Abstract
Signal transducer and activator of transcription-1 (STAT1) plays a role in the transduction of stress and cytokine responses, DNA damage, and activation of B and T cell immune responses. The ability of STAT1 to act as a pro- or anti-apoptotic signaling molecule depends upon the cellular environment and stimulus. Post-translational modifications provide the main method of control over the function of STAT1, however, recent data in the breast has shown that loss of STAT1 from both the tumor and the surrounding mammary epithelium greatly influence the development and response to treatment of breast cancers. Here, we discuss the recent findings of Chan et al. in Breast Cancer Research who described a new role for STAT1 in the development of estrogen receptor (ER)-positive and progesterone receptor (PR)-positive luminal breast cancers.
Keywords: STAT1, breast cancer, mouse, transcription, immunity
STAT1 is an important signaling protein in stress responses and is the main transducer of signals from the interferon (IFN)-γ receptor, it as an important player in the response to viral infection. In a similar manner to the well known tumor suppressors retinoblastoma protein and p53, STAT1 appears to play distinct roles depending on the cell type and tissue context. STAT1 is also activated upon exposure to UV irradiation, bacterial lipopolysaccharide, IFNα, interleukin (IL)-2, IL-12, B cell and T cell receptor crosslinking and other stumuli1 and has been identified as a potent activator of cell death. Downstream signaling from IFNγ receptors leads to activation of the Janus activate kinases (JAKs), which subsequently phosphorylate STAT1 at a conserved tyrosine residue in the C-terminal transactivation domain (Y701). Phosphorylation of Y701 by JAKs can lead to homo- or hetero-dimerization of STAT1, which then requires additional phosphorylation of a C-terminal serine (S727) for optimal transactivation activity. Mutation of this residue to alanine does not affect DNA binding but reduces activity at the interferon-regulatory factor 1 promoter by 80%.2 Multiple kinases have been identified as being responsible for S727 phosphorylation. These include CK23,4 and the double-stranded RNA activated protein kinase AKT.5-9 Phosphorylation of both Y701 and S727 has been shown to be essential for STAT1 dependent induction of cell death.10-12 Activated STAT1 dimers translocate to the nucleus where they can bind to DNA response elements located in the promoter of target genes and recruit co-factors such as CREB binding protein, which in-turn enhances binding of RNA polymerase to the promoter, initiating transcription.13
STAT1 has also been shown to be activated following DNA damage13 forming a complex which contains p53 (and possibly other proteins which bind to both p53 and STAT1 such as p7314) this enhances the transactivation activity of p53, resulting in increased expression of Bax, Noxa and FasL.15 Furthermore, STAT1 protein stability correlates with cell survival, thus having a powerful influence on cellular homeostasis,16 indicating that STAT1 may influence both tumor development and growth in certain tissue microenvironments.
In the past three years a number of groups have shown STAT1 to be important in the development of breast cancers.17-22 Chan et al. have recently added to this body of work and demonstrated that STAT1 is present at higher levels in stromal tissue than in tumorogenic tissue, whereas previous data had only suggested a role for STAT1 within tumors and the surrounding mammary epithelial tissue. They also demonstrate that STAT1 expression is higher in ER-negative, stromal tissue than in patient samples from ER-positive stromal tissue. These results suggest that not only does reduction of STAT1 expression correlate with development of breast carcinoma in humans but that ER expression reduces STAT1 expression, therefore suggesting that loss of STAT1 may be important in the development of ER-positive breast tumors. In addition to the role of STAT1 in breast tissue there is a possible role for STAT1 in immune cells which infiltrate the area of the mammary gland. This may stimulate stromal cells in a way that drives tumorogenesis. The role of STAT1 in IFNγ transduction as part of the innate immune system has been carefully dissected in cells with consideration to its role in “front line” immune protection, however, the role of STAT1 in the development of tumors as they escape immune surveillance has rarely been considered.
To date most mechanistic work has been performed in STAT1 mice which generate an N-terminally truncated STAT1 protein product23 (denoted here as Taconic mice). Chan et al. report in their recent Breast Cancer Research paper that STAT1−/− mammary tumors from Taconic STAT1−/− mice closely recapitulate ER-positive, PR-positive luminal breast cancers. They show well-defined progression kinetics and reproducible and homogenous characteristics at the histopathological, biological and molecular levels. These Taconic STAT1−/− tumors possess increased levels of ERα and PR plus other modulators, all of which are actively controlled by ERα and are sensitive to endocrine therapy. In a similar scenario to HER2/ErbB2 overexpression breast cancer models,18 Chan et al. demonstrated that Taconic STAT1−/− tumors grew more slowly in STAT1 sufficient than Taconic STAT1−/− recipients.19 In both cases multiparous mice showed a shorter mean time to tumor development, regardless of genotype. This was significant since previous work had shown that nulliparous mice display a higher percentage of STAT1 positive cells than cells from parous human breast tissue, suggesting that lower expression of STAT1 protein may reflect a susceptibility to breast cancer development, regardless of origin.20 STAT1−/− tumors transplanted into wild type STAT1 recipients continue to grow with the exception of ovarectomized mice; conversely in Taconic STAT1−/− recipients tumors grew more rapidly than the wild type controls. Here, the tumor size reduced over time, an effect which was not mirrored in the wild type recipients.19
This novel work suggests that STAT1 in the immune system and surrounding mammary epithelial cells of the breast are centrally important for regulating tumor development. In 2011 Klover et al. reported in a conditional mammary epithelial STAT1−/− ErbB2/Neu Tg model, breast cancer developed more rapidly than in the wild type counterpart initially, but that the significant difference between genotypes decreased with age.17 This further suggested that it is not only the immune cells in the STAT1 sufficient mice that are preventing growth of the STAT1 deficient tumor but that mammary epithelium also plays a role in tumor development.
One area that bears discussion is the nature of the STAT1 deletion in the various model systems that have been used. STAT1 has been shown to be phosphorylated by ER signaling20 and has the ability to transduce IFNγ signals which result in expression of IRF1. This in turn can sensitize anti-estrogen resistant cells to cell death from Fulvestrant.21 Chan et al. show no expression of the N-terminally truncated STAT1 in the spleen of Taconic STAT1−/− mice by immunohistochemistry (IHC) using a C-terminally-directed STAT1 antibody. Previously an N-terminally truncated STAT1 protein has been observed in the liver of these mice.23 Our own western blot data (Fig. 1) shows that this truncated protein also remains present in the spleen, albeit at a much lower level of expression compared with the full length form in wild type mice. Presumably the truncated form was not detected by IHC, as a result of the relatively low level of sensitivity of this technique and its low level of expression. The question that remains is whether this residual truncated protein might have a biological effect. In the same publication no significant difference in median time to tumor formation between Taconic STAT1−/− mice and complete STAT1 knockout mice generated by Levy and colleagues was reported.24 However, although not statistically significant, potentially due to a lack of tumor penetrance, the mean time for tumor formation in the Taconic STAT1−/− mice was approximately 23 mo compared with 14.5 mo for the complete knockout mouse, perhaps indicating that a residual STAT1 function remains in the Taconic STAT1−/− mice. As Chan et al. show that multiparous Taconic STAT1−/− mice show a faster time to tumor development (and are fully tumor penetrant) compared with nulliparous mice we feel that these data are incomplete without the comparison between Taconic STAT1−/− and complete STAT1−/− in multiparous mice. To be certain that the presence of a truncated STAT1 protein had no effect on the formation and maintenance of breast tumor development the experiments would need to be performed in mice in which no protein products can be detected. We have recently generated such a mouse in collaboration with Ozgene, where exons 18–20 of STAT1 were deleted, resulting in a mouse that has no residual protein expression (Fig. 1).
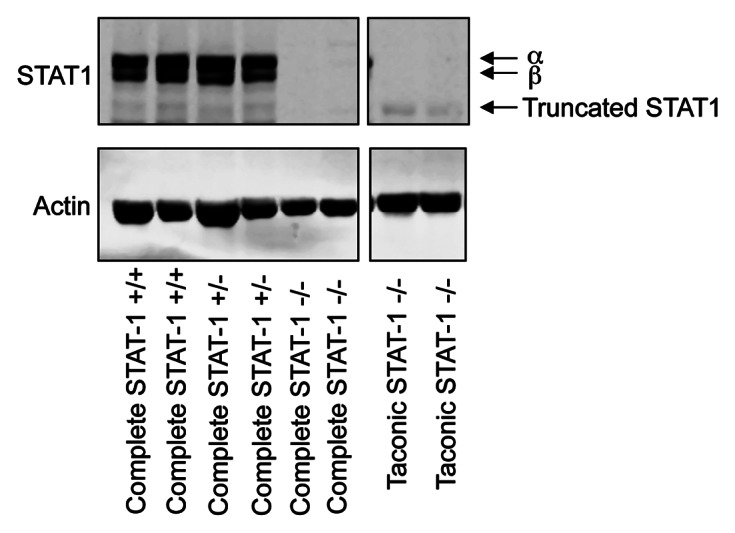
Figure 1. Truncated STAT1 can be detected in spleens of Taconic STAT1−/− mice but not in complete STAT1−/− mice. Spleens were taken from mice and homogenized in RIPA buffer. Thirty micrograms protein lysate was loaded per well on an 8% gel and separated by SDS PAGE. Western blotting was then performed using Santa Cruz p84/p91 STAT1 antibody clone E-23 (sc-346), which is directed to the C-terminus and β-actin antibody (Sigma). The immunoblotting demonstrates that the Taconic STAT1−/− mice express a small, truncated form of STAT1 not observed in our complete STAT1−/− mice. Note, a lane in between the two boxes was deleted for clarity yet the samples were run on the same gel.
Since Klover et al. have now generated a conditional knockout to ascertain the mechanisms behind mammary tumor development, we now require conditional knockouts of immune cells to determine their role in the development or suppression of breast cancers.17 These experiments would provide further insight into the role of STAT1 in the immune system and how this affects the development of breast cancer per se.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/20967
References
- 1.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–37. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 2.Wen Z, Zhong Z, Darnell JE., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 3.Timofeeva OA, Plisov S, Evseev AA, Peng S, Jose-Kampfner M, Lovvorn HN, et al. Serine-phosphorylated STAT1 is a prosurvival factor in Wilms’ tumor pathogenesis. Oncogene. 2006;25:7555–64. doi: 10.1038/sj.onc.1209742. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Yang Y, Sharma N, Tarasova NI, Timofeeva OA, Winkler-Pickett RT, et al. STAT1 activation regulates proliferation and differentiation of renal progenitors. Cell Signal. 2010;22:1717–26. doi: 10.1016/j.cellsig.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goh KC, Haque SJ, Williams BR. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 1999;18:5601–8. doi: 10.1093/emboj/18.20.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen H, Ramana CV, Bayes J, Stark GR. Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J Biol Chem. 2001;276:33361–8. doi: 10.1074/jbc.M105070200. [DOI] [PubMed] [Google Scholar]
- 7.Ramana CV, Grammatikakis N, Chernov M, Nguyen H, Goh KC, Williams BR, et al. Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J. 2000;19:263–72. doi: 10.1093/emboj/19.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takaoka A, Tanaka N, Mitani Y, Miyazaki T, Fujii H, Sato M, et al. Protein tyrosine kinase Pyk2 mediates the Jak-dependent activation of MAPK and Stat1 in IFN-gamma, but not IFN-alpha, signaling. EMBO J. 1999;18:2480–8. doi: 10.1093/emboj/18.9.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X, Wen Z, Xu LZ, Darnell JE., Jr Stat1 serine phosphorylation occurs independently of tyrosine phosphorylation and requires an activated Jak2 kinase. Mol Cell Biol. 1997;17:6618–23. doi: 10.1128/mcb.17.11.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, et al. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem. 2000;275:10002–8. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- 11.Stephanou A, Scarabelli TM, Townsend PA, Bell R, Yellon D, Knight RA, et al. The carboxyl-terminal activation domain of the STAT-1 transcription factor enhances ischemia/reperfusion-induced apoptosis in cardiac myocytes. FASEB J. 2002;16:1841–3. doi: 10.1096/fj.02-0150fje. [DOI] [PubMed] [Google Scholar]
- 12.Townsend PA, Scarabelli TM, Pasini E, Gitti G, Menegazzi M, Suzuki H, et al. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004;18:1621–3. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- 13.Townsend PA, Cragg MS, Davidson SM, McCormick J, Barry S, Lawrence KM, et al. STAT-1 facilitates the ATM activated checkpoint pathway following DNA damage. J Cell Sci. 2005;118:1629–39. doi: 10.1242/jcs.01728. [DOI] [PubMed] [Google Scholar]
- 14.Soond SM, Carroll C, Townsend PA, Sayan E, Melino G, Behrmann I, et al. STAT1 regulates p73-mediated Bax gene expression. FEBS Lett. 2007;581:1217–26. doi: 10.1016/j.febslet.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 15.Townsend PA, Scarabelli TM, Davidson SM, Knight RA, Latchman DS, Stephanou A. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J Biol Chem. 2004;279:5811–20. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 16.Soond SM, Townsend PA, Barry SP, Knight RA, Latchman DS, Stephanou A. ERK and the F-box protein betaTRCP target STAT1 for degradation. J Biol Chem. 2008;283:16077–83. doi: 10.1074/jbc.M800384200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Klover PJ, Muller WJ, Robinson GW, Pfeiffer RM, Yamaji D, Hennighausen L. Loss of STAT1 from mouse mammary epithelium results in an increased Neu-induced tumor burden. Neoplasia. 2010;12:899–905. doi: 10.1593/neo.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raven JF, Williams V, Wang S, Tremblay ML, Muller WJ, Durbin JE, et al. Stat1 is a suppressor of ErbB2/Neu-mediated cellular transformation and mouse mammary gland tumor formation. Cell Cycle. 2011;10:794–804. doi: 10.4161/cc.10.5.14956. [DOI] [PubMed] [Google Scholar]
- 19.Chan SR, Vermi W, Luo J, Lucini L, Rickert C, Fowler AM, et al. STAT1-deficient mice spontaneously develop estrogen receptor α-positive luminal mammary carcinomas. Breast Cancer Res. 2012;14:R16. doi: 10.1186/bcr3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blance RN, Sims AH, Anderson E, Howell A, Clarke RB. Normal breast tissue implanted into athymic nude mice identifies biomarkers of the effects of human pregnancy levels of estrogen. Cancer Prev Res (Phila) 2009;2:257–64. doi: 10.1158/1940-6207.CAPR-08-0161. [DOI] [PubMed] [Google Scholar]
- 21.Ning Y, Riggins RB, Mulla JE, Chung H, Zwart A, Clarke R. IFNgamma restores breast cancer sensitivity to fulvestrant by regulating STAT1, IFN regulatory factor 1, NF-kappaB, BCL2 family members, and signaling to caspase-dependent apoptosis. Mol Cancer Ther. 2010;9:1274–85. doi: 10.1158/1535-7163.MCT-09-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magkou C, Giannopoulou I, Theohari I, Fytou A, Rafailidis P, Nomikos A, et al. Prognostic significance of phosphorylated STAT-1 expression in premenopausal and postmenopausal patients with invasive breast cancer. Histopathology. 2012;60:1125–32. doi: 10.1111/j.1365-2559.2011.04143.x. [DOI] [PubMed] [Google Scholar]
- 23.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–42. doi: 10.1016/S0092-8674(00)81288-X. [DOI] [PubMed] [Google Scholar]
- 24.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–50. doi: 10.1016/S0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]


