Abstract
RPA2 is a subunit of a trimeric replication protein A (RPA) complex important for DNA repair and replication. Although it is known that RPA activity is regulated by post-translational modification, whether RPA expression is regulated and the mechanism therein is currently unknown. eIF3a, the largest subunit of eIF3, is an important player in translational control and has been suggested to regulate translation of a subset of messenger RNAs important for tumorigenesis, metastasis, cell cycle progression, drug response and DNA repair. In the present study, we show that RPA2 expression is regulated at translational level via internal ribosome entry site (IRES)-mediated initiation in response to DNA damage. We also found that eIF3a suppresses RPA2 synthesis and inhibits its cellular IRES activity by directly binding to the IRES element of RPA2 located at −50 to −150 bases upstream of the translation start site. Taken together, we conclude that RPA2 expression is translationally regulated via IRES and by eIF3a and that this regulation is partly accountable for cellular response to DNA damage and survival.
Introduction
Replication protein A (RPA) is a single-strand DNA-binding protein complex consisting of three subunits referred as RPA1, RPA2 and RPA3 (1). RPA plays a critical role in almost all DNA metabolic processes such as replication and DNA damage repair (2). Previously, it has been shown that inhibiting RPA binding to DNA affects nucleotide excision repair (NER) machinery and consequently changes the efficacy of DNA-damaging chemotherapeutics (3). Thus, it is a potential target of cancer therapy and RPA inhibitors have been identified for potential therapeutic development (3–5). Although it is known that RPA activity is regulated by post-translational modification (6), whether RPA expression is regulated and the mechanism therein is currently unknown.
Regulation of gene expression is complicated and involves several levels of control such as transcription and translation. Translational regulation occurs mainly at initiation, a rate-limiting step of protein synthesis. Translational initiation can occur via cap-dependent or cap-independent [e.g. internal ribosome entry site (IRES) mediated] mechanisms involving multiple translation initiation factors, named eIFs in eukaryotes (7–10). One of the eIFs, eIF3, is the largest and most complex one consisting of at least 13 subunits named eIF3a to eIF3m (10,11). Although some of these subunits are considered as core subunits of the eIF3 complex (12,13), various subcomplexes of eIF3 with different subunits have been shown to exist (14–16). Further, different eIF3 subunits have differential expression in tumors and were thought to have oncogenic or tumor suppressor functions (10). For example, the largest subunit, eIF3a, was found to be overexpressed in human cancers of breast (17), cervix (18), esophagus (19), stomach (20) and lung (21) and it may have oncogenic functions (22,23). Interestingly, cancer patients with high eIF3a level appeared to have better prognosis and response to chemotherapy than those with low eIF3a levels (18,24). This theory is supported by previous findings that eIF3a upregulates the synthesis of α-tubulin and ribonucleotide reductase M2 (22), but downregulates the synthesis of p27kip1 (25), Rad23B, xeroderma pigmentosum group proteins (XPA and XPC) and RPA (24,26) proteins. However, how eIF3a upregulates the translation of some messenger RNAs (mRNAs) while downregulates others is currently unknown.
One theory for the possible mechanism of eIF3a action in tumorigenesis and drug response is that eIF3a may play an important role in regulating translation of a subset of mRNAs that are critical in cell proliferation and survival (10). In this study, we investigated translational regulation of RPA using RPA2 as a model protein and found that RPA2 was regulated via IRES-mediated mechanism in response to DNA damage and that eIF3a suppresses RPA2 synthesis by inhibiting its IRES activity. Thus, the double-edged role of eIF3a in tumorigenesis and chemosensitivity is likely mediated by translational regulation of downstream target genes such as DNA repair proteins.
Materials and methods
Materials
Restriction enzymes, m7GpppG cap analog and Pfu polymerase were obtained from New England Biolabs, Amersham Biosciences and Agilent, respectively. Sp6, T7 RNA polymerases, RNasin, RNase-free DNase, rabbit reticulocyte lysate (RRL), dual luciferase reporter assay kit and Streptavidin MagneSphere® Paramagnetic Particles were purchased from Promega. RNeasy Mini Kit, QIAprep Spin Miniprep Kit and QIAGEN Plasmid Midi Kit were from Qiagen. Cell culture media, fetal bovine serum, Lipofectamine Plus and Lipofectin transfection reagent were from Invitrogen. eIF3a small interfering RNA (siRNA), RPA2 and β-actin antibody were from Santa Cruz, Abcam and Sigma, respectively. RNase T1 and Biotin-11-CTP were from Roche Diagnostics. α-32P ATP was from PerkinElmer. All other reagents were of molecular biology grade from Sigma or Fisher Scientific.
Construct engineering
The full-length and truncated 5′-untranslated region (UTR) sequences of human RPA2 were amplified from genomic DNA of H1299 cells using reverse transcription–polymerase chain reaction (RT–PCR) with a common reverse primer 5′-AATCCATGGTGTTCCACATCTTGGTCA-3′ and four individual forward primers: 5′-ATGCAAGCTTCGTAAAGATGGCCGC-3′ (for full-length RPA2 5′-UTR), 5′-TGCAAGCTTGCTCCGCCATTCGCGGGAAG-3′ (for Δ142 mutant), 5′-TCACTAGTAGCGGCGCAGTGGCGGCCGC-3′ (for Δ192 mutant) and 5′-TCACTAGTCCGCACCTTCTCGGCCTCTT-3′ (for Δ242 mutant). The PCR products were then cloned into pGEM-T Easy vector.
Dicistronic vectors phRF and pRF containing Renilla and firefly luciferase genes were used to engineer dicistronic constructs containing RPA2 5′-UTR (27–30). The RPA2 5′-UTR was released by digestion with HindIII and NcoI from pGEM-T Easy construct, blunted and cloned into phRF and pRF vectors at the blunted SpeI and NcoI sites, resulting in phR-RPA-F and pR-RPA-F, respectively.
To generate dicistronic transcripts with poly(A) tail, the full-length and truncated 5′-UTR sequences were digested with HindIII and NcoI from pGEM-T Easy constructs and subsequently cloned into pSP-R-HRV-FA30 (27–30) by replacing HRV (human rhinovirus) IRES sequence digested with SpeI and NcoI. All constructs were confirmed by double-strand DNA sequencing.
Cell culture, DNA and RNA transfections
H1299 cells were cultured using RPMI-1640 with 10% fetal bovine serum at 37°C with 5% CO2, whereas NIH3T3 cells without or with stable eIF3a overexpression (24) were maintained in Dulbecco's modified Eagle's medium with 10% horse serum at 37°C with 10% CO2.
For DNA transfection, 0.4 µg DNA was transfected into cells in 24-well plates using Lipofectamine Plus reagent according to manufacturer’s protocol. Cells were harvested after 24h and subjected to luciferase assay. RNA transfection was performed using Lipofectin as described previously (31). Briefly, 2×105 H1299 or NIH3T3 cells/well were seeded into 6-well plates on the day before transfection. Cells were washed once with Opti-MEMI reduced serum medium and incubated with a mixture containing 12.5 µg Lipofectin, 1–5 µg RNA and 1ml Opti-MEMI medium. At 8h after transfection, cells were harvested for luciferase assay. For siRNA transfection, H1299 cells were seeded into 6-well plates and transfected with 50nM eIF3a or scramble control siRNA using Lipofectamine 2000 according to manufacturer’s instructions. Cells were harvested 48h after transfection for further analysis.
Cytoplasmic extract (S100) preparation
Cytoplasmic extract was prepared as described previously (29). Briefly, 2×107 HeLa cells were collected and resuspended in 2ml extraction buffer H100 [10mM Tris–HCl (pH 7.4), 1.5mM MgCl2, 10mM KCl, 0.5mM dithiothreitol and 0.1mM phenylmethylsulfonyl fluoride (PMSF)]. The cells were homogenized with a Dounce homogenizer for 20 strokes on ice. Cell nuclei were removed by centrifugation at 2000g for 10min. The supernatant was adjusted to 150mM KCl and centrifuged at 100 000g for 90min at 4°C. The final supernatant was collected as S100 extract and stored at −80°C.
In vitro transcription and translation
In vitro transcription and translation in the absence or presence of HeLa cell extracts were performed as described previously (27–31). Briefly, DNA templates were linearized using EcoRI or NcoI, and transcripts with both 5′-cap and 3′-poly(A) tail were synthesized using T7 or SP6 RNA polymerases in the presence of 30mM m7GpppG. The in vitro RNA transcripts were then purified using Qiagen RNeasy mini kit. About 50ng of capped cRNA transcripts were used to program cell-free translation in RRL in a final volume of 10 µl containing 3.5 µl RRL, 4 µl of 5 µg/µl HeLa cell extract S100 or control extraction buffer H100.
To determine the RNA stability in RRL, in vitro transcription was performed in the presence of 70 µCi α[32P]-UTP (PerkinElmer) and dicistronic transcripts were purified as described above. In a 50 µl cell-free RRL translation reaction mixture with or without HeLa extract S100, 1.5×106 c.p.m. dicistronic RNAs were added and incubated at 30°C for different times followed by recovery of RNAs from the reactions using RNeasy mini Kit (Qiagen). The recovered RNAs were separated by 1.2% agarose denaturing gel for autoradiography analysis.
Protein sample preparation and western blot
Protein sample preparation and western blot were performed as described previously (24,32). Briefly, cells were collected and lysed using TNN-SDS buffer (50mM Tris–HCl, pH 7.5, 150mM NaCl, 0.5% Nonidet P-40, 50mM NaF, 1mM sodium orthovanadate, 1mM dithiothreitol, 0.1% sodium dodecyl sulfate and 2mM PMSF) at 4°C for 30min followed by centrifugation at 10 000g for 10min. The supernatant was saved and protein concentration was measured using Bradford method. The cell lysates were then separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to a polyvinylidene difluoride membrane for western blot analysis of eIF3a, RPA2 and β-actin.
UV cross-linking and pull-down assay
UV cross-linking was performed as described previously (29). Briefly, RNA probes were generated using in vitro transcription in the presence of 70 µCi α[32P]-UTP (PerkinElmer) and purified as described above. The RNA probe was diluted to 1×105 c.p.m./µl and mixed with cell extract followed by incubation at room temperature for 30min and irradiated by UV (254nm, 5.4 J/cm2) using Stratalinker 1800 (Stratagene). The mixture was then separated by SDS–PAGE for autoradiography.
For immunoprecipitation of cross-linked eIF3a, extracts from cells with overexpression of Flag-tagged eIF3a were used for cross-linking as described above. Unbound RNA probes were removed by digestion with 300U RNase T1 at 30°C for 15min and boiling in the presence of 0.5% sodium dodecyl sulfate to denature proteins. The reaction mixture was then diluted in the binding buffer (15mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.6, 50mM KCl, 10% glycerol, 5mM MgCl2 and 0.5mM PMSF) to make the final concentration of sodium dodecyl sulfate below 0.05% followed by immunoprecipitation using Flag antibody as described previously (29). The precipitates were separated by SDS–PAGE for autoradiography analysis.
For pull-down assay, biotin-labeled RNA probe was incubated with H1299 cell extracts at room temperature for 1h. The RNA–protein complexes were then isolated using Streptavidin MagneSphere® Paramagnetic Particles and washed for three times with wash buffer (10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 20mM KCl, 1mM MgCl2, 1mM dithiothreitol and 1mM PMSF). The pull-down materials were then separated by SDS–PAGE and analyzed using western blot probed by eIF3a antibody.
Real-time RT–PCR and luciferase reporter assay
Real-time RT–PCR was carried out as described previously (24,32). Briefly, total RNA was extracted using RNeasy Mini Kit and 1 µg RNA was used for reverse transcription using iScript cDNA synthesis kit (Bio-Rad) according to manufacturer’s protocol. The primers used for RPA2 were 5′-GAAGGCTCCAACCAACATTGTTTAC-3′ (forward) and 5′-GCCTGCCACTTTCACATATGTTTC-3′ (reverse) and for β-actin control were 5′-TGGCACCCAGCACAATGAA-3′ (forward) and 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (reverse). The reaction was performed in a 7500 real-time PCR system using SYBR Green PCR Master Mix (Applied Biosystems) according to manufacturer’s instructions. The threshold cycle value (C t) of each product was determined and normalized against that of the internal control β-actin.
Luciferase reporter assay was performed as described previously (30). Cells transfected with dicistronic reporter construct and β-galactosidase transfection efficiency control were lysed in a passive lysis buffer provided in the dual luciferase reporter assay kit. Both Renilla and firefly luciferase were then measured using the dual luciferase reporter assay kit according to manufacturer’s instructions.
Results
The 5′-UTR sequence of RPA2 contains an IRES element
Previously, we found that the expression of NER proteins Rad23B, XPA, XPC and RPA is regulated at the translational level (24,26). Examination of the available sequences of mRNAs of these proteins showed that some of them such as RPA2 have a long and structured 5′-UTR with upstream AUGs. This finding suggests that RPA2 mRNA may be subjected to regulation by IRES-mediated translational initiation.
To determine if the 5′-UTR of RPA2 has an IRES element, we first engineered a dicistronic reporter construct with the 5′-UTR of RPA2 inserted in the intergenic region (Figure 1A) and generated dicistronic transcript containing both 5′-cap and 3′-poly(A) tail using in vitro transcription (Figure 1B). This in vitro dicistronic transcript along with a negative control dicistronic transcript without any IRES and a positive control transcript with HRV IRES were transiently transfected into NIH3T3 cells for luciferase expression and determination of IRES activity. Translation of the second cistron, firefly luciferase, from the dicistronic transcript represents a potential IRES element in its preceding intergenic region, whereas translation of the first cistron, Renilla luciferase, is driven by cap-dependent mechanism. Figure 1C shows the relative ratio of firefly to Renilla luciferase (F/R) for each dicistronic transcripts in NIH3T3 cells, which represents the relative IRES activity. Clearly, the 5′-UTR sequence of RPA2 has similar level of IRES activity as that of the HRV IRES in NIH3T3 cells, ~2-fold increase compared with that of the negative control transcript.
Fig. 1.
Cellular IRES activity of RPA2 5′-UTR. (A) Schematic diagram of dicistronic transcripts with 5′-cap structure m7G and poly(A) tail (A30). (B) Agarose gel electrophoresis analysis of dicistronic transcripts synthesized in vitro. (C) IRES activity. NIH3T3 and H1299 cells were transiently transfected with dicistronic transcripts followed by determination of Renilla and firefly luciferase activities. The relative ratio of firefly to Renilla luciferase activity was used to represent relative IRES activity compared with the control RF (Renilla firefly) dicistronic transcript (*P < 0.05; n = 5). R: Renilla, F: Firefly.
To ensure that the above finding is not cell-line specific, we performed similar experiments using H1299 cells. Figure 1C shows that the IRES activity of the dicistronic transcript containing the 5′-UTR of RPA2 is again ~2-fold of that of the negative control transcript although the IRES activity of HRV is much higher in H1299 cells than that in NIH3T3 cells. Thus, the IRES activity of the 5′-UTR of RPA2 is not cell-line dependent, whereas that of the HRV IRES may be cell-type dependent.
Because the sequence and length of intergenic region in our dicistronic transcripts is different, it is possible that the observed IRES activity is a result of their effects on translational termination efficiency of the first cistron, Renilla luciferase. To exclude this possibility, we examined the absolute Renilla luciferase activity normalized to the quantity of dicistronic RNA used. As shown in Supplementary Figure S1A and B, available at Carcinogenesis Online, no significant difference in Renilla luciferase expression was detected between all dicistronic transcripts with different intergenic sequences, suggesting that translational termination efficiency is unlikely affected.
We next performed in vitro translation of the above dicistronic transcripts in RRL to determine the IRES activity. Previously, it has been shown that RRL lacks factors required for IRES-dependent translation (29) and, consequently, we supplemented the RRL system with cytoplasmic extract from HeLa cells (S100) prior to performing IRES activity assay as described previously (29). As shown in Figure 2A, the IRES activity of both the 5′-UTR of RPA2 and the HRV IRES appears to be suppressed in the control RRL, which is consistent with previous observations (29). However, the IRES activity of both RPA2 and HRV was significantly increased by the addition of HeLa cell extract (S100). This observation further confirms that the 5′-UTR of RPA2 may contain an IRES element.
Fig. 2.
In vitro translation and stability of dicistronic transcripts in RRL. (A) In vitro translation in RRL. Dicistronic transcripts shown in Figure 1 were used to program translation in RRL in the presence of HeLa cell cytoplasmic extract (S100) or buffer control (H100) followed by analysis of IRES activity as described in Figure 1 (*P < 0.05; n = 3). (B) Stability of dicistronic transcript containing RPA2 5′-UTR. [32P]-labeled dicistronic transcripts containing RPA2 5′-UTR were incubated in RRL in the absence or presence of S100 followed by recovery and analysis using agarose gel electrophoresis.
The major argument against the existence of cellular IRES using dicistronic assay is the existence of cryptic promoters in the dicistronic DNA constructs and alternative splicing of the dicistronic transcripts (27–30). Since we did not use DNA constructs in the above assays, the potential misleading effects from cryptic promoters can be eliminated. However, it is possible that the observed expression of the firefly luciferase in above studies may potentially be due to unexpected splicing or degradation of the dicistronic transcripts, leading to production of monocistronic firefly luciferase cistron that may use cap-dependent translation. To eliminate this possibility, we determined if the dicistronic transcripts have been converted to monocistronic transcripts following incubation in RRL supplemented with HeLa cell extract. As shown in Figure 2B, no clear shorter transcripts representing a monocistronic RNA was observed and addition of HeLa extract S100 did not accelerate degradation of the dicistronic transcripts in RRL. Thus, the IRES activity of RPA2 is unlikely due to unexpected splicing or degradation of the dicistronic transcripts.
To rule out another possibility that expression of the second cistron firefly luciferase is due to potential read-through or ribosome shunting in the presence of RPA2 5′-UTR sequence in the intergenic region, we engineered a new construct containing a synthetic hairpin in front of the first cistron. As described previously (27), this hairpin structure is expected to inhibit translation of the first cistron and, thus, potential ribosome read-through or shunting. It will not affect translational initiation of the second cistron mediated by IRES. As shown in Supplementary Figure S2, available at Carcinogenesis Online, insertion of the hairpin structure dramatically reduced the expression of the first cistron Renilla luciferase. However, it did not significantly reduce the expression of the second cistron firefly luciferase. Thus, the IRES activity of RPA2 is unlikely due to the potential read-through or ribosome shunting from the first cistron.
Location of the IRES element in the 5′-UTR of RPA2
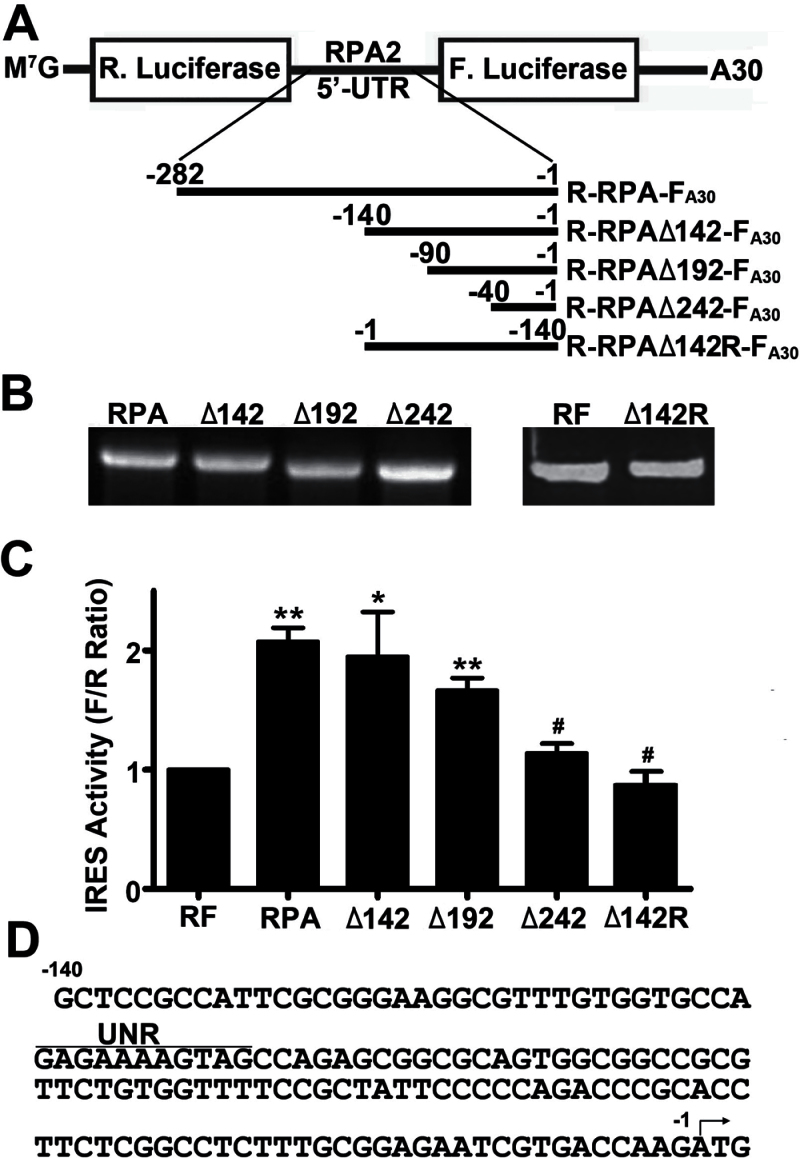
We next attempted to locate the IRES element in the 5′-UTR of RPA2 by systematically deleting the 5′-UTR from the 5′-end and generated dicistronic transcripts (Figure 3A and B) to analyze the effects of deletion on IRES activity. Figure 3C shows that the 5′-UTR with 142 bases removed at the 5′-end (R-RPAΔ142-FA30) has nearly full IRES activity compared with the full-length 5′-UTR (R-RPA-FA30). Deletion of additional 50 bases from the 5′-end (R-RPAΔ192-FA30) partially reduced the IRES activity, whereas further deletion of additional 100 bases (R-RPAΔ242-FA30) essentially eliminated its IRES activity. Thus, the IRES activity of the 5′-UTR may reside in the region ranging from −40 to −140 bases upstream of the translation start site.
Fig. 3.
Mapping of the IRES element in the 5′-UTR of RPA2. (A) Schematic diagram of dicistronic transcripts with sequential deletions from the 5′-end of the 5′-UTR of RPA2 in the intergenic region. (B) Agarose gel electrophoresis analysis of in vitro synthesized dicistronic transcripts containing full-length (RPA) and deletion mutant 5′-UTRs of RPA2. (C) Relative IRES activity of different RPA2 5′-UTRs. Dicistronic transcripts containing full-length (RPA) and deletion mutant 5′-UTRs, and the control complimentary sequence (Δ142R) were transiently transfected into H1299 cells followed by analysis of relative IRES activity as described in Figure 1 (**P < 0.01; *P < 0.05; # P > 0.05; n = 3–4). (D) Sequence of 5′-UTR. The translation start site and the putative UNR-binding site are shown by an arrow and a line, respectively.
To ensure that the IRES activity of R-RPAΔ142-FA30 was not due to spatial effect of the 140 bases 5′-UTR sequence in the dicistronic transcripts, we tested its complementary sequence (R-RPAΔ142R-FA30) using the same assay as described above. As shown in Figure 3C, no IRES activity was observed with R-RPAΔ142R-FA30 containing the complementary sequence of the 140 bases of RPA2 5′-UTR. Thus, we conclude that the IRES activity observed with the 140 bases of RPA2 5′-UTR is unlikely due to spatial effect of the sequence on the expression of the second cistron.
We next performed a sequence analysis of the 140 bases of RPA2 5′-UTR to identify putative RNA-binding consensus sequences using an online tool UTRScan (http://itbtools.ba.itb.cnr.it/utrscan). As shown in Figure 3D, a putative consensus sequence of 5′-GAGAAAAUGAG-3′ was identified for binding by UNR (upstream of N-ras), a cold-shock domain-containing RNA-binding protein that is known to mediate IRES-dependent translation initiation (see Discussion). This observation further supports the conclusion that the 140 bases at the 3′-end of the 5′-UTR of RPA2 contains a putative element for IRES-dependent translational initiation.
IRES activity of RPA2 during DNA damage
Because RPA2 is an important protein in DNA damage repair and its expression is known to be upregulated by DNA damages (6,33), it is possible that this IRES-mediated translation initiation of RPA2 mRNA is an important process during DNA damage response and repair. To test this possibility, we first tested if the IRES activity of RPA2 changes in response to DNA damage. For this purpose, dicistronic transcripts with (R-RPA-FA30) or without (R-RPAΔ242-FA30) IRES element of RPA2 were transiently transfected into cisplatin-treated H1299 cells followed by determination of IRES activity. As shown in Figure 4A, the IRES activity in the 5′-UTR of RPA2 (R-RPA-FA30) is significantly enhanced by ~30% in the cisplatin-treated cells compared with the dimethyl sulfoxide-treated control cells. However, no change was detected between the two cells for the R-RPAΔ242-FA30 transcript lacking RPA2 IRES element. Thus, the IRES activity of RPA2 is likely increased in cells experiencing DNA damages. This increase may elevate RPA2 translation in response to DNA damage. To test this possibility, we performed a western blot and real-time RT–PCR analyses of the endogenous level of RPA2 protein and mRNA, respectively. Figure 4B and C shows that the level of endogenous RPA2 protein is increased, whereas the level of its mRNA remains unchanged following cisplatin treatment. Together, these observations suggest that the RPA2 expression is translationally upregulated in response to DNA damage possibly via increasing its IRES activity.
Fig. 4.
IRES activity and expression of RPA2 following DNA damage. (A) Effect of cisplatin treatment on RPA2 IRES activity. H1299 cells were treated with 5 µM cisplatin or dimethyl sulfoxide control for 16h followed by transfection with dicistronic transcripts R-RPA-FA30 (RPA) or R-RPAΔ242-FA30 (Δ242) and determination of relative IRES activity. (B and C) Effect of cisplatin treatment on expression of endogenous RPA2. H1299 cells were treated with 5 µM cisplatin or dimethyl sulfoxide control for 24h followed by determination of RPA2 level using western blot (B) and real-time RT–PCR (C) analyses. β-Actin was used as loading control for western blot and as an internal control for real-time RT–PCR (*P < 0.05; # P > 0.05; n = 3–5).
eIF3a regulates RPA2 IRES activity
In the above analysis of the effect of cisplatin treatment on RPA2 expression, we also found that eIF3a expression is downregulated in the cisplatin-treated cells (Figure 4B). Previously, it has been shown that eIF3a may regulate hepatitis C virus IRES by binding to it (34) and cleavage of eIF3a inhibited assembly of ribosomal complexes to the IRES element of Theller’s murine encephalomyelitis virus (35). It has also been shown that eIF3a suppresses RPA2 synthesis (24). Thus, we hypothesize that eIF3a may regulate RPA2 synthesis via regulating its IRES activity and RPA2 expression in response to DNA damages.
To test this hypothesis, we first knocked down eIF3a expression in H1299 cells using siRNA followed by transient transfection of dicistronic transcripts with (R-RPA-FA30) or without (R-RPAΔ242-FA30) the IRES element of RPA2 and determined the effect of eIF3a expression on RPA2 IRES activity. Figure 5A shows that knocking down eIF3a expression significantly increases the IRES activity of RPA2. It has no effect on the expression of firefly luciferase in the construct with truncated 5′-UTR (R-RPAΔ242-FA30) that has no IRES.
Fig. 5.
eIF3a regulation of RPA2 expression and IRES activity. H1299 cells (A and C) transfected with eIF3a (Si) or scramble control (Scr) siRNAs and NIH3T3 cells (B and D) with stable eIF3a overexpression (eIF3a) or transfected with vector control (Vec) were transiently transfected with dicistronic transcripts R-RPA-FA30 (RPA) or R-RPAΔ242-FA30 (Δ242). Cells were then harvested for determination of relative IRES activity (A and B) or western blot analyses of eIF3a and RPA2 protein level (C and D). β-Actin was used as loading control for western blot. The relative protein level was determined using gel densitometer (**P < 0.01; *P < 0.05; # P > 0.05; n = 3–5).
We next performed a reverse experiment to determine if overexpressing eIF3a can suppress the IRES activity of RPA2. For this purpose, the previously established NIH3T3 stable cell line with eIF3a overexpression was transiently transfected with the dicistronic reporter transcripts as described above followed by determination of IRES activity. Figure 5B shows that the IRES activity of RPA2 is significantly suppressed in the cells with eIF3a overexpression compared with the vector-transfected control cells. On the other hand, it has no effect on the expression of firefly luciferase in the truncated construct (R-RPAΔ242-FA30) that has no IRES element.
To further determine if eIF3a affects the expression of the endogenous RPA2, similar to the IRES activity of RPA2, we performed a western blot analysis of RPA2 in the above transfected cells. Figure 5C and D shows that the endogenous RPA2 is increased by eIF3a knockdown in H1299 cells and decreased by eIF3a overexpression in NIH3T3 cells. Thus, probably eIF3a regulates RPA2 synthesis by regulating its IRES activity.
eIF3a binds to RPA2 IRES element
The above studies suggest that eIF3a suppresses RPA2 synthesis possibly by inhibiting IRES-dependent translation of RPA2 mRNA. To determine if eIF3a possibly bind to the IRES element in the 5′-UTR of RPA2 mRNA, we first performed a UV cross-linking experiment. For this purpose, 32P-labeled 5′-UTR of RPA2 RNA was synthesized in vitro and incubated with H1299 cell extracts followed by UV cross-linking and analysis of cross-linked proteins by SDS–PAGE and autoradiography. Figure 6A shows that many proteins including one with a molecular weight of ~170kDa (similar size to that of eIF3a) are cross-linked to this probe, suggesting that eIF3a may bind to the 5′-UTR of RPA2.
Fig. 6.
Interaction between eIF3a and RPA2 IRES element. (A) SDS–PAGE analysis of RPA2 IRES-bound proteins. H1299 cell extracts were incubated with [32P]-labeled RPA2 IRES probe followed by UV irradiation. The cross-linked proteins were then subjected to separation on SDS–PAGE and autoradiograph analysis. (B) Immunoprecipitation of RPA2 IRES-bound eIF3a. Extracts from cells transfected with Flag-tagged eIF3a were incubated with [32P]-labeled RPA2 IRES probe (RPA) or its complementary sequence control (Rev) followed by UV irradiation and immunoprecipitation using Flag antibody or normal control immunoglobulin G. The precipitate was then subjected to separation on SDS–PAGE and autoradiography analysis. (C and D) Pull-down assay. H1299 cell extracts were incubated with biotin-labeled probes containing the 5′-UTR sequences of RPA2 from −282 to −1 (RPA), −140 to −1 (Δ142), −40 to −1 (Δ242) and the complementary sequences (Rev) of the 5′-UTR of RPA2. The RNA–protein complexes were then pulled down using streptavidin-conjugated beads for separation on SDS–PAGE and analysis of eIF3a using western blot. Probes without biotin were used as controls.
To further determine if the 170kDa protein bound to the 5′-UTR probe of RAP2 possibly represents eIF3a, we performed an immunoprecipitation of Flag-tagged eIF3a following UV cross-linking the probe to proteins in cell extracts before subjecting the precipitate to SDS–PAGE and autoradiography analysis. As shown in Figure 6B, only one protein of ~170kDa cross-linked to the 32P-labeled 5′-UTR probe of RPA2 is precipitated by Flag antibody but not by the control immunoglobulin G. Furthermore, the control probe derived from the complementary sequence of the 5′-UTR of RPA (Rev) did not result in a cross-linked complex that could be precipitated by Flag antibody. Thus, eIF3a probably can bind directly to the 5′-UTR of RPA2.
To verify eIF3a binding to the IRES element in the 5′-UTR of RPA2, we performed a pull-down assay of eIF3a using biotin-labeled probes corresponding to the regions from −1 to −282 (RPA), −1 to −140 (Δ142) and −1 to −40 (Δ242) of the 5′-UTR of RPA2. The pull-down materials were then analyzed by western blot for eIF3a. As shown in Figure 6C, eIF3a is pulled down successfully by both full-length (RPA) and truncated Δ142 probes as expected since both contain the IRES element of RPA2 (see Figure 3). However, the truncated Δ242 probe without IRES element is unable to pull down eIF3a, suggesting that it does not bind to eIF3a. We also found that a probe (Rev) representing the complimentary sequence of the 5′-UTR of RPA2 did not bind to eIF3a (Figure 6D), further confirming that the binding of eIF3a to the IRES element of RPA2 in its 5′-UTR is sequence dependent. Thus, eIF3a likely regulates the IRES activity of RPA2 by binding to its IRES element in the region −40 to −140 of the 5′-UTR of RPA2.
Discussion
In this study, we showed that the 5′-UTR sequence of human RPA2 mRNA has IRES activity, which is upregulated during cellular response to DNA damage to upregulate RPA2 expression at translational level. We also showed that the IRES element of RPA2 mRNA is located in the region 40–140 bases upstream of the translation start site and that eIF3a can directly bind to this site to suppress the IRES-dependent translation of RPA2 mRNAs. It appears that reduced eIF3a expression level due to DNA damage releases eIF3a suppression of the IRES activity of RPA2, leading to the increased expression of RPA2 for repair of DNA damages.
Cap-independent translation initiation via IRES is an important mechanism of initiation for protein synthesis. Initially identified for translation of viral poly-cistronic transcripts (36), IRES has also been reported to exist in many cellular mRNAs (http://www.iresite.org). However, various issues have been raised with cellular IRES, mainly due to the use of dicistronic DNA constructs in reporter assays, which have inherited problems of cryptic promoter activity in DNA constructs and alternative splicing event of dicistronic transcripts (37). Later studies have demonstrated that some of the previously identified cellular IRESs are indeed cryptic promoter activities in the cDNA encoding the 5′-UTRs (27–30) or due to aberrant splicing event (38). These previous studies suggest that the use of dicistronic transcript reporters will be more appropriate than the dicistronic DNA constructs to help eliminate the two major concerns: cryptic promoter activity and alternative splicing event.
In the present study, dicistronic transcripts were used to assess the IRES in the 5′-UTR of human RPA2 to eliminate the potential cryptic promoter issue. We have also eliminated the possible effect of splicing or degradation of dicistronic transcripts as well as potential ribosome shunting and read-through that can potentially be misled as IRES. The IRES activity of RPA2 mRNA is relatively weak in H1299 cells compared with the known viral HRV IRES. However, it is similar in NIH3T3 cells in which the HRV IRES activity is reduced to that of RPA2 IRES. Clearly, the IRES activity of RPA2 is not dependent on cell lines used although the HRV IRES is much higher in H1299 than in NIH3T3 cells. Previously, we found that the activity of HRV IRES in HeLa cells is also very high. The reason for the difference of HRV IRES activity between NIH3T3 and H1299 cells is currently unknown. Perhaps, cancer cells such as H1299 and HeLa cells have different factors important for HRV IRES from the fibroblast cell line such as NIH3T3. It is also possible that the human IRES of HRV is more active in human cell lines (H1299 and HeLa) than in the mouse cell line (NIH3T3). These possibilities await further investigation.
Cellular IRES has been shown to play important roles for protein synthesis in various cellular processes such as during mitosis (39) and apoptosis (40) and during pathological and stress conditions (41). For example, Johannes et al. (42) identified cellular IRES elements using a cDNA microarray in HeLa cells and found that some IRES-harboring mRNAs encode proteins that are involved in stress response such as inflammation and angiogenesis. Here, we found that RPA2 IRES was activated following cisplatin treatment to induce stress and DNA damage. Because RPA2 is an important protein component of RPA with responsibilities in NER of cisplatin-induced DNA damages (43), the increase in IRES activity of RPA2 and its protein level in response to cisplatin treatment is consistent with its roles in repair of DNA damages. IRES-mediated translational control may be another level of regulation for RPA2 in response to DNA damages. Whether expression of the other subunits of RPA complex is also subjected to IRES regulation is currently unknown and needs to be investigated in the future.
It is also noteworthy that eIF3a expression was decreased after treating cells with cisplatin (Figure 5) and that eIF3a has been found to suppress RPA2 synthesis (24). Although it is currently unknown how cisplatin treatment reduces eIF3a expression, the reduced eIF3a expression appears to release its suppression of the IRES activity of RPA2 mRNA. The eIF3a suppression of the IRES activity of RPA2 may be due to its direct binding to the IRES sequence. This observation is consistent with a previous study where it was found that eIF3a can directly bind to the IRES element of hepatitis C virus (34). However, the binding of eIF3a to the hepatitis C virus IRES promotes its IRES activity, whereas eIF3a binding to RPA2 IRES suppresses the IRES activity. It is currently unknown why eIF3a binding to different IRES play different roles in promoting or suppressing the activity of these IRESs. Considering that eIF3a may promote the synthesis of some proteins such as ribonucleotide reductase (22) while suppressing the synthesis of other proteins such as p27 (25), it is not surprising to find that eIF3a may activate some while inhibit other IRESs by directly binding to these IRES elements. This observation is also consistent with an earlier finding that eIF3a affects the activity of some but not other IRES elements (35).
Interestingly, we found that the 5′-UTR of human RPA2 contains a consensus sequence for UNR, which is known to mediate IRES-dependent translation initiation (44). UNR has been shown to be required for HRV IRES activity both in vitro and in vivo (45,46). It has also been shown that UNR interacts with the IRES element of PITSLRE kinase and that the UNR consensus binding site is essential for its IRES activity (47). IRES-dependent translation initiation appears to require trans-acting proteins that are RNA-binding proteins (48) and many trans-acting factors such as polypyrimidine tract-binding protein and UNR have been identified (40,41,45–49). It is, thus, tempting to speculate that increased eIF3a level may compete with UNR for binding to the IRES element of human RPA2, resulting in reduced IRES-dependent translation initiation and lower level of RPA2 production. We are currently testing this hypothesis.
eIF3a expression has been associated with tumorigenesis and malignant transformation (10,22,23), metastasis (50) and cancer prognosis (18,19). Findings that eIF3a suppresses the synthesis of DNA repair proteins suggest that elevation of eIF3a may reduce cellular protection against DNA damages by decreasing DNA repair, resulting in higher frequency of mutation for tumorigenesis or reduced sensitivity of cancer cells to DNA-damaging anticancer drugs. The finding that eIF3a regulation of RPA2 synthesis at its IRES level suggests that this mechanism of regulation may be widespread although further studies are needed to determine if this is true. It is also interesting to note that the IRES activity of RPA2 is relatively low compared with viral IRES. Thus, it is not clear how much the IRES-mediated translation of RPA2 mRNA contributes to the total level of RPA2 protein. However, considering that RPA2 mRNA has a long, stable and highly structured (−109 kcal/mol) 5′-UTR, cap-dependent translation of RPA2 may not be efficient, making the IRES-dependent translation more important. Examining the available 5′-UTR sequences in the mRNAs of other NER proteins shows that XPF and XPG both also have a long and highly structured 5′-UTR, suggesting that they may also be under IRES control via eIF3a.
Supplementary material
Supplementary Figures S1 and S2 can be found online at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (CA140582 to J.T.Z.) and National Science Foundation of China (81173129 to Z.-Q.L., 81202595 to J.Y.Y.). J.Y.Y. was also supported, in part, by the China Scholarship Council and Scholarship Award for Excellent Doctoral Student granted by the Ministry of Education of China to study in the USA.
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- IRES
internal ribosome entry site
- mRNA
messenger RNA
- NER
nucleotide excision repair
- PMSF
phenylmethylsulfonyl fluoride
- RPA
replication protein A
- RRL
rabbit reticulocyte lysate
- RT–PCR
reverse transcription–polymerase chain reaction
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- siRNA
small interfering RNA
- UNR
upstream of N-ras
- UTR
untranslated region
- RHV
human rhinovirus.
References
- 1. Fanning E, et al. (2006). A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res, 34, 4126–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zou Y, et al. (2006). Functions of human replication protein A (RPA): from DNA replication to DNA damage and stress responses. J. Cell. Physiol, 208, 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neher T.M, et al. (2011). Novel irreversible small molecule inhibitors of replication protein A display single-agent activity and synergize with cisplatin. Mol. Cancer Ther, 10, 1796–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrews B.J, et al. (2004). Development of a high-throughput screen for inhibitors of replication protein A and its role in nucleotide excision repair. Mol. Cancer Ther, 3, 385–391 [PubMed] [Google Scholar]
- 5. Shuck S.C, et al. (2010). Targeted inhibition of replication protein A reveals cytotoxic activity, synergy with chemotherapeutic DNA-damaging agents, and insight into cellular function. Cancer Res, 70, 3189–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wold M.S. (1997). Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem, 66, 61–92 [DOI] [PubMed] [Google Scholar]
- 7. Sarnow P. (2003). Viral internal ribosome entry site elements: novel ribosome-RNA complexes and roles in viral pathogenesis. J. Virol, 77, 2801–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Livingstone M, et al. (2010). Mechanisms governing the control of mRNA translation. Phys. Biol, 7, 021001 [DOI] [PubMed] [Google Scholar]
- 9. Hinnebusch A.G. (2011). Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol. Mol. Biol. Rev, 75, 434–467, first page of table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yin J.Y, et al. (2011). Translational control gone awry: a new mechanism of tumorigenesis and novel targets of cancer treatments. Biosci. Rep, 31, 1–15 [DOI] [PubMed] [Google Scholar]
- 11. Dong Z, et al. (2006). Initiation factor eIF3 and regulation of mRNA translation, cell growth, and cancer. Crit. Rev. Oncol. Hematol, 59, 169–180 [DOI] [PubMed] [Google Scholar]
- 12. Asano K, et al. (1998). Complex formation by all five homologues of mammalian translation initiation factor 3 subunits from yeast Saccharomyces cerevisiae. J. Biol. Chem, 273, 18573–18585 [DOI] [PubMed] [Google Scholar]
- 13. Masutani M, et al. (2007). Reconstitution reveals the functional core of mammalian eIF3. EMBO J, 26, 3373–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaudhuri J, et al. (1997). Biochemical characterization of mammalian translation initiation factor 3 (eIF3). Molecular cloning reveals that p110 subunit is the mammalian homologue of Saccharomyces cerevisiae protein Prt1. J. Biol. Chem, 272, 30975–30983 [DOI] [PubMed] [Google Scholar]
- 15. Fraser C.S, et al. (2004). The j-subunit of human translation initiation factor eIF3 is required for the stable binding of eIF3 and its subcomplexes to 40 S ribosomal subunits in vitro. J. Biol. Chem, 279, 8946–8956 [DOI] [PubMed] [Google Scholar]
- 16. Zhou C, et al. (2005). PCI proteins eIF3e and eIF3m define distinct translation initiation factor 3 complexes. BMC Biol, 3, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bachmann F, et al. (1997). Cloning of a novel protein overexpressed in human mammary carcinoma. Cancer Res, 57, 988–994 [PubMed] [Google Scholar]
- 18. Dellas A, et al. (1998). Expression of p150 in cervical neoplasia and its potential value in predicting survival. Cancer, 83, 1376–1383 [DOI] [PubMed] [Google Scholar]
- 19. Chen G, et al. (1999). p150 expression and its prognostic value in squamous-cell carcinoma of the esophagus. Int. J. Cancer, 84, 95–100 [DOI] [PubMed] [Google Scholar]
- 20. Chen G, et al. (2004). p150 overexpression in gastric carcinoma: the association with p53, apoptosis and cell proliferation. Int. J. Cancer, 112, 393–398 [DOI] [PubMed] [Google Scholar]
- 21. Pincheira R, et al. (2001). Identification of a 170-kDa protein over-expressed in lung cancers. Br. J. Cancer, 84, 1520–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong Z, et al. (2004). Role of eIF3 p170 in controlling synthesis of ribonucleotide reductase M2 and cell growth. Oncogene, 23, 3790–3801 [DOI] [PubMed] [Google Scholar]
- 23. Zhang L, et al. (2007). Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J. Biol. Chem, 282, 5790–5800 [DOI] [PubMed] [Google Scholar]
- 24. Yin J.Y, et al. (2011). Effect of eIF3a on response of lung cancer patients to platinum-based chemotherapy by regulating DNA repair. Clin. Cancer Res, 17, 4600–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong Z, et al. (2003). EIF3 p170, a mediator of mimosine effect on protein synthesis and cell cycle progression. Mol. Biol. Cell, 14, 3942–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu R.Y, et al. (2011). Role of eIF3a in regulating cisplatin sensitivity and in translational control of nucleotide excision repair of nasopharyngeal carcinoma. Oncogene, 30, 4814–4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han B, et al. (2003). Regulation of constitutive expression of mouse PTEN by the 5′-untranslated region. Oncogene, 22, 5325–5337 [DOI] [PubMed] [Google Scholar]
- 28. Han B, et al. (2003). Tight control of platelet-derived growth factor B/c-sis expression by interplay between the 5′-untranslated region sequence and the major upstream promoter. J. Biol. Chem, 278, 46983–46993 [DOI] [PubMed] [Google Scholar]
- 29. Han B, et al. (2002). Regulation of gene expression by internal ribosome entry sites or cryptic promoters: the eIF4G story. Mol. Cell. Biol, 22, 7372–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Z, et al. (2005). Regulation of expression by promoters versus internal ribosome entry site in the 5′-untranslated sequence of the human cyclin-dependent kinase inhibitor p27kip1. Nucleic Acids Res, 33, 3763–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong Z, et al. (2005). Regulation of ribonucleotide reductase M2 expression by the upstream AUGs. Nucleic Acids Res, 33, 2715–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yin J.Y, et al. (2009). Characterization and analyses of multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphisms in Chinese population. Pharmacogenet. Genomics, 19, 206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu J.S, et al. (2006). DNA damage-induced RPA focalization is independent of gamma-H2AX and RPA hyper-phosphorylation. J. Cell. Biochem, 99, 1452–1462 [DOI] [PubMed] [Google Scholar]
- 34. Buratti E, et al. (1998). Functional analysis of the interaction between HCV 5′UTR and putative subunits of eukaryotic translation initiation factor eIF3. Nucleic Acids Res, 26, 3179–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baugh J.M, et al. (2004). 20S proteasome differentially alters translation of different mRNAs via the cleavage of eIF4F and eIF3. Mol. Cell, 16, 575–586 [DOI] [PubMed] [Google Scholar]
- 36. Pelletier J, et al. (1988). Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature, 334, 320–325 [DOI] [PubMed] [Google Scholar]
- 37. Kozak M. (2001). New ways of initiating translation in eukaryotes? Mol. Cell. Biol, 21, 1899–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Eden M.E, et al. (2004). Demonstrating internal ribosome entry sites in eukaryotic mRNAs using stringent RNA test procedures. RNA, 10, 720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin X, et al. (2004). Preferential translation of internal ribosome entry site-containing mRNAs during the mitotic cycle in mammalian cells. J. Biol. Chem, 279, 13721–13728 [DOI] [PubMed] [Google Scholar]
- 40. Bushell M, et al. (2006). Polypyrimidine tract binding protein regulates IRES-mediated gene expression during apoptosis. Mol. Cell, 23, 401–412 [DOI] [PubMed] [Google Scholar]
- 41. Komar A.A, et al. (2011). Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle, 10, 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johannes G, et al. (1999). Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc. Natl Acad. Sci. U.S.A, 96, 13118–13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shuck S.C, et al. (2008). Eukaryotic nucleotide excision repair: from understanding mechanisms to influencing biology. Cell Res, 18, 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang T.C, et al. (2004). UNR, a new partner of poly(A)-binding protein, plays a key role in translationally coupled mRNA turnover mediated by the c-fos major coding-region determinant. Genes Dev, 18, 2010–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boussadia O, et al. (2003). Unr is required in vivo for efficient initiation of translation from the internal ribosome entry sites of both rhinovirus and poliovirus. J. Virol, 77, 3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hunt S.L, et al. (1999). unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev, 13, 437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tinton S.A, et al. (2005). Regulation of the cell-cycle-dependent internal ribosome entry site of the PITSLRE protein kinase: roles of Unr (upstream of N-ras) protein and phosphorylated translation initiation factor eIF-2alpha. Biochem. J, 385, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spriggs K.A, et al. (2005). Internal ribosome entry segment-mediated translation during apoptosis: the role of IRES-trans-acting factors. Cell Death Differ, 12, 585–591 [DOI] [PubMed] [Google Scholar]
- 49. Lewis S.M, et al. (2008). For IRES trans-acting factors, it is all about location. Oncogene, 27, 1033–1035 [DOI] [PubMed] [Google Scholar]
- 50. Saletta F, et al. (2010). The translational regulator eIF3a: the tricky eIF3 subunit!. Biochim. Biophys. Acta, 1806, 275–286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.