Abstract
What does it take to make a heart? Even in the fruit fly, in which matters of the heart don’t extend to either pop music or pulp fiction, making a heart requires big decisions and processes of surprising complexity.
Keywords: heart, development, Drosophila, tinman, E(spl)-C
Although lacking the intricacy of the vertebrate cardiovascular system, the humble fruit fly does have both blood and a heart—a combination that circulates hemolymph around the larval and adult body. While, it has long been known that JAK-STAT signaling is involved in the proliferation and differentiation of Drosophila blood cells (reviewed in refs. 1 and 2), work by Aaron Johnson and colleagues in the lab of Eric Olson has recently extended the known roles of JAK-STAT pathway signaling to the heart itself.3
The Drosophila heart starts out as a straight tube made up of two lines of cardioblasts framed by rows of excretory pericardial support cells.4 This structure then develops into an anterior aorta and a posterior lumen, or “heart proper,” where hemolymph inflow openings termed ostia are also located (Fig. 1). Surprisingly, although flies are only the most distant of relatives, their hearts are remarkably similar to ours in two fundamental respects. First, the tubular morphology of the dorsal vessel reminds of a very early stage of the developing vertebrate heart, and second, the rhythmic contractions of a fly heart work with the same mechanics as the human heartbeat—a contrast to the much more closely related vertebrate zebrafish heart, which has been suggested to function more like a dynamic “suction pump.”5 These similarities make the Drosophila cardiac system a potentially important low complexity model for the study of cardiac development and defects.
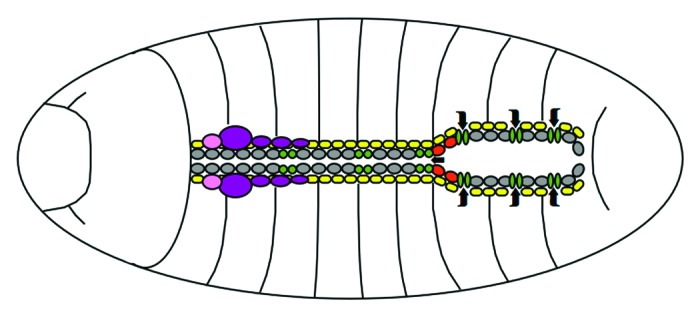
Figure 1. Schematic outline of the Drosophila heart. In the late stage embryo, cardioblasts (gray) shape the anterior tubular aorta and the posterior lumen of the dorsal vessel, interspersed with ostia (green) and aortic valves (red) to allow hemolymph flow (indicated by arrows). Adjacent pericardial cells (yellow) provide support and excretory functions. The lymph gland (purple) and ring gland (pink) lie associated to the cardiac system. Dorsal view, anterior is to the left.
The initial stages of Drosophila heart development begin in the embryonic dorsal mesoderm. In this tissue, stepwise specification drives mesodermal cells to become cardiogenic primordia, which are progressively determined to become heart precursors, cardioblasts and finally mature heart cells.6 Many well-known signaling pathways are essential for this process, including the fibroblast growth factor (FGF), decapentaplegic (Dpp), wingless (Wg), notch (N) and hedgehog (Hh) pathways. However, one of the busiest proteins in the dorsal mesoderm must be the NK2 homeobox containing transcription factor tinman (Tin). Drosophila mutants lacking tin not only lack a heart (as does its eponymous namesake in the Wizard of Oz), but also show severe visceral and somatic muscle defects, identifying Tin as an essential early regulator of mesodermal tissue specification. Given this key role, it is potentially very significant that Tin directly regulates StatTAT92E expression in the dorsal mesoderm, and that JAK-STAT signaling, downstream of Tin, is essential for myogenesis.7
However, this is not the only role of JAK-STAT signaling in the dorsal mesoderm. Johnson et al. now describe that not only the muscle, but also the heart vitally depends on JAK-STAT signaling, and that the tin mutant cannot overcome its heartlessness without the instructive input of the JAK-STAT signal. The authors based this important finding on the analysis of upd and stat92E mutants, which show severe defects including abnormal cell-cell adhesion, cell aggregation and improper lumen formation, phenotypes that called for a more detailed analysis of JAK-STAT signaling in this context.
Not disheartened by the fact that expression of the JAK-STAT pathway ligand upd is absent from the region where heart development takes place, the authors were also unfazed by the finding that even though stat92E was identified as a target gene of Tin, STAT92E protein also inhibits tin expression. Instead, the authors unravel an intricate mechanism of spatial and temporal regulation by Upd and demonstrate a novel auto-inhibitory relationship linking Tin and STAT92E.
Although Upd is only briefly expressed in the ventral ectoderm, this pulse of ligand is sufficient to induce long range JAK-STAT signaling in the underlying precardiac mesoderm (Fig. 2). Following this signal, the tin expression pattern changes dramatically from a broad to a more confined pattern. This restriction of tin expression is both JAK-STAT dependent and mesoderm autonomous. The latter being shown by elegant rescue experiments using mesodermally expressed STAT92E in a stat92E mutant background. It is this early regulation of tin expression, controlled by JAK-STAT signaling, that is the key to normal heart development, with failure of JAK-STAT to curb tin resulting in too many tin positive pericardial cells later on.
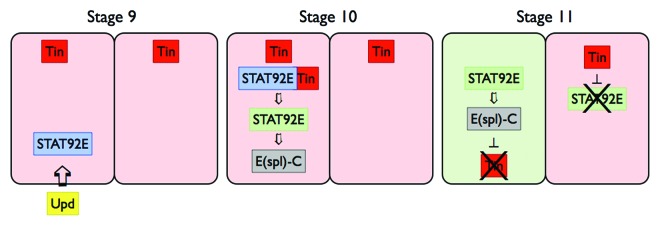
Figure 2. JAK-STAT signaling restricts tin expression pattern. Maternally contributed JAK-STAT pathway components are stimulated by Upd ligand expressed by the ventral ectoderm to induce segmental STAT92E activation (blue) in tin (red) expressing dorsal mesoderm (stage 9). STAT92E together with Tin causes the expression of zygotic STAT92E (green), which in turn drives expression of target genes such as E(spl)-C (gray) (stage 10). By stage 11, tin expression in alternating segments leads to stripes of tin-on (pink segments) and tin-off (green segments).
Finally, Johnson and colleagues show that the mechanism for downstream, STAT92E-mediated repression of tin expression involves E(spl)-C and the T-box transcription factor H15. As such, Tin is required for the initial expression of STAT92E yet also regulates its own inhibition via the STAT92E target genes (Fig. 2).
Although superficially complex, this description of a role for JAK-STAT in the cardiac development of Drosophila highlights how a model system with relatively straightforward morphology and low pathway complexity, combined with excellent developmental/genetic tools can be used to unravel even the deepest mysteries of the heart. However, the key question now relates to potential implications for understanding JAK-STAT cardiac functions in mammalian models.
Intriguingly, a handful of reports have also implicated JAK-STAT signaling in vertebrate cardiac development. For example, loss of pathway components has been associated with defects and delays in heart development. An affect noted for the cytokine cardiotrophin-1,8 as well as for JAK29 and gp-130.10 Furthermore, JAK-STAT also plays important homeostatic roles later in development, where activation of JAK1 or JAK2 and STAT3 function to protect the heart from oxidative stress and cardiac damage (reviewed in ref. 11).
While the precise factors linking JAK-STAT signaling in the vertebrate and invertebrate heart remain to be determined, the molecular dissection of a role for JAK-STAT signaling in Drosophila heart development opens up a new field of investigation with important implications for invertebrate as well as vertebrate cardiac development.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/21361
References
- 1.Meister M, Lagueux M. Drosophila blood cells. Cell Microbiol. 2003;5:573–80. doi: 10.1046/j.1462-5822.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 2.Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–26. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson AN, Mokalled MH, Haden TN, Olson EN. JAK/Stat signaling regulates heart precursor diversification in Drosophila. Development. 2011;138:4627–38. doi: 10.1242/dev.071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev Biol. 2002;246:14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- 5.Forouhar AS, Liebling M, Hickerson A, Nasiraei-Moghaddam A, Tsai HJ, Hove JR, et al. The embryonic vertebrate heart tube is a dynamic suction pump. Science. 2006;312:751–3. doi: 10.1126/science.1123775. [DOI] [PubMed] [Google Scholar]
- 6.Tao Y, Schulz RA. Heart development in Drosophila. Semin Cell Dev Biol. 2007;18:3–15. doi: 10.1016/j.semcdb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Liu YH, Jakobsen JS, Valentin G, Amarantos I, Gilmour DT, Furlong EE. A systematic analysis of Tinman function reveals Eya and JAK-STAT signaling as essential regulators of muscle development. Dev Cell. 2009;16:280–91. doi: 10.1016/j.devcel.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Jougasaki M. Cardiotrophin-1 in cardiovascular regulation. Adv Clin Chem. 2010;52:41–76. doi: 10.1016/S0065-2423(10)52002-X. [DOI] [PubMed] [Google Scholar]
- 9.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–95. doi: 10.1016/S0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci U S A. 1996;93:407–11. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurdi M, Booz GW. JAK redux: a second look at the regulation and role of JAKs in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1545–56. doi: 10.1152/ajpheart.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


