Abstract
Janus kinases (JAKs) are non-receptor tyrosine kinases essential for activation of signaling mediated by cytokine receptors that lack catalytic activity, including receptors for erythropoietin, thrombopoietin, most interleukins and interferon. Upon hormone binding, JAKs phosphorylate tyrosine residues in the receptor cytoplasmic domains and in JAKs themselves leading to recruitment and activation of downstream signaling proteins such as signal transducer and activator of transcription (STAT). The JAK-STAT pathway is important for functional hematopoiesis and several activating mutations in JAK proteins have recently been described as underlying cause of blood disorders. One of the best studied examples is the JAK2 V617F mutant which is found in 95% of polycythemia vera patients and 50% of patients suffering from essential thrombocythemia and primary myelofibrosis. Much effort has been made to understand how the JAK2 V617F affects hematopoietic stem cell (HSC) renewal and lineage differentiation, since convincing evidence has been provided to support the notion that the mutation is acquired at the HSC level. We discuss several in vivo models that support contrary conclusions with respect to the advantage given to HSCs by JAK2 V617F. Moreover, we provide the current knowledge about STAT5 activation and its link to HSC expansion as well as amplification of the erythroid compartment. Evidence for both JAK2 V617F mutated HSCs exhibiting skewed differentiation potential and for amplification occurring after erythroid commitment has been provided, and we will discuss whether this evidence is relevant for the disease.
Keywords: JAK-STAT, JAK2 V617F, myeloproliferative disorders, hematopoietic stem cells, mouse models
Introduction
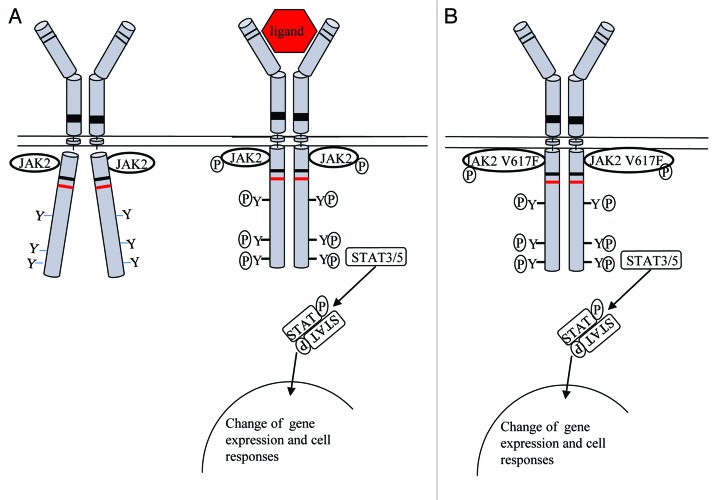
Type I cytokine receptors, such as receptors for erythropoietin (Epo), thrombopoietin (Tpo), granulocyte colony stimulating factor (G-CSF) and most interleukins (IL) receptors, and type II interferons (IFN) lack intrinsic catalytic activity. In order to initiate downstream signaling these receptors bind one or several members of cytoplasmic tyrosine kinases of the Janus kinase (JAK) family (Fig. 1A). After binding of the cytokine to the receptor extracellular domain two scenarios are possible: (1) the receptor exists as preformed dimer at the cell surface and ligand binding induces a conformational change; or (2) dimerization or oligomerization is induced after ligand binding and thus induces a conformational change of the receptor. The conformational change brings the cognate JAK proteins in close enough proximity that they transphosphorylate each other (Fig. 1A). In turn, activated JAK proteins phosphorylate tyrosine residues in the receptor cytoplasmic domain and in JAKs themselves and provide docking sites for signaling proteins such as signal transducer and activators of transcription (STAT). STAT proteins become thus substrates and are phosphorylated by JAKs, form dimers and translocate to the nucleus where they modify gene expression. Several activating mutations have been identified in JAK proteins. In particular the JAK2 V617F mutant has been studied in great detail and our current understanding about its function is highlighted in this review.
Figure 1. Signaling via the JAK-STAT pathway. (A) Ligand binding induces a conformational change of the cytokine receptor and allows transphosphorylation of JAK proteins. Activated JAKs phosphorylate tyrosine residues in the receptor cytoplasmic domain and provide docking site for STAT proteins. Phosphorylated STATs dissociate from the receptor, dimerize and translocate to the nucleus where they modulate gene expression. (B) In case of the JAK2 V617F, mutant cytosolic tyrosines are constantly phosphorylated, leading to constitutive activation of STAT proteins.
The JAK2 V617F Mutant and Myeloproliferative Disorders
Myeloproliferative neoplasms (MPNs) are a group of blood disorders characterized by an overproduction of one or more of the myelo-erythroid lineages (Fig. 2B).1 The three most common MPNs are polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF). Features of PV are the overproduction of mature, functional red blood cells, leading to an increased red-cell mass and hematocrit, while ET patients have an increase in platelet numbers and PMF is characterized by scarring of the bone marrow with fibrosis and extramedullary hematopoiesis. The JAK2 V617F mutation was first described in 2005 as the major underlying molecular event of Bcr-Abl negative MPN.2-5 It is found in approximately 95% of PV and 50% of ET and PMF patients.2-7 The point mutation occurs in the pseudokinase domain of JAK2 and causes a substitution of valine at position 617 by phenylalanine (JAK2 V617F). The pseudokinase domain has high sequence homology to kinase domains, but lacks key residues necessary for catalytic activity. It is thought that the V617F mutation either releases the inhibitory function of the pseudokinase domain on the JAK2 catalytic domain,8 or that it induces a conformational change to the pseudokinase domain that in turn activates the kinase domain9 (Fig. 1B). As a consequence of the JAK2 V617F kinase domain activation, STAT proteins, especially STAT5 and STAT3, become constitutively activated.2 It has been shown that overexpression of JAK2 V617F promotes cytokine independent growth of the IL-3 dependent pro B cell line Ba/F3 (Basel F3 cell line) in the absence of cytokines;2,3,10 however, at low JAK2 V617F expression, dimeric cytokine receptors such as EpoR, TpoR or G-CSFR have to be present to promote cytokine independent BaF3 growth.11 It is thought that the JAK2 V617F mutant gains full transforming activity only when the mutant JAK2 dimerization is promoted by receptor dimerization. This is supported by the need for oncogenic JAK2 V617F activation of an intact FERM (homologous to protein 4.1, ezrin, radixin, moesin)-like domain,12 a domain that normally mediates JAK binding to cytokine receptors. This is the case for EpoR, TpoR or G-CSFR, but other receptors can support JAK2 V617F activation. A second model predicts that the JAK2 V617F mutant is a weak, constitutively active kinase and that receptor binding is necessary to induce its full kinase activity. In any case, binding of JAK2 V617F to EpoR, TpoR or G-CSFR is predicted to stimulate expansion of the erythroid, megakaryocytic and granulocytic lineages which are affected in PV, ET and PMF.
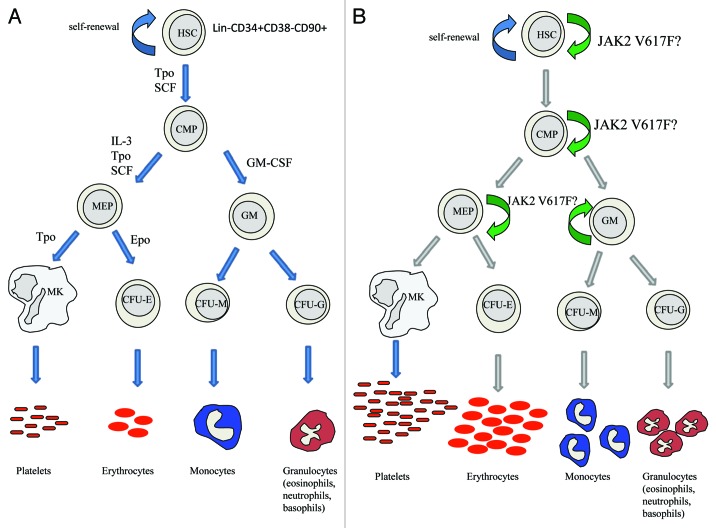
Figure 2. (A) Myeloid blood cell development starts with the hematopoietic stem cell (HSC) that either self-renews or gives rise to a common myeloid progenitor (CMP). The CMP in turn differentiates into more restricted progenitors of the megakaryocyte (MK)-erythroid (E) lineages or granulocyte-monocyte progenitor (GM). These cells give then rise to terminally differentiated erythrocytes, megakaryocytes/platelets, granulocytes (eosinophils, neutrophils and basophils) and monocytes. Cytokines and their receptors control cell proliferation and differentiation during hematopoiesis and ensure the balance between renewal and cell differentiation. (B) Several reports have confirmed that the JAK2 V617F mutation occurs at the level of an HSC. There is, however, still controversy to whether the JAK2 V617F mutant expands the true HSC pool or whether it acts downstream on a more committed progenitor. Further studies are necessary to clarify the exact role of JAK2 V617F on HSC or progenitor expansion. Since the JAK2 V617F mutant only influences myeloid differentiation, we have restricted the schematic to the myeloid lineage.
The JAK2 V617F Mutant and STAT Activation
The JAK2 V617F mutant induces several downstream signaling pathways, recapitulating in a persistent manner the pathways transiently activated by cytokines, i.e., STAT5/3, MAP-kinase, PI-3′-kinase/Akt and mTOR pathways. Interestingly, there are differences in STAT signaling between different MPNs. PV patients exhibit high STAT5 and STAT3 phosphorylation, while ET patients exhibit high STAT3 but low STAT5 phosphorylation.13 In contrast, myelofibrosis patients exhibit low STAT5 and low STAT3 phosphorylation. Several lines of evidence indicate that constitutive activation of JAK2-STAT5 and STAT3 signaling is the basis of PV, ET and PMF. Recently, it was shown that knockout of STAT5A/STAT5B prevents induction of the myeloproliferative phenotype by JAK2 V617F,14,15 thereby establishing an obligatory role of STAT5 in MPNs. Furthermore, the choice and relative levels of activated STATs can have profound consequences on the lineage that is amplified. For example, down-modulation of STAT5 in CD34+ cells promotes megakaryocyte differentiation, while activation of STAT5 promotes erythropoiesis.16 More recently, STAT1 was shown to be specifically associated with megakaryopoiesis.17
Mouse Models of JAK2 V617F
X-linked polymorphism studies showed that MPNs are clonal disorders18 and several groups could show that the JAK2 V617F mutation can be detected in cells with an HSC signature (Lin- CD34+CD38−CD90+19 and CD34+CD38−20), confirming that the JAK2 V617F mutant arises in an HSC. Previously, Delhommeau et al. reported that JAK2 V617F is present in a multipotent cell that can give rise to both myeloid and lymphoid cells.21 Much effort has been made to study the in vivo significance of JAK2 V617F with mouse models in order to decipher the molecular mechanisms causing initiation and progression of MPNs. Several groups performed reconstitution assays using bone marrow cells ectopically expressing the JAK2 V617F mutant and showed that mice indeed developed hallmarks of PV.22-24 Shortly after, Shide et al. and Xing et al. generated stable transgenic mouse models expressing the mouse JAK2 V617F cDNA under control of the major histocompatibility complex H-2Kb promoter25 or the human JAK2 V617F cDNA under control of the Vav promoter,26 respectively. Both H-2Kb and Vav promoters drive transgene expression efficiently throughout the nucleated hematopoietic cell types27,28 and both groups demonstrated that the mice developed key features of PV or ET with erythrocytosis and thrombocytosis.25,26 Interestingly, a later study used a floxed version of the human JAK2 V617F cDNA under control of a human JAK2 promoter and showed that the disease outcome correlates with the relative expression levels of endogenous wild type (wt) vs. human mutant JAK2.29 For example, lower levels of human JAK2 V617F mutant than endogenous wtJAK2 induced an ET-like phenotype, whereas higher JAK2 V617F levels induced a PV-like phenotype.29 More recently, several knock-in models have been published where the JAK2 V617F mutant is expressed from the endogenous JAK2 locus thereby allowing expression in blood cells at physiological levels. Akada et al. observed a PV-like phenotype in hetero- and homozygous mice including increased number of red blood cells and hematocrit levels with homozygous mice frequently progressing to myelofibrosis.30 Additionally, in this particular model heterozygous and homozygous JAK2 V617F mice showed significant expansion of hematopoietic stem cells (HSC) [Lin−Sca1+cKit+ (LSK) population] and myeloid progenitor cells in the bone marrow. In another study by Marty et al. where a murine JAK2 V617F was knocked-in,31 mice developed a marked PV-like phenotype accompanied by erythrocytosis, leukocytosis and splenomegaly and myelofibrosis around 9 mo of age.31 A third study showed similar results with the JAK2 V617F knock-in mouse developing a PV phenotype.32 However, the mice progressed faster to PMF and had an average survival rate of only 150 d, while mice described by Marty et al. developed PMF after 9 mo.31 Mullaly and colleagues further showed that the HSC compartment has the unique capacity for disease initiation, but does not exhibit a competitive advantage over wild-type HSCs.32 In contrast, myeloid progenitor populations are expanded and skewed toward the erythroid lineage, but could not transplant the disease.32 Consistent with these data are microarray analysis that indicated that gene expression profiles of LSK cells were similar to those of wild-type LSK cells.32
In a fourth study, Li et al. generated knock-in mice encoding the human JAK2 V617F cDNA.33 In contrast to the above mentioned models, the authors could only observe an ET-like phenotype with mild increase in platelets and hematocrit, and no sign of myelofibrosis.33 Interestingly, Li et al. reported that mice at 26 weeks of age had 50% reduced numbers of LSK with increased DNA damage, reduced cell cycling and reduced apoptosis.33 Also, bone marrow transplantation experiments showed decreased stem cell activity in cells expressing JAK2 V617F compared with wild-type controls. Consistent with this, direct comparison of the wild-type vs. JAK2 V617F bone marrow cells in competitive transplantation experiments demonstrated that JAK2 V617F confers a mild but significant disadvantage to HSCs, an effect that was most striking in secondary transplantations.33
JAK2 V617F and Human Hematopoietic Stem Cells
The above described mouse models (summarized in Table 1) were addressing the question of how the JAK2 V617F mutant affects HSC expansion. The reasons behind the opposite conclusions between the study of Li et al. showing a reduction of the HSC pool, and that of Akada et al. reported HSC expansion remains to be determined.30,33 Several studies have directly focused on the role of JAK2 V617F in primary MPN patient samples. Jamieson and colleagues isolated HSC and myeloid progenitors from patients and healthy controls in order to investigate whether JAK2 V617F positive cells have altered differentiation potential.19 The authors analyzed cells with a HSC signature (Lin−CD34+CD38−CD90+) and showed that the majority of HSC obtained from PV patients harbor the JAK2 V617F mutation with the mutation being transmitted to more committed progenitors.19 Moreover, the authors demonstrated that the HSC numbers and the common myeloid progenitor (CMP) pool are increased and that differentiation is skewed toward the erythroid lineage.19 In contrast to this report are studies performed by James et al. and Anand et al. The first study by James and colleagues used xenograft assays to study the properties of JAK2 V617F positive CD34+ cells from PV, PMF and post-PV MF patients and demonstrated that the JAK2 V617F repopulating cells are present in the three diseases, but the proportion of JAK2 V617F compared with JAK2wt was markedly different between PV and PMF.20 The authors did however not find major differences in the proliferation and self-renewal properties of JAK2 V617F repopulating cells between PV, PMF and post-PV MF indicating that the JAK2 V617F mutant does not confer a proliferation advantage to the HSC compartment.20 The second study by Anand et al. examined the effects of the JAK2 V617F mutant on HSC homeostasis through analysis of bone marrow samples from PV, ET and MF patients.34 The study revealed that the JAK2 V617F mutant does not alter the Lin−CD34+CD38−, CMP and granulocyte-monocyte progenitor (GMP) compartments in ET and PV patients, but rather affects the later erythroid CD34−CD71+GPA+ cell compartment.34 A third study by Ishii et al. examined the engraftment potential of CD34+ isolated from PV patients in xenograft assays.35 The authors showed that CD34+ cells from healthy donors as well as IMF patients and PV patients with high JAK2 V617F burden reconstituted mice, whereas cells from PV patients with low JAK2 V617F burden did not.35 One possible explanation for this observation is a qualitative difference between low- and high-burden JAK2 V617F HSC. As a second possibility is that high burden JAK2 V617F cells contain higher numbers of authentic long-term HSCs, while CD34+ cell fractions from patients with low JAK2V617F burden contain less HSCs that engraft in immuno-deficient mice. Based on the result of latter study the level of JAK2 V617F decides whether or not JAK2 V617F positive HSCs have an engraftment and repopulating advantage compared with healthy HSC (Fig. 2). In conclusion, the results obtained by the different studies have to be compared in order to define the differences in cell populations used and to determine what the possible explanations are for the discrepancies obtained with the different analyses.
Table 1. Summary of transplantation, transgenic or knock-in JAK2 V617F mouse models.
| Mouse model | References | Activation | Platelets | Hematocrit | MF |
|---|---|---|---|---|---|
| Retroviral overexpression |
Lacout et al.22 |
N/A |
Normal |
> 55% |
Yes |
| Wernig et al.23 |
Normal |
> 70% |
Yes |
||
| Zaleskas et al.24 |
Normal |
> 65% |
Yes |
||
| Transgenic JAK2 V617F mouse models |
Tiedt et al.29 |
MX1-Cre |
3,710 ± 488 |
49–62% |
Yes |
| Shide et al.25 |
Not inducible |
> 1,400 |
50% |
Yes |
|
| Xing et al.26 |
Not inducible |
2,708 ± 712 |
51% |
Yes |
|
| Knock-in JAK2 V617F mouse models | Akada et al.30 |
MX1-Cre |
~1.5-fold increase |
Up to 80% |
Mild heterozygous High homozygous |
| Mullally et al.32 |
Constitutive |
No increase |
Up to 80% |
No |
|
| Marty et al.31 |
Constitutive |
~4-fold increase |
Up to 70% |
Yes |
|
| Li et al.33 | MX1-Cre | ~1.5-fold increase | Up to 60% | No |
STAT5 and HSCs
STAT5 was reported to be involved in self-renewal of mouse and human HSCs and to regulate renewal vs. differentiation as a function of its levels of expression.36-38 Using a tamoxifen-inducible STAT5A(1*6)-estrogen receptor fusion protein, it was shown that high STAT5 levels block myelopoiesis and promote erythropoiesis, while intermediate levels of transactivation induce HSC and CD34+ proliferation.37 Self-renewal of HSCs was promoted by the same intermediate levels of STAT5 activation. These results indicate therefore that levels of STAT5 activation are decoded in self-renewal vs. differentiation signals. One caveat of such models is that a double mutant STAT5 is used,39 which is constitutively active, but might have properties different from those of cytokine-activated STAT5. Indeed, in cell lines persistently activated STAT5 was shown to induce common, but also different genes than transiently activated STAT5.40,41 STAT5 was shown to induce long-term self-renewal only in human HSCs, not in progenitors.38 One target gene of STAT5 that is induced by STAT5 in HSCs was identified to be hypoxia-induced factor 2α (HIF2α). Expression of this gene is apparently required for STAT5-induced HSC amplification, but not for HSC differentiation. One model has been proposed where STAT5 maintains expression of hypoxia-induced genes, in the absence of hypoxia, in order to promote HSC self-renewal.38 Another gene that is modulated by STAT5 is C/EBPα, and this gene is the major downregulated gene by STAT5 in HSCs. Re-expression of C/EBPα impairs the effects of constitutively active STAT5 on HSCs, indicating that part of the mechanism by which mutant STAT5 expands long-term HSCs is represented by down-modulation of C/EBPα.42
Interferons, HSC and IFNs Treatment of MPNs
While interferons are classical antiproliferative agents, several studies reported that IFN-α and IFN-γ induce cycling of HSCs.43,44 IFN-α binds to the interferon α receptor 1 and 2 (IFNAR1 and IFNAR2) and thus activates the JAK tyrosine kinase family members JAK1 and TYK2 to signal via a complex formed of STAT1/STAT2/IRF9 (reviewed in ref. 45). IFN gamma signal activates JAK1 and JAK2 via the interferon gamma receptor 1 (IFNgR1) and IFNgR2 receptor subunits and signals via STAT1 homodimers.
In response to treatment of mice with IFN-α, signaling via STAT1 and Akt induces the transition of HSCs from quiescence into cell cycle.43,46 STAT1 and the surface protein Sca-1 are required for IFN-α-induced HSC proliferation. Similarly, IFN-γ induced in several infections, signals via STAT1 and induce HSC cycling.44
The implications of these data might be relevant for the treatment of MPNs with IFN-α. At this moment, approximately 40% of PV and ET patients respond positively to IFN-α treatment, and for JAK2 V617F patients, this leads to a decrease or disappearance of JAK2 V617F positivity. The effect is quite slow, requiring many month to one year, suggesting that the effect is mainly on HSCs, and that somehow IFN treatment leads to exhaustion of the mutated clone, with preservation of the normal clones.47 In patients with MPNs where the JAK2 V617F mutation occurs on the background of a clone where TET (ten 11 translocation) methylcytosine dioxygenase 2 (TET2), a protein that converts 5-methylcytosine in DNA to 5-hydroxymetylcytosine48 is deleted/mutated on both alleles,49 clonal analysis showed that IFNα-2a treatment targets the JAK2 V617F-positive clone, but does not affect the TET2 clone.50 Interestingly, the presence of biallelic loss of TET2 did not impair the effect of IFNα on JAK2 V617F HSCs. It will therefore be important to determine the molecular basis of the effect of IFNα specifically on the JAK2 V617F HSCs.
Concluding Remarks and Perspectives
The roles of JAK-STAT signaling in HSC renewal and amplification remain poorly defined. Results obtained from studies examining the role of STAT5 in HSC homeostasis on one hand, and the effects of interferons on the other, provided evidence that the JAK-STAT pathway can profoundly influence HSCs. The recent discovery of the unique somatic acquired JAK2 V617F mutation, which is at the basis of the majority of BCR-ABL negative MPNs provided novel models for exploring the question of whether JAK-STAT pathway plays an important role at the HSC level.
There are two major biological questions that to date have been answered with apparently contradictory reports: (1) does JAK2 V617F confer an advantage to HSCs, in terms of proliferation, self-renewal, differentiation or transplantation (Fig. 2), and (2) is JAK2 V617F at the HSC level inducing a skew in the type of downstream differentiation, in contrast to mere amplification of the downstream progenitors without any skew of HSC potential?
While the acquisition of JAK2 V617F at the HSC level has been confirmed by several groups, the reasons behind it remain unclear. It is still not known whether the mutation is constantly acquired (like BCR-ABL), but only some individuals select for it (i.e., hypersensitivity to cytokines due to SNPs or unknown previous mutations), whether the mutation protects against an insult, and its selection is favored by such pressure, or whether there are DNA replication defects that favor such mutation acquisition. The G to T mutation occurs in a region with repeated TT sequences; it is possible that it occurs from slipped mispairing, however, the actual mechanism of mutation acquisition remains still to be determined.
How can we explain the contradictory results coming from mouse models and from xenotransplantation and colony assays, where both an advantage and a disadvantage of the mutated HSCs (or no change) were observed, or where differentiation skewing or absence of it was each observed? One possibility is that any transplantation approach requires homing and renewal in a foreign niche, events that are not relevant for the disease. Another is that, as opposed to mouse models where all HSCs are knocked-in or transgenic, in humans the disease starts from one mutated cell that must make its way to differentiation and gain clonal dominance. It is possible that events linked to the latter are very important, and remain unexplored by mouse models. Finally, HSCs exist in several subtypes, such as myeloid-biased, lymphoid-biased and several others51 and we have little idea at the moment in which such HSC JAK2 V617F must arise in order to induce ET, or PV or PMF and which events are necessary for progression of ET and PV to secondary MF. In any case, it appears that the JAK2 V617F burden at CD34+ or at HSC level is the most important,52 and that MF is associated with a loss of wt HSCs and preservation of mutated HSCs, which also exit the marrow, have down-modulated CXCR453 and can be found in periphery. Directly linked to the subtype of HSCs targeted by the mutation, recently it was shown that a precursor of endothelial/HSC cells can also acquire the mutation, and might be involved in the deep venous thrombosis events linked to JAK2 V617F.54 Clearly a much more detailed understanding of the HSC subtypes, their niches, pressures and response to cytokines will be necessary for understanding of relevant effects of JAK2 V617F on HSCs.
The major goal of therapy in MPNs is to reduce the source of cytokine-independent myeloid progenitors that is the HSCs with mutations. For this purpose a major advance is required to distinguish mutated disease-initiating HSCs from possibly non-disease initiating HSCs and non-mutated HSCs. The prediction is that the effect of JAK-STAT signaling in each such cell will be different.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/22071
References
- 1.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–5. [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Cancer Genome Project Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 6.Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106:2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 7.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–92. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saharinen P, Vihinen M, Silvennoinen O. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol Biol Cell. 2003;14:1448–59. doi: 10.1091/mbc.E02-06-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dusa A, Mouton C, Pecquet C, Herman M, Constantinescu SN. JAK2 V617F constitutive activation requires JH2 residue F595: a pseudokinase domain target for specific inhibitors. PLoS One. 2010;5:e11157. doi: 10.1371/journal.pone.0011157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staerk J, Kallin A, Demoulin JB, Vainchenker W, Constantinescu SN. JAK1 and Tyk2 activation by the homologous polycythemia vera JAK2 V617F mutation: cross-talk with IGF1 receptor. J Biol Chem. 2005;280:41893–9. doi: 10.1074/jbc.C500358200. [DOI] [PubMed] [Google Scholar]
- 11.Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci U S A. 2005;102:18962–7. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE, et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood. 2008;111:3751–9. doi: 10.1182/blood-2007-07-102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teofili L, Martini M, Cenci T, Petrucci G, Torti L, Storti S, et al. Different STAT-3 and STAT-5 phosphorylation discriminates among Ph-negative chronic myeloproliferative diseases and is independent of the V617F JAK-2 mutation. Blood. 2007;110:354–9. doi: 10.1182/blood-2007-01-069237. [DOI] [PubMed] [Google Scholar]
- 14.Yan D, Hutchison RE, Mohi G. Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood. 2012;119:3539–49. doi: 10.1182/blood-2011-03-345215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walz C, Ahmed W, Lazarides K, Betancur M, Patel N, Hennighausen L, et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and JAK2(V617F) in mice. Blood. 2012;119:3550–60. doi: 10.1182/blood-2011-12-397554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olthof SG, Fatrai S, Drayer AL, Tyl MR, Vellenga E, Schuringa JJ. Downregulation of signal transducer and activator of transcription 5 (STAT5) in CD34+ cells promotes megakaryocytic development, whereas activation of STAT5 drives erythropoiesis. Stem Cells. 2008;26:1732–42. doi: 10.1634/stemcells.2007-0899. [DOI] [PubMed] [Google Scholar]
- 17.Chen E, Beer PA, Godfrey AL, Ortmann CA, Li J, Costa-Pereira AP, et al. Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell. 2010;18:524–35. doi: 10.1016/j.ccr.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fialkow PJ. Clonal origin of human tumors. Annu Rev Med. 1979;30:135–43. doi: 10.1146/annurev.me.30.020179.001031. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson CH, Gotlib J, Durocher JA, Chao MP, Mariappan MR, Lay M, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc Natl Acad Sci U S A. 2006;103:6224–9. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James C, Mazurier F, Dupont S, Chaligne R, Lamrissi-Garcia I, Tulliez M, et al. The hematopoietic stem cell compartment of JAK2V617F-positive myeloproliferative disorders is a reflection of disease heterogeneity. Blood. 2008;112:2429–38. doi: 10.1182/blood-2008-02-137877. [DOI] [PubMed] [Google Scholar]
- 21.Delhommeau F, Dupont S, Tonetti C, Massé A, Godin I, Le Couedic JP, et al. Evidence that the JAK2 G1849T (V617F) mutation occurs in a lymphomyeloid progenitor in polycythemia vera and idiopathic myelofibrosis. Blood. 2007;109:71–7. doi: 10.1182/blood-2006-03-007146. [DOI] [PubMed] [Google Scholar]
- 22.Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–60. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 23.Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–81. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaleskas VM, Krause DS, Lazarides K, Patel N, Hu Y, Li S, et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS One. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shide K, Shimoda HK, Kumano T, Karube K, Kameda T, Takenaka K, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22:87–95. doi: 10.1038/sj.leu.2405043. [DOI] [PubMed] [Google Scholar]
- 26.Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–17. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dominici M, Tadjali M, Kepes S, Allay ER, Boyd K, Ney PA, et al. Transgenic mice with pancellular enhanced green fluorescent protein expression in primitive hematopoietic cells and all blood cell progeny. Genesis. 2005;42:17–22. doi: 10.1002/gene.20121. [DOI] [PubMed] [Google Scholar]
- 28.Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989;8:2283–90. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–40. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- 30.Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010;115:3589–97. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marty C, Lacout C, Martin A, Hasan S, Jacquot S, Birling MC, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010;116:783–7. doi: 10.1182/blood-2009-12-257063. [DOI] [PubMed] [Google Scholar]
- 32.Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17:584–96. doi: 10.1016/j.ccr.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Kent DG, Chen E, Green AR. Mouse models of myeloproliferative neoplasms: JAK of all grades. Dis Model Mech. 2011;4:311–7. doi: 10.1242/dmm.006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anand S, Stedham F, Beer P, Gudgin E, Ortmann CA, Bench A, et al. Effects of the JAK2 mutation on the hematopoietic stem and progenitor compartment in human myeloproliferative neoplasms. Blood. 2011;118:177–81. doi: 10.1182/blood-2010-12-327593. [DOI] [PubMed] [Google Scholar]
- 35.Ishii T, Zhao Y, Sozer S, Shi J, Zhang W, Hoffman R, et al. Behavior of CD34+ cells isolated from patients with polycythemia vera in NOD/SCID mice. Exp Hematol. 2007;35:1633–40. doi: 10.1016/j.exphem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Schuringa JJ, Chung KY, Morrone G, Moore MA. Constitutive activation of STAT5A promotes human hematopoietic stem cell self-renewal and erythroid differentiation. J Exp Med. 2004;200:623–35. doi: 10.1084/jem.20041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wierenga AT, Vellenga E, Schuringa JJ. Maximal STAT5-induced proliferation and self-renewal at intermediate STAT5 activity levels. Mol Cell Biol. 2008;28:6668–80. doi: 10.1128/MCB.01025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fatrai S, Wierenga AT, Daenen SM, Vellenga E, Schuringa JJ. Identification of HIF2alpha as an important STAT5 target gene in human hematopoietic stem cells. Blood. 2011;117:3320–30. doi: 10.1182/blood-2010-08-303669. [DOI] [PubMed] [Google Scholar]
- 39.Onishi M, Nosaka T, Misawa K, Mui AL, Gorman D, McMahon M, et al. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–9. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moucadel V, Constantinescu SN. Differential STAT5 signaling by ligand-dependent and constitutively active cytokine receptors. J Biol Chem. 2005;280:13364–73. doi: 10.1074/jbc.M407326200. [DOI] [PubMed] [Google Scholar]
- 41.Girardot M, Pecquet C, Boukour S, Knoops L, Ferrant A, Vainchenker W, et al. miR-28 is a thrombopoietin receptor targeting microRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood. 2010;116:437–45. doi: 10.1182/blood-2008-06-165985. [DOI] [PubMed] [Google Scholar]
- 42.Wierenga AT, Schepers H, Moore MA, Vellenga E, Schuringa JJ. STAT5-induced self-renewal and impaired myelopoiesis of human hematopoietic stem/progenitor cells involves down-modulation of C/EBPalpha. Blood. 2006;107:4326–33. doi: 10.1182/blood-2005-11-4608. [DOI] [PubMed] [Google Scholar]
- 43.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–8. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 44.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–7. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzé G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- 46.Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10:201–9. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 47.Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood. 2011;117:4706–15. doi: 10.1182/blood-2010-08-258772. [DOI] [PubMed] [Google Scholar]
- 48.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Massé A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 50.Kiladjian JJ, Massé A, Cassinat B, Mokrani H, Teyssandier I, le Couédic JP, et al. French Intergroup of Myeloproliferative Neoplasms (FIM) Clonal analysis of erythroid progenitors suggests that pegylated interferon alpha-2a treatment targets JAK2V617F clones without affecting TET2 mutant cells. Leukemia. 2010;24:1519–23. doi: 10.1038/leu.2010.120. [DOI] [PubMed] [Google Scholar]
- 51.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–78. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein BL, Williams DM, Rogers O, Isaacs MA, Spivak JL, Moliterno AR. Disease burden at the progenitor level is a feature of primary myelofibrosis: a multivariable analysis of 164 JAK2 V617F-positive myeloproliferative neoplasm patients. Exp Hematol. 2011;39:95–101. doi: 10.1016/j.exphem.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosti V, Massa M, Vannucchi AM, Bergamaschi G, Campanelli R, Pecci A, et al. Italian Registry of Myelofibrosis with Myeloid Metaplasia. Myeloproliferative Disorders Research Consortium The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol Dis. 2007;38:280–6. doi: 10.1016/j.bcmd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Sozer S, Ishii T, Fiel MI, Wang J, Wang X, Zhang W, et al. Human CD34+ cells are capable of generating normal and JAK2V617F positive endothelial like cells in vivo. Blood Cells Mol Dis. 2009;43:304–12. doi: 10.1016/j.bcmd.2009.08.005. [DOI] [PubMed] [Google Scholar]