
receptor
- HIV
human immunodeficiency virus
- CMV
cytomegalovirus
- ROP16
rhoptry kinase of T. gondii
- PRR
pattern recognition receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/19918
References
- 1.Lang R. Tuning of macrophage responses by Stat3-inducing cytokines: molecular mechanisms and consequences in infection. Immunobiology. 2005;210:63–76. doi: 10.1016/j.imbio.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–9. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 3.Najjar I, Fagard R. STAT1 and pathogens, not a friendly relationship. Biochimie. 2010;92:425–44. doi: 10.1016/j.biochi.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Haan JM, Kraal G, Bevan MJ. Cutting edge: Lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J Immunol. 2007;178:5429–33. doi: 10.4049/jimmunol.178.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson MS, Cheever AW, White SD, Thompson RW, Wynn TA. IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni. PLoS Pathog. 2011;7:e1002171. doi: 10.1371/journal.ppat.1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan A, Lynch M, Smith SM, Amu S, Nel HJ, McCoy CE, et al. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 2011;7:e1002076. doi: 10.1371/journal.ppat.1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duell BL, Carey AJ, Tan CK, Cui X, Webb RI, Totsika M, et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J Immunol. 2012;188:781–92. doi: 10.4049/jimmunol.1101231. [DOI] [PubMed] [Google Scholar]
- 8.Specht S, Taylor MD, Hoeve MA, Allen JE, Lang R, Hoerauf A. Over expression of IL-10 by macrophages overcomes resistance to murine filariasis. Exp Parasitol. 2012;132:90–6. doi: 10.1016/j.exppara.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–20. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 10.Kirschning CJ, Schumann RR. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr Top Microbiol Immunol. 2002;270:121–44. doi: 10.1007/978-3-642-59430-4_8. [DOI] [PubMed] [Google Scholar]
- 11.Ramos HC, Rumbo M, Sirard JC. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 2004;12:509–17. doi: 10.1016/j.tim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Yeganegi M, Leung CG, Martins A, Kim SO, Reid G, Challis JR, et al. Lactobacillus rhamnosus GR-1-induced IL-10 production in human placental trophoblast cells involves activation of JAK/STAT and MAPK pathways. Reprod Sci. 2010;17:1043–51. doi: 10.1177/1933719110377237. [DOI] [PubMed] [Google Scholar]
- 15.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/S1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 17.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–45. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duell BL, Tan CK, Carey AJ, Wu F, Cripps AW, Ulett GC. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol Med Microbiol. 2012;64:295–313. doi: 10.1111/j.1574-695X.2012.00931.x. [DOI] [PubMed] [Google Scholar]
- 19.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 20.Cheon H, Rho YH, Choi SJ, Lee YH, Song GG, Sohn J, et al. Prostaglandin E2 augments IL-10 signaling and function. J Immunol. 2006;177:1092–100. doi: 10.4049/jimmunol.177.2.1092. [DOI] [PubMed] [Google Scholar]
- 21.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 22.Tang-Feldman YJ, Lochhead GR, Lochhead SR, Yu C, Pomeroy C. Interleukin-10 repletion suppresses pro-inflammatory cytokines and decreases liver pathology without altering viral replication in murine cytomegalovirus (MCMV)-infected IL-10 knockout mice. Inflamm Res. 2011;60:233–43. doi: 10.1007/s00011-010-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilliams M, Movahedi K, Bosschaerts T, VandenDriessche T, Chuah MK, Hérin M, et al. IL-10 dampens TNF/inducible nitric oxide synthase-producing dendritic cell-mediated pathogenicity during parasitic infection. J Immunol. 2009;182:1107–18. doi: 10.4049/jimmunol.182.2.1107. [DOI] [PubMed] [Google Scholar]
- 24.van der Sluis M, Bouma J, Vincent A, Velcich A, Carraway KL, Büller HA, et al. Combined defects in epithelial and immunoregulatory factors exacerbate the pathogenesis of inflammation: mucin 2-interleukin 10-deficient mice. Lab Invest. 2008;88:634–42. doi: 10.1038/labinvest.2008.28. [DOI] [PubMed] [Google Scholar]
- 25.Balcewicz-Sablinska MK, Gan H, Remold HG. Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNF-alpha activity. J Infect Dis. 1999;180:1230–7. doi: 10.1086/315011. [DOI] [PubMed] [Google Scholar]
- 26.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–7. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 27.Beamer GL, Flaherty DK, Assogba BD, Stromberg P, Gonzalez-Juarrero M, de Waal Malefyt R, et al. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J Immunol. 2008;181:5545–50. doi: 10.4049/jimmunol.181.8.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–51. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–73. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 30.Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW. A receptor for interleukin 10 is related to interferon receptors. Proc Natl Acad Sci U S A. 1993;90:11267–71. doi: 10.1073/pnas.90.23.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Y, Qin L, Zamarin D, Kotenko SV, Pestka S, Moore KW, et al. Differential IL-10R1 expression plays a critical role in IL-10-mediated immune regulation. J Immunol. 2001;167:6884–92. doi: 10.4049/jimmunol.167.12.6884. [DOI] [PubMed] [Google Scholar]
- 32.Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513–21. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- 33.Schaljo B, Kratochvill F, Gratz N, Sadzak I, Sauer I, Hammer M, et al. Tristetraprolin is required for full anti-inflammatory response of murine macrophages to IL-10. J Immunol. 2009;183:1197–206. doi: 10.4049/jimmunol.0803883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, et al. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith AM, Qualls JE, O’Brien K, Balouzian L, Johnson PF, Schultz-Cherry S, et al. A distal enhancer in Il12b is the target of transcriptional repression by the STAT3 pathway and requires the basic leucine zipper (B-ZIP) protein NFIL3. J Biol Chem. 2011;286:23582–90. doi: 10.1074/jbc.M111.249235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–90. [PubMed] [Google Scholar]
- 37.Wehinger J, Gouilleux F, Groner B, Finke J, Mertelsmann R, Weber-Nordt RM. IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes. FEBS Lett. 1996;394:365–70. doi: 10.1016/0014-5793(96)00990-8. [DOI] [PubMed] [Google Scholar]
- 38.Giordano V, De Falco G, Chiari R, Quinto I, Pelicci PG, Bartholomew L, et al. Shc mediates IL-6 signaling by interacting with gp130 and Jak2 kinase. J Immunol. 1997;158:4097–103. [PubMed] [Google Scholar]
- 39.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 40.Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997;16:5894–903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber-Nordt RM, Riley JK, Greenlund AC, Moore KW, Darnell JE, Schreiber RD. Stat3 recruitment by two distinct ligand-induced, tyrosine-phosphorylated docking sites in the interleukin-10 receptor intracellular domain. J Biol Chem. 1996;271:27954–61. doi: 10.1074/jbc.271.44.27954. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber T, Ehlers S, Heitmann L, Rausch A, Mages J, Murray PJ, et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–12. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Y, Chen D, Tarcsafalvi A, Su R, Qin L, Bromberg JS. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J Immunol. 2003;170:1383–91. doi: 10.4049/jimmunol.170.3.1383. [DOI] [PubMed] [Google Scholar]
- 44.Johnston JA. Are SOCS suppressors, regulators, and degraders? J Leukoc Biol. 2004;75:743–8. doi: 10.1189/jlb.1003507. [DOI] [PubMed] [Google Scholar]
- 45.Prêle CM, Keith-Magee AL, Yerkovich ST, Murcha M, Hart PH. Suppressor of cytokine signalling-3 at pathological levels does not regulate lipopolysaccharide or interleukin-10 control of tumour necrosis factor-alpha production by human monocytes. Immunology. 2006;119:8–17. doi: 10.1111/j.1365-2567.2006.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qasimi P, Ming-Lum A, Ghanipour A, Ong CJ, Cox ME, Ihle J, et al. Divergent mechanisms utilized by SOCS3 to mediate interleukin-10 inhibition of tumor necrosis factor alpha and nitric oxide production by macrophages. J Biol Chem. 2006;281:6316–24. doi: 10.1074/jbc.M508608200. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, et al. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci U S A. 2000;97:6493–8. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoiber D, Kovarik P, Cohney S, Johnston JA, Steinlein P, Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J Immunol. 1999;163:2640–7. [PubMed] [Google Scholar]
- 49.Cyktor JC, Turner J. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect Immun. 2011;79:2964–73. doi: 10.1128/IAI.00047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN. Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J Immunol. 2007;179:5966–76. doi: 10.4049/jimmunol.179.9.5966. [DOI] [PubMed] [Google Scholar]
- 52.Lee EB, Kim A, Kang K, Kim H, Lim JS. NDRG2-mediated Modulation of SOCS3 and STAT3 Activity Inhibits IL-10 Production. Immune Netw. 2010;10:219–29. doi: 10.4110/in.2010.10.6.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–72. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 54.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–5. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 55.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, et al. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–50. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 56.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–6. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 57.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–16. doi: 10.1016/S0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 58.Duplomb L, Baud’huin M, Charrier C, Berreur M, Trichet V, Blanchard F, et al. Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology. 2008;149:3688–97. doi: 10.1210/en.2007-1719. [DOI] [PubMed] [Google Scholar]
- 59.Yagil Z, Nechushtan H, Kay G, Yang CM, Kemeny DM, Razin E. The enigma of the role of protein inhibitor of activated STAT3 (PIAS3) in the immune response. Trends Immunol. 2010;31:199–204. doi: 10.1016/j.it.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Ivashkiv LB, Hu X. Signaling by STATs. Arthritis Res Ther. 2004;6:159–68. doi: 10.1186/ar1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larsen L, Röpke C. Suppressors of cytokine signalling: SOCS. APMIS. 2002;110:833–44. doi: 10.1034/j.1600-0463.2002.1101201.x. [DOI] [PubMed] [Google Scholar]
- 62.Greenhalgh CJ, Hilton DJ. Negative regulation of cytokine signaling. J Leukoc Biol. 2001;70:348–56. [PubMed] [Google Scholar]
- 63.Antoniv TT, Ivashkiv LB. Interleukin-10-induced gene expression and suppressive function are selectively modulated by the PI3K-Akt-GSK3 pathway. Immunology. 2011;132:567–77. doi: 10.1111/j.1365-2567.2010.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SH, Kim KE, Hwang HY, Kim TY. Regulatory effect of SOCS on NF-kappaB activity in murine monocytes/macrophages. DNA Cell Biol. 2003;22:131–9. doi: 10.1089/104454903321515931. [DOI] [PubMed] [Google Scholar]
- 65.Hottiger MO, Felzien LK, Nabel GJ. Modulation of cytokine-induced HIV gene expression by competitive binding of transcription factors to the coactivator p300. EMBO J. 1998;17:3124–34. doi: 10.1093/emboj/17.11.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, et al. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102:4123–9. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- 67.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 68.Williams L, Jarai G, Smith A, Finan P. IL-10 expression profiling in human monocytes. J Leukoc Biol. 2002;72:800–9. [PubMed] [Google Scholar]
- 69.Cunha FQ, Moncada S, Liew FY. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–9. doi: 10.1016/0006-291X(92)91852-H. [DOI] [PubMed] [Google Scholar]
- 70.de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 72.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–8. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latifi SQ, O’Riordan MA, Levine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun. 2002;70:4441–6. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higgins SC, Lavelle EC, McCann C, Keogh B, McNeela E, Byrne P, et al. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J Immunol. 2003;171:3119–27. doi: 10.4049/jimmunol.171.6.3119. [DOI] [PubMed] [Google Scholar]
- 75.Sekine K, Fujishima S, Sasaki J, Ishizaka A, Aiso S, Aikawa N. In vivo IL-18 supplementation ameliorates lethal acute lung injury in burn-primed endotoxemic mice: a novel anti-inflammatory role of IL-18. Shock. 2009;32:554–62. doi: 10.1097/SHK.0b013e31819e2db6. [DOI] [PubMed] [Google Scholar]
- 76.Sakamori R, Takehara T, Ohnishi C, Tatsumi T, Ohkawa K, Takeda K, et al. Signal transducer and activator of transcription 3 signaling within hepatocytes attenuates systemic inflammatory response and lethality in septic mice. Hepatology. 2007;46:1564–73. doi: 10.1002/hep.21837. [DOI] [PubMed] [Google Scholar]
- 77.Fang M, Dai H, Yu G, Gong F. Gene delivery of SOCS3 protects mice from lethal endotoxic shock. Cell Mol Immunol. 2005;2:373–7. [PubMed] [Google Scholar]
- 78.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–87. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez S, Jose P, Avdiushko MG, Kaplan AM, Cohen DA. Inhibition of IL-10 receptor function in alveolar macrophages by Toll-like receptor agonists. J Immunol. 2004;172:2613–20. doi: 10.4049/jimmunol.172.4.2613. [DOI] [PubMed] [Google Scholar]
- 80.Sing A, Reithmeier-Rost D, Granfors K, Hill J, Roggenkamp A, Heesemann J. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc Natl Acad Sci U S A. 2005;102:16049–54. doi: 10.1073/pnas.0504728102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Depaolo RW, Tang F, Kim I, Han M, Levin N, Ciletti N, et al. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4:350–61. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brubaker RR. Interleukin-10 and inhibition of innate immunity to Yersiniae: roles of Yops and LcrV (V antigen) Infect Immun. 2003;71:3673–81. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Textoris J, Ban LH, Capo C, Raoult D, Leone M, Mege JL. Sex-related differences in gene expression following Coxiella burnetii infection in mice: potential role of circadian rhythm. PLoS One. 2010;5:e12190. doi: 10.1371/journal.pone.0012190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meghari S, Capo C, Raoult D, Mege JL. Deficient transendothelial migration of leukocytes in Q fever: the role played by interleukin-10. J Infect Dis. 2006;194:365–9. doi: 10.1086/505227. [DOI] [PubMed] [Google Scholar]
- 85.Ghigo E, Pretat L, Desnues B, Capo C, Raoult D, Mege JL. Intracellular life of Coxiella burnetii in macrophages. Ann N Y Acad Sci. 2009;1166:55–66. doi: 10.1111/j.1749-6632.2009.04515.x. [DOI] [PubMed] [Google Scholar]
- 86.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–21. [PubMed] [Google Scholar]
- 87.Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J Immunol. 2002;168:3402–11. doi: 10.4049/jimmunol.168.7.3402. [DOI] [PubMed] [Google Scholar]
- 88.Meghari S, Bechah Y, Capo C, Lepidi H, Raoult D, Murray PJ, et al. Persistent Coxiella burnetii infection in mice overexpressing IL-10: an efficient model for chronic Q fever pathogenesis. PLoS Pathog. 2008;4:e23. doi: 10.1371/journal.ppat.0040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Leary S, O’Sullivan MP, Keane J. IL-10 blocks phagosome maturation in mycobacterium tuberculosis-infected human macrophages. Am J Respir Cell Mol Biol. 2011;45:172–80. doi: 10.1165/rcmb.2010-0319OC. [DOI] [PubMed] [Google Scholar]
- 90.Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med. 2006;203:1021–31. doi: 10.1084/jem.20052333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakaya M, Hamano S, Kawasumi M, Yoshida H, Yoshimura A, Kobayashi T. Aberrant IL-4 production by SOCS3-over-expressing T cells during infection with Leishmania major exacerbates disease manifestations. Int Immunol. 2011;23:195–202. doi: 10.1093/intimm/dxq472. [DOI] [PubMed] [Google Scholar]
- 92.Biswas A, Bhattacharya A, Kar S, Das PK. Expression of IL-10-triggered STAT3-dependent IL-4Rα is required for induction of arginase 1 in visceral leishmaniasis. Eur J Immunol. 2011;41:992–1003. doi: 10.1002/eji.201040940. [DOI] [PubMed] [Google Scholar]
- 93.Bokil NJ, Totsika M, Carey AJ, Stacey KJ, Hancock V, Saunders BM, et al. Intramacrophage survival of uropathogenic Escherichia coli: differences between diverse clinical isolates and between mouse and human macrophages. Immunobiology. 2011;216:1164–71. doi: 10.1016/j.imbio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 94.Berry RE, Klumpp DJ, Schaeffer AJ. Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli. Infect Immun. 2009;77:2762–72. doi: 10.1128/IAI.00323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ulett GC, Adderson EE. Nitric oxide is a key determinant of group B streptococcus-induced murine macrophage apoptosis. J Infect Dis. 2005;191:1761–70. doi: 10.1086/429693. [DOI] [PubMed] [Google Scholar]
- 97.Ulett GC, Bohnsack JF, Armstrong J, Adderson EE. Beta-hemolysin-independent induction of apoptosis of macrophages infected with serotype III group B streptococcus. J Infect Dis. 2003;188:1049–53. doi: 10.1086/378202. [DOI] [PubMed] [Google Scholar]
- 98.Ulett KB, Benjamin WH, Jr., Zhuo F, Xiao M, Kong F, Gilbert GL, et al. Diversity of group B streptococcus serotypes causing urinary tract infection in adults. J Clin Microbiol. 2009;47:2055–60. doi: 10.1128/JCM.00154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ulett GC, Maclean KH, Nekkalapu S, Cleveland JL, Adderson EE. Mechanisms of group B streptococcal-induced apoptosis of murine macrophages. J Immunol. 2005;175:2555–62. doi: 10.4049/jimmunol.175.4.2555. [DOI] [PubMed] [Google Scholar]
- 100.Van Grol J, Subauste C, Andrade RM, Fujinaga K, Nelson J, Subauste CS. HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PLoS One. 2010;5:e11733. doi: 10.1371/journal.pone.0011733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc Natl Acad Sci U S A. 2000;97:1695–700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stacey MA, Marsden M, Wang EC, Wilkinson GW, Humphreys IR. IL-10 restricts activation-induced death of NK cells during acute murine cytomegalovirus infection. J Immunol. 2011;187:2944–52. doi: 10.4049/jimmunol.1101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY. IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. J Immunol. 2005;174:3148–52. doi: 10.4049/jimmunol.174.6.3148. [DOI] [PubMed] [Google Scholar]
- 104.Ulett GC, Adderson EE. Regulation of Apoptosis by Gram-Positive Bacteria: Mechanistic Diversity and Consequences for Immunity. Curr Immunol Rev. 2006;2:119–41. doi: 10.2174/157339506776843033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Butcher BA, Fox BA, Rommereim LM, Kim SG, Maurer KJ, Yarovinsky F, et al. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 2011;7:e1002236. doi: 10.1371/journal.ppat.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ong YC, Reese ML, Boothroyd JC. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J Biol Chem. 2010;285:28731–40. doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whitmarsh RJ, Gray CM, Gregg B, Christian DA, May MJ, Murray PJ, et al. A critical role for SOCS3 in innate resistance to Toxoplasma gondii. Cell Host Microbe. 2011;10:224–36. doi: 10.1016/j.chom.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin T, Bost KL. STAT3 activation in macrophages following infection with Salmonella. Biochem Biophys Res Commun. 2004;321:828–34. doi: 10.1016/j.bbrc.2004.07.039. [DOI] [PubMed] [Google Scholar]