Abstract
JAK-STAT is an efficient and highly regulated system mainly dedicated to the regulation of gene expression. Primarily identified as functioning in hematopoietic cells, its role has been found critical in all cell types, including neurons. This review will focus on JAK-STAT functions in the mature central nervous system. Our recent research suggests the intriguing possibility of a non-nuclear role of STAT3 during synaptic plasticity. Dysregulation of the JAK-STAT pathway in inflammation, cancer and neurodegenerative diseases positions it at the heart of most brain disorders, highlighting the importance to understand how it can influence the fate and functions of brain cells.
Keywords: synaptic plasticity, long term depression, JAK2, STAT3, inflammation, cancer, NMDA receptor, hormonal regulation
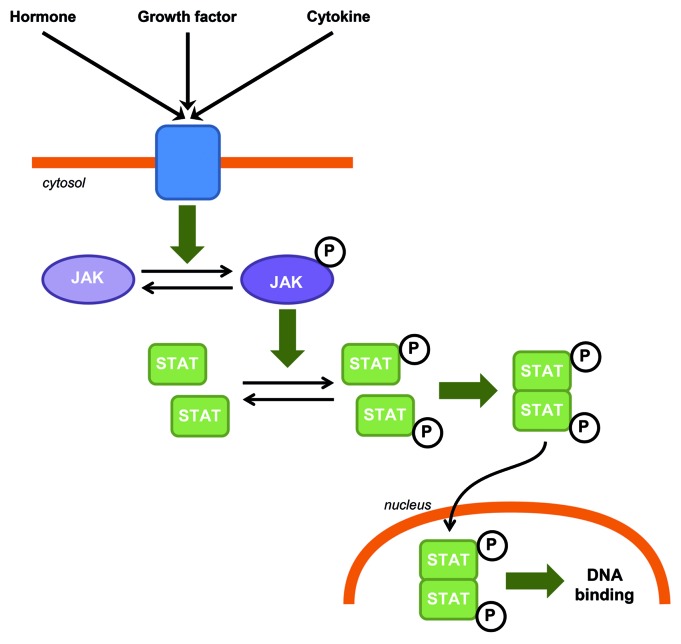
JAK-STAT is a generic name for intracellular signaling pathways involving the activation of two families of proteins discovered and cloned in the early 1990s: the Janus kinase (JAK), which comprise four tyrosine kinases (JAK1, JAK2, JAK3 and TYK2), and the signal transducer and activator of transcription (STAT) containing seven transcription factors (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6). The JAK-STAT pathway is an efficient and highly regulated system mainly dedicated to the regulation of gene expression. This pathway involves the activation of a receptor by polypeptides such as hormones, growth factors or cytokines which leads to the activation of JAK. JAK phosphorylates STATs which then dimerize. The STAT dimer is translocated to the nucleus where it binds to the DNA and regulates transcription (Fig. 1). The control of activity of this pathway involves different mechanisms including regulation of the phosphorylation state of JAK and STAT by phosphatases, or of the JAK kinase activity by SOCS (suppressor of cytokine signaling) for example.
Figure 1. JAK-STAT canonical signaling pathway in CNS. Hormones, growth factors and cytokines can induce JAK phosphorylation and activation. Activated JAKs phosphorylate STATs which in turn homo or heterodimerize. STAT dimers are then translocated to the nucleus where they bind to DNA.
JAK-STAT signaling pathway is evolutionarily conserved in eukaryotes and is involved in cell growth, survival, development and differentiation. Initially described in hematopoietic cells, its role has been found critical in many cell types. Its deregulation can be associated with pathologies such as cancers, immune disorders and cardiovascular diseases. Our review will focus on the functions of JAK-STAT signaling pathways in the mature central nervous system (CNS).
In the CNS, the JAK-STAT signaling pathway is mainly associated with gene regulation during development, hormone release, inflammation or tumorigenesis. Although JAK and STAT expression in the CNS is weaker than in other systems, different studies have nonetheless shown that these proteins could be expressed in several areas of the brain, for example in the cerebral cortex, hippocampus, hypothalamus and cerebellum. The expression of these proteins also varies during development; they are expressed at high levels during embryonic stages (particularly JAK2, JAK1, STAT3, STAT6 and STAT1) and expression gradually decreases during growth and into adulthood.1 We recently discovered the involvement of both JAK2 and STAT3 in hippocampal synaptic plasticity (a phenomenon associated with learning and memory), independently of their ability to regulate gene expression. Together with other recent publications, this observation presented an intriguing question on to their non-transcriptional role.2-4
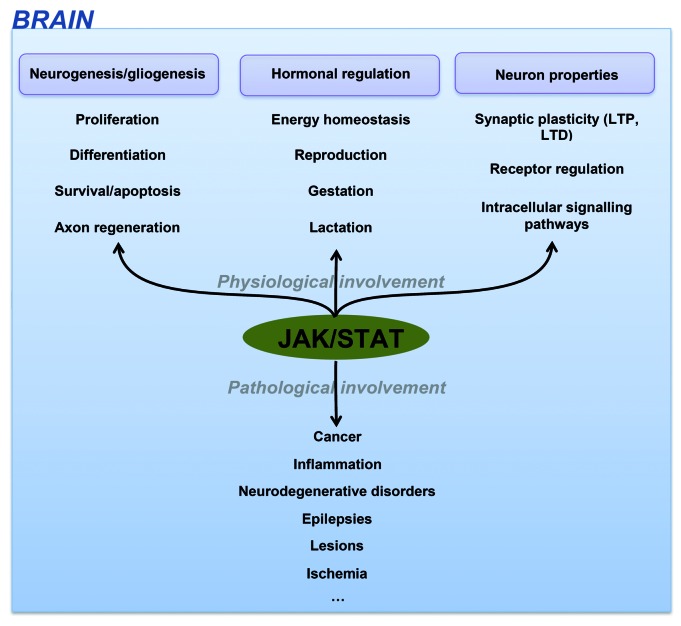
CNS dysregulation of the JAK-STAT pathway is mainly related to brain inflammation processes and neuronal/glial survival. It has consequently been involved in most brain disorders including epilepsies, brain cancer, lesions, ischemia and neurodegenerative disorders like Alzheimer disease (AD) highlighting the importance to decipher how this pathway can influence the fate and functions of brain cells (Fig. 2).
Figure 2. JAK-STAT functions in CNS.
The Role of the JAK-STAT Pathway in Cell Proliferation, Differentiation and Survival in Adult Brain
Proliferation and differentiation
Most brain cells are generated during development from neural stem cells (NSC), or neural progenitor cells (NPC), which can differentiate into neurons, astrocytes or oligodendrocytes. Some populations of NSC can also be found in the adult brain.5 The subventricular zone (SVZ) of the olfactory bulbs and the dentate gyrus (DG) of the hippocampus are the two main neurogenic regions in the adult brain. The newly differentiated cells from these populations can migrate to the olfactory bulb or the granule cell layer of the DG, respectively.6
The proliferation of NSC is regulated by the JAK-STAT pathway. Cytokines like interleukin-15 (IL-15) which is expressed by the adult NSC of the SVZ, induces an activation of STAT1, STAT3 and STAT5 and proliferation is blocked by JAK inhibitors.7,8 Leptin can also regulate neuroproliferation in the DG of adult mice via activation of STAT3 and Akt, both in vitro and in vivo.9 The action of interferon β (IFN-β, the primary treatment for multiple sclerosis), in proliferation and differentiation is controversial in murine NPC.10,11 It can either inhibit,11 have no effect10 or enhance proliferation of NPC12 but in all cases can activate STATs.
One of the first studies to show the role of JAK-STAT in glial differentiation was performed by Bonni et al. in 1997.13 They showed, in embryonic cortical precursor cells, that activation of the ciliary neurotrophic factor (CNTF) receptor leads to the activation of JAK1, STAT1 and STAT3 and triggers the differentiation of these precursor cells into astrocytes. Such differentiation can also be induced by microglia-derived IL-6 and leukemia inhibitory factor (LIF) cytokines.14 Prolactin also allows proliferation and differentiation of astrocytes during development partly via JAK2, STAT1 and STAT3 activation.15 Further studies have then emphasized the role of STAT3 in glial differentiation.14,16,17 One study in particular showed that STAT3 knock-down enhanced embryonic neurogenesis while inhibiting astrogliogenesis in conditional knockout mice.16 This effect on NSC fate could be mediated by the downregulation of genes such as notch1, notch2 and hes5. However, in adult dentate gyrus, neurogenesis is dependent on STAT3 activation,18 a divergence that could be explained by the differences in gene expression profile and signaling pathways observed between embryonic and adult NSCs.19
Neuronal differentiation and neurite outgrowth involve inhibitory proteins of the JAK-STAT pathway such as SOCS2, 3 and 6, which can negatively regulate the signaling pathways induced by, for example, growth hormone (GH) and insulin-like growth factor-1 (IGF-1).20,21 SOCS2 expression is found in NPC and neurons and can be stimulated by LIF receptor signaling. Overexpression of SOCS2 in NSC from SOCS2−/− mice inhibits GH-signaling and increases neuronal differentiation while neurogenesis is impaired in SOCS2−/− mice.21 Interestingly, SOCS6 is overexpressed after JAK2/STAT5 activation but also inhibits this pathway when associated with the IGF-1 receptor leading to a negative feedback loop.20
Concerning the JAK isoforms, it seems that JAK1 is more involved in astrocytic differentiation13 while JAK2, which can be activated by leptin receptor, seems essential for NSC proliferation.9,22 However, modulation of JAK3 signaling is involved in differentiation since knock-down of JAK3 in NPC induces differentiation in neurons and oligodendrocytes.22
Survival/apoptosis and regeneration
Although the JAK-STAT pathway is usually linked to cell proliferation and pathologies such as cancers (see Yu et al.23 for a review), STAT activation could also lead to apoptosis. For example, IFN-β treatment of human SH-SY5Y neuroblastoma cells, which transiently activates STAT1 and STAT3, increases apoptosis via cytochrome C release and caspase activation.24 Similarly IFN-γ, which also activates STAT1, reduces proliferation and induces apoptosis by upregulating p21 and caspase-3 signaling in NPCs.25 On the other hand, IL-9, which also activates the JAK-STAT pathway, protects neonatal neurons (where IL-9R is predominantly expressed) from apoptosis. STAT1 and STAT3 are activated after IL-9 treatment in vitro and AG490, an inhibitor of the JAK-STAT pathway can prevent this anti-apoptotic effect in vivo.26
Differences in the model used for these studies and the duration of treatment may partly explain the different data obtained. A quick, non-transcriptional function of STATs should also be distinguished from their more prolonged effect on transcription. STAT activation can lead to a rapid caspase activation but also to the transcription of non-apoptotic proteins.27 The ratio of STAT1 over STAT3 or STAT5 activation also seems to play a role in apoptosis, with STAT3 and STAT5 being more anti-apoptotic than STAT1.27 Furthermore, the other pathways that are activated or inhibited by these different cytokine treatments and may interfere with the JAK-STAT pathway also have to be taken into account. For example, the downregulation of the PI3K/Akt pathway and downstream upregulation of GSK3β which can accompany the JAK-STAT activation can also trigger cell apoptosis.24 Leptin, however, has a neuroprotective effect via activation of the JAK-STAT pathway but also the PI3K/Akt and ERK pathways.28
Following CNS insults, the JAK-STAT pathway is directly involved in neuronal regeneration and glia scar formation around the lesion. After an axon injury, STAT3 is overexpressed and activated specifically in regenerating neurons.29 In adult mouse, deletion of SOCS3 promotes axon regeneration after optical nerve injury.30 Such a deletion would promote gp130-mediated signaling pathway (including JAK-STAT) as the one mediated by CNTF. Astrogliosis after CNS injury is dependent on STAT3 activation, a necessary step for glia scar formation and reduction of the propagation of inflammation.31
The Role of the JAK-STAT Pathway in Hormonal Regulation
In endocrine cells, including neurons, the JAK-STAT pathway is involved in the control of hormone and peptide release from CNS structures such as the hypothalamus. It influences the regulation of different processes like energy homeostasis and reproduction in the CNS. Indeed, mice with a neural-specific disruption of STAT3 or STAT5 have neuroendocrine defects leading to obesity, diabetes and infertility.32,33
Regulation of energy homeostasis
Leptin is known as a satiety hormone, synthesized by adipocytes and regulating development, growth, metabolism and reproduction. It acts mainly on the hypothalamus to regulate food intake, energy expenditure and reproduction. When leptin binds to the b isoform of the leptin receptor (LepRb or ObRb), JAK2 is activated and leads to the activation of STAT3, STAT5 and SHP2 (see Villanueva et al.34 for a review). In the hypothalamic arcuate nucleus (ARC), activation of the LepR results in an increase of anorexygenic peptide synthesis and a decrease of orexygenic peptide synthesis.35 A similar effect can be observed when IL-7 is injected in an animal model of obesity, therefore preventing obesity. STAT3 (but not STAT5) and c-fos were activated in the ventromedial part of the ARC after injection of IL-7 in this model and an increase of anorexygenic peptide production was observed.36
Activation of LepRb also leads to an increase in SOCS protein expression. This is mediated by STAT3, and/or activation of tyrosine phosphatases such as SHP2 or PTP1B and results in attenuation of this pathway and a desensitization of the LepRb, which can be responsible for obesity.34,37 A similar desensitizing effect can be observed with the Angiotensin II (AngII) receptor AT1. AngII has a dypsogenic effect via activation of the AT1 receptor in the hypothalamus. Repeated intra-cerebro-ventricular injection of AngII leads to a desensitization and a loss of the dypsogenic effect. Activation of the AT1R and JAK-STAT as a downstream signaling pathway would indeed lead to an increase in expression of SOCS3.38
Regulation of reproduction, gestation and lactation
JAK2, STAT3 and STAT5 are expressed in gonadotropin-releasing hormone (GnRH) neurons and mediate the signaling of cytokines such as LIF or CNTF.39 GnRH neurons are present in the hypothalamus and secretion of GnRH regulates the expression of Luteinizing hormone (LH) and follicule-stimulating hormone (FSH) by the anterior pituitary. These hormones regulate gonad function and reproductive behavior. In GnRH neuron-specific conditional JAK2 KO mice, the level of GnRH secreted was reduced and, as a consequence, reproductive development and fertility were impaired in female mice, suggesting an important role for JAK2 in the control of GnRH production.40
The secretion of prolactin by the anterior pituitary is negatively regulated, in part, by hypothalamic neuroendocrine dopaminergic neurons, which are in turn regulated by prolactin as a feed-back to modulate the secretion of dopamine. It has been shown that prolactin-induced regulation of these neurons requires STAT5B activation.41 During late pregnancy and lactation, a decrease in STAT5B activation and an overexpression of SOCS can be observed in these neurons, contributing to the increase in prolactin synthesis at these stages.42
During pregnancy both prolactin and leptin secretion are increased. These hormones promote the synthesis of orexygenic and anorexygenic peptides, respectively. However, desensitization of leptin function is also observed, leading overall to an increase of food intake.43 As seen above, both leptin and prolactin can lead to overexpression of SOCS. Interestingly, the desensitization of the leptin effect seen during pregnancy does not seem to come from the overexpression of SOCS mediated by the prolactin receptor. First these receptors are not expressed in the same type of cells in ARC43 and second, when both receptors are expressed in the same cells, the pathways mediated by their respective activation do not inhibit each other.44 Instead, the desensitization of leptin could partly come from a downstream loss of responsiveness to α-melanocyte-stimulating hormone (α-MSH) in targeted neurons such as oxytocin neurons, which are also negatively regulated by prolactin.43,45
The Role of the JAK-STAT Pathway in Synaptic Plasticity and Modulation of Receptor Function
Many upstream regulators of the JAK-STAT pathway (cytokines, hormones and growth factors) have been shown to modulate and/or alter synaptic activity.46 The ability of neurons to modify the strength of their synaptic transmission is called synaptic plasticity. Two major forms of long-lasting synaptic plasticity have been characterized in the mammalian brain—long-term potentiation (LTP) and long-term depression (LTD)—which are, by definition, a long-lasting increase or decrease of the synaptic activity, respectively. Some data suggest a role for JAK-STAT in the modulation of receptors involved in this process such as the 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA), N-methyl-D-aspartate (NMDA) or muscarinic receptors in the hippocampus and a role of this pathway in learning and memory.
Regulation of synaptic receptors and plasticity by cytokines/hormones
AMPA and NMDA receptor-mediated EPSPs (excitatory post-synaptic potentials) are increased following application of GH to hippocampal slices. This GH-induced potentiation and a tetanus-induced LTP are mutually occluded, suggesting a common signaling mechanism. Furthermore, the GH effect is reduced by AG490, a JAK2 inhibitor.47 CPEB-1 (cytoplasmic polyadenylation element binding protein 1) also plays a role in synaptic plasticity by controlling GH transcription.48 CPEB-1 KO mice have impaired synaptic plasticity, decreased GH expression in the hippocampus compared with WT and a reduction of the activated form of JAK2 and STAT3. GH application induced an enhancement of synaptic transmission in the CA1 area of the hippocampus which was smaller in slices from KO compared with WT animals and the phosphorylation of JAK2 was detectable only in WT samples. Furthermore, a theta-burst stimulation able to induce LTP in slices from WT animals failed to do so in these KOs.47
On the other hand, treatment of rat hippocampal slices with IL-6 reduced LTP in CA1 pyramidal cells, compared with vehicle treated slices. This effect was blocked by application of a tyrosine kinase inhibitor (lavendustin A).49 Moreover, following the IL-6 mediated reduction of LTP, STAT3 phosphorylation levels were increased while there was an inhibition of MAPK/ERK phosphorylation and both effects were reduced when using lavendustin A. Another study also showed that IL-6 could increase the calcium influx through NMDA receptors and that this effect was mediated by JAK-STAT although the mechanism remains unclear.50
Brain derived neurotrophic factor (BDNF) can have a longer lasting effect on synaptic receptors by indirectly modifying the expression of gamma-amino-butyric-acid receptors (GABARs). An increase in CREB phosphorylation and inducible cAMP early repressor (ICER) expression in the DG mediates the repression of Gabra1 (rat gene for GABAAR α1 subunit) transcription following status epilepticus in vivo.51 BDNF (whose expression increases after seizures) increases ICER via the JAK-STAT pathway in vitro and inhibiting this pathway before status epilepticus induction prevents the decrease in Gabra1 transcription.
One study investigated a potential relationship between aging and LTP,52 focusing on the imbalance between a pro-inflammatory (IL-1β) and an anti-inflammatory cytokine (IL-4, known to use the JAK-STAT signaling pathway). In vivo electrophysiological recordings were made in aged animals at synapses between the perforant path and DG in the rat hippocampus. LTP was routinely observed following tetanic stimulation in all young rats. However it was only present in some of the aged rats. IL-1β levels were significantly elevated in aged rats and especially so in those where LTP could not be induced. Conversely, IL-4 was decreased in aged rats which failed to sustain LTP, and so was the phosphorylation of JAK1 and STAT6.
Involvement of JAK-STAT in NMDA receptor-dependent synaptic plasticity
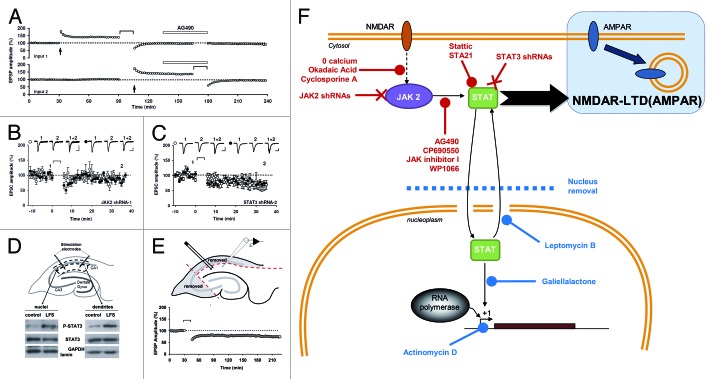
All these data and the fact that AG490 can impair spatial memory53 suggested that JAK-STAT can play a role in synaptic plasticity. However, the requirement of this pathway for NMDA receptor (NMDAR)-dependent plasticity, one of the most common forms of synaptic plasticity in the brain, had until recently not been investigated. We have recently found that, in rat hippocampal slices, induction of NMDAR-LTD, but not LTP or depotentiation of AMPA receptor (AMPAR)-mediated synaptic transmission, can be selectively blocked by different JAK inhibitors.2,4 Knockdown of the JAK2 isoform, which is expressed at synapses, in organotypic slices also abolished NMDAR-LTD. The protocol used to induce NMDAR-LTD caused a transient increase in phosphorylated JAK2 (tyr1007–1008) and this was dependent on NMDAR activation as well as calcium entry, two key features of this form of LTD at the CA1 synapses of pyramidal neurons. The requirement for JAK2 activation is probably specific to NMDAR-LTD since it was not involved in either carbachol- or DHPG-induced LTD, agonists of muscarinic acetylcholine receptors (mAChR) and metabotropic glutamate receptors (group I), respectively. We also found an increase in STAT3 activation after NMDAR-LTD and, indeed, inhibiting STAT3 or knocking it down blocked NMDAR-LTD. Although we found that STAT3 translocates to the nucleus after LTD induction, we also found that its action within the nucleus was not required for the induction process. Different inhibitors of nuclear transport and inhibitors of STAT3 binding to DNA were all unable to block LTD. In addition, we performed extracellular recording in the dendritic area on slices where the CA1 cell bodies have been removed (and so the nuclei of all CA1 pyramidal neurons were absent) and could still induce NMDAR-LTD. Thus, the JAK-STAT pathway is involved in LTD and cytoplasmic activity of STAT3 plays a major role in synaptic plasticity (Fig. 3).
Figure 3. A non-nuclear role for JAK-STAT in synaptic plasticity (adapted from Nicolas et al.2). (A) Pooled data of field recordings showing that a JAK inhibitor (AG490, 10 µM) can block the induction (but not the maintenance) of NMDAR-LTD. (B–C) Pooled data and representative currents of patch-clamp recordings (CA1 cells) in organotypic slices transfected with a JAK2 shRNA (B) and a STAT3 shRNA (C) showing that no LTD can be induced. (D) After stimulation, the area surrounding the stimulating electrodes (dendrites) and the CA1 cells bodies were microdissected. The nuclei were isolated from the cell bodies by centrifugation. The blots of P-STAT3 and STAT3 show that the phosphorylation of STAT3 was increased in both compartments after LTD. (E) Pooled data of field-recording performed on slices where the cell bodies have been removed, showing that the nuclei are not required for the induction of NMDAR-LTD. (F) Schematic summary of the data: Activation of NMDARs during synaptic stimulation leads to JAK2 phosphorylation and activation via a pathway involving Ca2+, protein phosphatase 2B (PP2B, inhibited by cyclosporine A) and Protein phosphatase 1 (PP1, inhibited by okadaic acid). JAK2 activates STAT3 which then translocates to the nucleus, but only the cytoplasmic actions of STAT3 are required for NMDAR-LTD. The 11 treatments in red are all able to inhibit LTD, whereas the four treatments in blue do not.
Involvement of JAK-STAT in the modulation of receptor signaling
Humanin is a short peptide known to abolish amyloid-β (Aβ) toxicity and its derivative colivelin (CLN) acts via the JAK-STAT pathway. CLN can increase ERK phosphorylation induced by carbachol, a mAChR agonist, and this effect is mediated by JAK2 and STAT3. Indeed, intra-cerebro-ventricular injection of AG490, which induces spatial working memory impairment, reduced the number of ACh-producing enzyme (ChAT) positive neurons. It also prevented the CLN-induced increase in ERK phosphorylation induced by carbachol, therefore desensitizing the mAChRs. Expression of a dominant negative STAT3 also prevented the CLN effect on ERK phosphorylation.53
The serotonin receptor 5HT2A can activate phospholipase C (PLC) in rat frontal cortex. Olanzapine, an atypical antipsychotic drug, can desensitize the 5HT2AR-stimulated PLC. This mechanism is mediated by the JAK-STAT pathway in the frontal cortex.54 In vivo olanzapine injection induced desensitization of this receptor in the rat hypothalamic paraventricular nucleus (HPN) as well as in the frontal cortex but only in the latter did olanzapine increase the level of phospho-JAK2. Consistently, AG490 could only prevent the desensitization of 5HT2AR-stimulated PLC in the frontal cortex but not the 5HT2AR-stimulated-hormone release in the HPN.
JAK-STAT Signaling Pathway in Brain Pathologies
Brain tumors
WHO (World Health Organization) classifies brain tumors using histological criteria and grades. The histological criteria are used to predict the biological behavior of the neoplasm whereas the grades (I to IV) determine the malignancy scale of the tumor, IV being the most aggressive and lethal.55 Glioma are the most common CNS tumors observed in humans and up to 70% of all glioma are classified as grade III and IV. Up to 20% of children with primary brain tumors present medulloblastoma (the main form of neuroepithelial embryonic tumors).56,57 Both grade III/IV glioma and medulloblastoma are associated with alteration of the JAK-STAT pathway leading to STAT aberrant activation.58-60 The main STAT isoform deregulated is the pro-oncogenic STAT3.
STAT3 is not activated in healthy brains under basal conditions, whereas in human brain tumors, high levels of STAT3 tyrosine 705 and STAT3 serine 727 phosphorylation are detected,61 demonstrating a constitutive and maximal activation of this enzyme. STAT3 targets genes that promote cell cycle and inhibit apoptosis, two key mechanisms underlying tumorigenesis. Tumor recognition by the immune system is also blocked by STAT3 over-activation due to increased secretion of factors that inhibit the anti-neoplastic activity of microglia and macrophages.62-66
Over-activation of STAT3 is probably mediated by several mechanisms. Many signaling pathways have been shown to be involved in progression of brain tumors and most of them converge onto STAT3, such as epidermal growth factor (EGF), LIF, interferons and other interleukins or erythropoietin receptor (EPOR).67 Positive feedback loops are probably a key mechanism as STAT3 overexpression leads to increased secretion of these factors or expression of their receptors. Decreased activity of the endogenous inhibitors of STAT3 such as protein inhibitors of activated STAT (PIAS)68 and SOCS69 is also observed in parallel.
Inhibition of aberrant STAT3 activation in human brain cancer models by genetic manipulation, pharmacology or molecular biology techniques results in a regulation of tumor proliferation and an increase of apoptosis, leading to a stabilization or regression of the tumor.61,70-77 Inhibition of STAT3 could also play a central role in sensitizing resistant glioma to radiotherapies75 and chemotherapies.60,70
Few data are available regarding the involvement of other STAT isoforms in brain cancer. Recent studies involved STAT678 and STAT5B79 in glioblastoma proliferation and invasion. Knocking down these STATs reduces cell line proliferation and inhibits in vitro migration/invasion. The role of STAT1 in brain tumor growth is more controversial, because even if it is commonly associated with tumor suppressor activity,80,81 it could also have a pro-oncogenic effect in glioma.82
The role of JAK is less well documented. Inhibition of JAK2 activity with AG490,83,84 cucurbitacin-I (JSI-124),60 WP1066,85 inhibitor 2,86 sorafenib,87 curcumin88 or WP119389 downregulates STAT3 activity, inhibits the proliferation and migratory/invasive behavior of glioma cell lines and stimulates their progression toward apoptosis. Drugs like cucurbitacin-I sensitize malignant glioma to chemotherapy, but the exact role of JAK in this process is not clearly characterized (cucurbitacin-I inhibits both JAK2 and STAT3).60
CNS inflammation
Inflammation in the CNS occurs mainly through the activation of microglia and astrocytes and the release of numerous factors like cytokines, chemokines or growth factors, leading to a complex crosstalk between different brain cell types (microglia, astrocytes, neurons, endothelial cells, etc.). The inflammatory locus is then characterized by an increase of the inflammation associated gene expression. CNS inflammation is necessary to protect the brain against insults but can also be an intrinsic component or worsen many diseases or conditions that affect the brain, including cerebral lesions, epilepsies, brain cancer, ischemia and neurodegenerative diseases like Alzheimer disease (AD).90-92
An important component of the factors released during inflammation is activators of the JAK-STAT signaling pathway. In particular, anti-inflammatory cytokines like IL-10 activate STAT3 whereas pro-inflammatory cytokines, such as IFN-γ, induces STAT1 phosphorylation via JAK2.81 Oxidative stress and some cytokines (e.g., IL-6) activate both STAT1 and STAT3 by a JAK2-dependent mechanism.92 The main consequence of the activation of this pathway is to promote inflammation-associated gene expression but also to regulate survival-associated gene expression. Depending on the STAT isoform activated it is possible to have different effects on inflammation, survival, proliferation or differentiation. The role of STAT3 during brain inflammation is still controversial. It can either promote cell death and contribute to brain damage or be implicated in neuronal survival.93 The role of STAT1 is more consensual as it promotes cell death.
Neurodegenerative diseases
Neurodegenerative diseases include a large variety of diseases including AD, leukodystrophies and multiple sclerosis and involving neuron and/or glia degeneration. JAK2/STAT3 activation has been shown to protect neurons and alteration of this pathway could be one of the mechanisms involved in neurodegenerative diseases like AD independently of any inflammation process. Aβ, which is believed to play a key role in this pathology, is neurotoxic under certain conditions. Nicotinic Acetylcholine receptors can reduce Aβ neurotoxicity by activating JAK2/STAT3, but whether the neuroprotection requires STAT3 gene regulation is not known (see Buckingham et al.94 for a detailed review). Humanin and its derivatives abolish Aβ neurotoxicity by activating the JAK2/STAT3 pathway and maintain cholinergic activity.95
Within the CNS, myelin formation is supported by oligodendrocytes. Death of these cells and the resulting demyelination is one of the main features of leukodystrophies and multiple sclerosis, two of the main white matter disorders.96 STAT1 and/or STAT3 activation by cytokines97,98 or STAT5 by glucocorticoid receptor99 promote the survival of oligodendrocytes, an effect counteracted by SOCS3.98 Pharmacological modulation of SOCS and STAT activity could provide exciting potential therapeutic strategies for the treatment of demyelinating diseases.
Conclusion
The JAK-STAT pathway, by its strong links to cell proliferation, differentiation, survival and to inflammation, is one of the most important signaling pathways involved in the regulation of neural function. Its dysregulation in brain pathologies has been clearly demonstrated both in human and animal models, highlighting its great therapeutic potential. However, extensive research is required to understand how the JAK-STAT pathway exerts its effect in the brain. Indeed, while activation of the different JAK and STAT isoforms in the CNS has been until now mainly associated to gene regulation, emerging studies unraveled mechanisms of action independent of any nuclear effect in neurons.
Acknowledgments
This work was supported by grants from the MRC and BBSRC, Inserm, Université Paris Diderot, PremUP, Fondation Roger de Spoelberch, Fondation Grace de Monaco and Leducq Foundation. G.L.C. is WCU International Scholars and supported by WCU program through the KOSEF funded by the MEST (R31–10089). P.D. is supported by Université Denis Diderot-Paris 7, Assistance publique-Hôpitaux de Paris (AP-HP) (Contrat d’Interface), Association pour la Recherche sur le Cancer (ARC) and Fédération pour la Recherche sur le Cerveau (FRC).
Glossary
Abbreviations:
- 5HT
serotonin
- Aβ
amyloid beta peptide
- ACTH
adrenocorticotropic hormone
- AD
Alzheimer disease
- Akt
proto-oncogene proteins c-akt/protein kinase B
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- Ang
angiotensin
- ARC
arcuate nucleus
- AT1R
angiotensin receptor 1
- BDNF
brain derived neurotrophic factor
- ChAT
acetyl choline-producing enzyme
- CLN
colivelin
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- CPEB-1
cytoplasmic polyadenylation element binding protein 1
- CREB
CRE binding protein
- DG
dentate gyrus
- DHPG
dihydroxyphenylglycine
- EPO
erythropoietin
- EPSP
excitatory post-synaptic potential
- ERK
mitogen-activated protein kinase
- FSH
follicule-stimulating hormone
- GABA
gamma-amino-butyric-acid
- GH
growth hormone
- GnRH
gonadotropin-releasing hormone
- gp130
glycoprotein 130
- GSK3
glycogen synthase kinase 3
- HPN
hypothalamic paraventricular nucleus
- ICER
inducible cAMP early repressor
- IFN
interferon
- IGF-1
insulin-like growth factor-1
- IL
interleukin
- JAK
Janus kinase
- LepR
leptin receptor
- LH
luteinizing hormone
- LIF
leukemia inhibitory factor
- LTD
long term depression
- LTP
long term potentiation
- mAChR
muscarinic acetylcholine receptors
- MSH
melanocyte stimulating hormone
- NMDA
N-methyl-D-aspartate
- NPC
neural progenitor cells
- NSC
neural stem cells
- PC1
prohormone convertase 1
- PI3K
phosphatidylinositol 3-kinase
- PIAS
protein inhibitors of activated STAT
- PLC
phospholipase C
- POMC
pro-opiomelanocortin
- PTP1B
protein tyrosine phosphatase 1B
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription
- SVZ
subventricular zone
- WHO
World Health Organization
- WT
wild type
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/22925
References
- 1.De-Fraja C, Conti L, Magrassi L, Govoni S, Cattaneo E. Members of the JAK/STAT proteins are expressed and regulated during development in the mammalian forebrain. J Neurosci Res. 1998;54:320–30. doi: 10.1002/(SICI)1097-4547(19981101)54:3<320::AID-JNR3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Nicolas CS, Peineau S, Amici M, Csaba Z, Fafouri A, Javalet C, et al. The Jak/STAT pathway is involved in synaptic plasticity. Neuron. 2012;73:374–90. doi: 10.1016/j.neuron.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V, et al. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol. 2006;172:245–57. doi: 10.1083/jcb.200503021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann HD, Kirsch M. JAK2-STAT3 signaling: A novel function and a novel mechanism. JAK-STAT. 2012;1:191–3. doi: 10.4161/jkst.20446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–8. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 6.Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer S. Cytokine control of adult neural stem cells. Ann N Y Acad Sci. 2009;1153:48–56. doi: 10.1111/j.1749-6632.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 8.Gómez-Nicola D, Valle-Argos B, Pallas-Bazarra N, Nieto-Sampedro M. Interleukin-15 regulates proliferation and self-renewal of adult neural stem cells. Mol Biol Cell. 2011;22:1960–70. doi: 10.1091/mbc.E11-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–47. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch M, Knight J, Tobita M, Soltys J, Panitch H, Mao-Draayer Y. The effect of interferon-beta on mouse neural progenitor cell survival and differentiation. Biochem Biophys Res Commun. 2009;388:181–6. doi: 10.1016/j.bbrc.2009.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lum M, Croze E, Wagner C, McLenachan S, Mitrovic B, Turnley AM. Inhibition of neurosphere proliferation by IFNgamma but not IFNbeta is coupled to neuronal differentiation. J Neuroimmunol. 2009;206:32–8. doi: 10.1016/j.jneuroim.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Arscott WT, Soltys J, Knight J, Mao-Draayer Y. Interferon β-1b directly modulates human neural stem/progenitor cell fate. Brain Res. 2011;1413:1–8. doi: 10.1016/j.brainres.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–83. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–58. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- 15.Mangoura D, Pelletiere C, Leung S, Sakellaridis N, Wang DX. Prolactin concurrently activates src-PLD and JAK/Stat signaling pathways to induce proliferation while promoting differentiation in embryonic astrocytes. Int J Dev Neurosci. 2000;18:693–704. doi: 10.1016/S0736-5748(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 16.Cao F, Hata R, Zhu P, Nakashiro K, Sakanaka M. Conditional deletion of Stat3 promotes neurogenesis and inhibits astrogliogenesis in neural stem cells. Biochem Biophys Res Commun. 2010;394:843–7. doi: 10.1016/j.bbrc.2010.03.092. [DOI] [PubMed] [Google Scholar]
- 17.He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, et al. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci. 2005;8:616–25. doi: 10.1038/nn1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller S, Chakrapani BP, Schwegler H, Hofmann HD, Kirsch M. Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells. 2009;27:431–41. doi: 10.1634/stemcells.2008-0234. [DOI] [PubMed] [Google Scholar]
- 19.Marei HE, Ahmed AE, Michetti F, Pescatori M, Pallini R, Casalbore P, et al. Gene expression profile of adult human olfactory bulb and embryonic neural stem cell suggests distinct signaling pathways and epigenetic control. PLoS One. 2012;7:e33542. doi: 10.1371/journal.pone.0033542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Mishra K, Surolia A, Banerjee K. Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition. PLoS One. 2011;6:e26674. doi: 10.1371/journal.pone.0026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnley AM, Faux CH, Rietze RL, Coonan JR, Bartlett PF. Suppressor of cytokine signaling 2 regulates neuronal differentiation by inhibiting growth hormone signaling. Nat Neurosci. 2002;5:1155–62. doi: 10.1038/nn954. [DOI] [PubMed] [Google Scholar]
- 22.Kim YH, Chung JI, Woo HG, Jung YS, Lee SH, Moon CH, et al. Differential regulation of proliferation and differentiation in neural precursor cells by the Jak pathway. Stem Cells. 2010;28:1816–28. doi: 10.1002/stem.511. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedoni S, Olianas MC, Onali P. Interferon-β induces apoptosis in human SH-SY5Y neuroblastoma cells through activation of JAK-STAT signaling and down-regulation of PI3K/Akt pathway. J Neurochem. 2010;115:1421–33. doi: 10.1111/j.1471-4159.2010.07046.x. [DOI] [PubMed] [Google Scholar]
- 25.Mäkelä J, Koivuniemi R, Korhonen L, Lindholm D. Interferon-gamma produced by microglia and the neuropeptide PACAP have opposite effects on the viability of neural progenitor cells. PLoS One. 2010;5:e11091. doi: 10.1371/journal.pone.0011091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontaine RH, Cases O, Lelièvre V, Mesplès B, Renauld JC, Loron G, et al. IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell Death Differ. 2008;15:1542–52. doi: 10.1038/cdd.2008.79. [DOI] [PubMed] [Google Scholar]
- 27.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 28.Tang BL. Leptin as a neuroprotective agent. Biochem Biophys Res Commun. 2008;368:181–5. doi: 10.1016/j.bbrc.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 29.Schwaiger FW, Hager G, Schmitt AB, Horvat A, Hager G, Streif R, et al. Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT) Eur J Neurosci. 2000;12:1165–76. doi: 10.1046/j.1460-9568.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–23. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–43. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–6. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, et al. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS One. 2008;3:e1639. doi: 10.1371/journal.pone.0001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villanueva EC, Myers MG., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes (Lond) 2008;32(Suppl 7):S8–12. doi: 10.1038/ijo.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 36.Macia L, Viltart O, Delacre M, Sachot C, Héliot L, Di Santo JP, et al. Interleukin-7, a new cytokine targeting the mouse hypothalamic arcuate nucleus: role in body weight and food intake regulation. PLoS One. 2010;5:e9953. doi: 10.1371/journal.pone.0009953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knobelspies H, Zeidler J, Hekerman P, Bamberg-Lemper S, Becker W. Mechanism of attenuation of leptin signaling under chronic ligand stimulation. BMC Biochem. 2010;11:2. doi: 10.1186/1471-2091-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torsoni MA, Carvalheira JB, Calegari VC, Bezerra RM, Saad MJ, Gontijo JA, et al. Angiotensin II (AngII) induces the expression of suppressor of cytokine signaling (SOCS)-3 in rat hypothalamus - a mechanism for desensitization of AngII signaling. J Endocrinol. 2004;181:117–28. doi: 10.1677/joe.0.1810117. [DOI] [PubMed] [Google Scholar]
- 39.Magni P, Dozio E, Ruscica M, Watanobe H, Cariboni A, Zaninetti R, et al. Leukemia inhibitory factor induces the chemomigration of immortalized gonadotropin-releasing hormone neurons through the independent activation of the Janus kinase/signal transducer and activator of transcription 3, mitogen-activated protein kinase/extracellularly regulated kinase 1/2, and phosphatidylinositol 3-kinase/Akt signaling pathways. Mol Endocrinol. 2007;21:1163–74. doi: 10.1210/me.2006-0270. [DOI] [PubMed] [Google Scholar]
- 40.Wu S, Divall S, Hoffman GE, Le WW, Wagner KU, Wolfe A. Jak2 is necessary for neuroendocrine control of female reproduction. J Neurosci. 2011;31:184–92. doi: 10.1523/JNEUROSCI.2974-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma FY, Anderson GM, Gunn TD, Goffin V, Grattan DR, Bunn SJ. Prolactin specifically activates signal transducer and activator of transcription 5b in neuroendocrine dopaminergic neurons. Endocrinology. 2005;146:5112–9. doi: 10.1210/en.2005-0770. [DOI] [PubMed] [Google Scholar]
- 42.Anderson GM, Beijer P, Bang AS, Fenwick MA, Bunn SJ, Grattan DR. Suppression of prolactin-induced signal transducer and activator of transcription 5b signaling and induction of suppressors of cytokine signaling messenger ribonucleic acid in the hypothalamic arcuate nucleus of the rat during late pregnancy and lactation. Endocrinology. 2006;147:4996–5005. doi: 10.1210/en.2005-0755. [DOI] [PubMed] [Google Scholar]
- 43.Ladyman SR, Augustine RA, Grattan DR. Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol. 2010;22:805–17. doi: 10.1111/j.1365-2826.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- 44.Roy AF, Benomar Y, Bailleux V, Vacher CM, Aubourg A, Gertler A, et al. Lack of cross-desensitization between leptin and prolactin signaling pathways despite the induction of suppressor of cytokine signaling 3 and PTP-1B. J Endocrinol. 2007;195:341–50. doi: 10.1677/JOE-07-0321. [DOI] [PubMed] [Google Scholar]
- 45.Ladyman SR, Fieldwick DM, Grattan DR. Suppression of leptin-induced hypothalamic JAK/STAT signalling and feeding response during pregnancy in the mouse. Reproduction. 2012;144:83–90. doi: 10.1530/REP-12-0112. [DOI] [PubMed] [Google Scholar]
- 46.Moult PR, Harvey J. Hormonal regulation of hippocampal dendritic morphology and synaptic plasticity. Cell Adh Migr. 2008;2:269–75. doi: 10.4161/cam.2.4.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmoud GS, Grover LM. Growth hormone enhances excitatory synaptic transmission in area CA1 of rat hippocampus. J Neurophysiol. 2006;95:2962–74. doi: 10.1152/jn.00947.2005. [DOI] [PubMed] [Google Scholar]
- 48.Zearfoss NR, Alarcon JM, Trifilieff P, Kandel E, Richter JD. A molecular circuit composed of CPEB-1 and c-Jun controls growth hormone-mediated synaptic plasticity in the mouse hippocampus. J Neurosci. 2008;28:8502–9. doi: 10.1523/JNEUROSCI.1756-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tancredi V, D’Antuono M, Cafè C, Giovedì S, Buè MC, D’Arcangelo G, et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–43. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- 50.Orellana DI, Quintanilla RA, Gonzalez-Billault C, Maccioni RB. Role of the JAKs/STATs pathway in the intracellular calcium changes induced by interleukin-6 in hippocampal neurons. Neurotox Res. 2005;8:295–304. doi: 10.1007/BF03033983. [DOI] [PubMed] [Google Scholar]
- 51.Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, et al. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–28. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Chiba T, Yamada M, Aiso S. Targeting the JAK2/STAT3 axis in Alzheimer’s disease. Expert Opin Ther Targets. 2009;13:1155–67. doi: 10.1517/14728220903213426. [DOI] [PubMed] [Google Scholar]
- 54.Singh RK, Jia C, Garcia F, Carrasco GA, Battaglia G, Muma NA. Activation of the JAK-STAT pathway by olanzapine is necessary for desensitization of serotonin2A receptor-stimulated phospholipase C signaling in rat frontal cortex but not serotonin2A receptor-stimulated hormone release. J Psychopharmacol. 2010;24:1079–88. doi: 10.1177/0269881109103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudà R, Trevisan E, Soffietti R. Low-grade gliomas. Handb Clin Neurol. 2012;105:437–50. doi: 10.1016/B978-0-444-53502-3.00001-X. [DOI] [PubMed] [Google Scholar]
- 57.Packer RJ, Macdonald T, Vezina G, Keating R, Santi M. Medulloblastoma and primitive neuroectodermal tumors. Handb Clin Neurol. 2012;105:529–48. doi: 10.1016/B978-0-444-53502-3.00007-0. [DOI] [PubMed] [Google Scholar]
- 58.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–13. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 59.Abou-Ghazal M, Yang DS, Qiao W, Reina-Ortiz C, Wei J, Kong LY, et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008;14:8228–35. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lo HW, Cao X, Zhu H, Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res. 2008;14:6042–54. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu J, Li G, Sun T, Su Y, Zhang X, Shen J, et al. Blockage of the STAT3 signaling pathway with a decoy oligonucleotide suppresses growth of human malignant glioma cells. J Neurooncol. 2008;89:9–17. doi: 10.1007/s11060-008-9590-9. [DOI] [PubMed] [Google Scholar]
- 62.Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–6. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 63.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–84. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57:1458–67. doi: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 65.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9:67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Liu W, Alizadeh D, Zhao D, Farrukh O, Lin J, et al. S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59:486–98. doi: 10.1002/glia.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brantley EC, Nabors LB, Gillespie GY, Choi YH, Palmer CA, Harrison K, et al. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res. 2008;14:4694–704. doi: 10.1158/1078-0432.CCR-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ehrmann J, Strakova N, Vrzalikova K, Hezova R, Kolar Z. Expression of STATs and their inhibitors SOCS and PIAS in brain tumors. In vitro and in vivo study. Neoplasma. 2008;55:482–7. [PubMed] [Google Scholar]
- 70.Akasaki Y, Liu G, Matundan HH, Ng H, Yuan X, Zeng Z, et al. A peroxisome proliferator-activated receptor-gamma agonist, troglitazone, facilitates caspase-8 and -9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1B on human glioma cells. J Biol Chem. 2006;281:6165–74. doi: 10.1074/jbc.M505266200. [DOI] [PubMed] [Google Scholar]
- 71.Kong LY, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14:5759–68. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fuh B, Sobo M, Cen L, Josiah D, Hutzen B, Cisek K, et al. LLL-3 inhibits STAT3 activity, suppresses glioblastoma cell growth and prolongs survival in a mouse glioblastoma model. Br J Cancer. 2009;100:106–12. doi: 10.1038/sj.bjc.6604793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen J, Li R, Li G. Inhibitory effects of decoy-ODN targeting activated STAT3 on human glioma growth in vivo. In Vivo. 2009;23:237–43. [PubMed] [Google Scholar]
- 74.Chen F, Xu Y, Luo Y, Zheng D, Song Y, Yu K, et al. Down-regulation of Stat3 decreases invasion activity and induces apoptosis of human glioma cells. J Mol Neurosci. 2010;40:353–9. doi: 10.1007/s12031-009-9323-3. [DOI] [PubMed] [Google Scholar]
- 75.Gao L, Li F, Dong B, Zhang J, Rao Y, Cong Y, et al. Inhibition of STAT3 and ErbB2 suppresses tumor growth, enhances radiosensitivity, and induces mitochondria-dependent apoptosis in glioma cells. Int J Radiat Oncol Biol Phys. 2010;77:1223–31. doi: 10.1016/j.ijrobp.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 76.Tang C, Xue HL, Huang HB, Wang XG. Tanshinone IIA inhibits constitutive STAT3 activation, suppresses proliferation, and induces apoptosis in rat C6 glioma cells. Neurosci Lett. 2010;470:126–9. doi: 10.1016/j.neulet.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 77.Ball S, Li C, Li PK, Lin J. The small molecule, LLL12, inhibits STAT3 phosphorylation and induces apoptosis in medulloblastoma and glioblastoma cells. PLoS One. 2011;6:e18820. doi: 10.1371/journal.pone.0018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merk BC, Owens JL, Lopes MB, Silva CM, Hussaini IM. STAT6 expression in glioblastoma promotes invasive growth. BMC Cancer. 2011;11:184. doi: 10.1186/1471-2407-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang QC, Xiong H, Zhao ZW, Jia D, Li WX, Qin HZ, et al. Inhibition of transcription factor STAT5b suppresses proliferation, induces G1 cell cycle arrest and reduces tumor cell invasion in human glioblastoma multiforme cells. Cancer Lett. 2009;273:164–71. doi: 10.1016/j.canlet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Adámková L, Soucková K, Kovarík J. Transcription protein STAT1: biology and relation to cancer. Folia Biol (Praha) 2007;53:1–6. [PubMed] [Google Scholar]
- 81.Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol. 2008;19:351–9. doi: 10.1016/j.semcdb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Adach-Kilon A, Swiatek-Machado K, Kaminska B, Dabrowski M. Signal transducer and activator of transcription 1 (Stat1) maintains basal mRNA expression of pro-survival stat3-target genes in glioma C6 cells. J Cell Biochem. 2011;112:3685–94. doi: 10.1002/jcb.23305. [DOI] [PubMed] [Google Scholar]
- 83.Jane EP, Premkumar DR, Pollack IF. AG490 influences UCN-01-induced cytotoxicity in glioma cells in a p53-dependent fashion, correlating with effects on BAX cleavage and BAD phosphorylation. Cancer Lett. 2007;257:36–46. doi: 10.1016/j.canlet.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Senft C, Priester M, Polacin M, Schröder K, Seifert V, Kögel D, et al. Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells. J Neurooncol. 2011;101:393–403. doi: 10.1007/s11060-010-0273-y. [DOI] [PubMed] [Google Scholar]
- 85.Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26:2435–44. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 86.Swiatek-Machado K, Mieczkowski J, Ellert-Miklaszewska A, Swierk P, Fokt I, Szymanski S, et al. Novel small molecular inhibitors disrupt the JAK/STAT3 and FAK signaling pathways and exhibit a potent antitumor activity in glioma cells. Cancer Biol Ther. 2012;13:657–70. doi: 10.4161/cbt.20083. [DOI] [PubMed] [Google Scholar]
- 87.Yang F, Brown C, Buettner R, Hedvat M, Starr R, Scuto A, et al. Sorafenib induces growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3. Mol Cancer Ther. 2010;9:953–62. doi: 10.1158/1535-7163.MCT-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weissenberger J, Priester M, Bernreuther C, Rakel S, Glatzel M, Seifert V, et al. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res. 2010;16:5781–95. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- 89.Sai K, Wang S, Balasubramaniyan V, Conrad C, Lang FF, Aldape K, et al. Induction of cell-cycle arrest and apoptosis in glioblastoma stem-like cells by WP1193, a novel small molecule inhibitor of the JAK2/STAT3 pathway. J Neurooncol. 2012;107:487–501. doi: 10.1007/s11060-011-0786-z. [DOI] [PubMed] [Google Scholar]
- 90.Degos V, Favrais G, Kaindl AM, Peineau S, Guerrot AM, Verney C, et al. Inflammation processes in perinatal brain damage. J Neural Transm. 2010;117:1009–17. doi: 10.1007/s00702-010-0411-x. [DOI] [PubMed] [Google Scholar]
- 91.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.04.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Planas AM, Gorina R, Chamorro A. Signalling pathways mediating inflammatory responses in brain ischaemia. Biochem Soc Trans. 2006;34:1267–70. doi: 10.1042/BST0341267. [DOI] [PubMed] [Google Scholar]
- 93.Tsai MC, Chen WJ, Tsai MS, Ching CH, Chuang JI. Melatonin attenuates brain contusion-induced oxidative insult, inactivation of signal transducers and activators of transcription 1, and upregulation of suppressor of cytokine signaling-3 in rats. J Pineal Res. 2011;51:233–45. doi: 10.1111/j.1600-079X.2011.00885.x. [DOI] [PubMed] [Google Scholar]
- 94.Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signalling: roles in Alzheimer’s disease and amyloid neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiba T, Yamada M, Sasabe J, Terashita K, Shimoda M, Matsuoka M, et al. Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol Psychiatry. 2009;14:206–22. doi: 10.1038/mp.2008.105. [DOI] [PubMed] [Google Scholar]
- 96.Werner HB, Jahn O. Myelin matters: proteomic insights into white matter disorders. Expert Rev Proteomics. 2010;7:159–64. doi: 10.1586/epr.09.105. [DOI] [PubMed] [Google Scholar]
- 97.Valerio A, Ferrario M, Dreano M, Garotta G, Spano P, Pizzi M. Soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro differentiation of purified rat oligodendroglial lineage cells. Mol Cell Neurosci. 2002;21:602–15. doi: 10.1006/mcne.2002.1208. [DOI] [PubMed] [Google Scholar]
- 98.Emery B, Cate HS, Marriott M, Merson T, Binder MD, Snell C, et al. Suppressor of cytokine signaling 3 limits protection of leukemia inhibitory factor receptor signaling against central demyelination. Proc Natl Acad Sci U S A. 2006;103:7859–64. doi: 10.1073/pnas.0602574103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu J, Chen S, Chen H, Xiao Q, Hsu CY, Michael D, et al. STAT5 mediates antiapoptotic effects of methylprednisolone on oligodendrocytes. J Neurosci. 2009;29:2022–6. doi: 10.1523/JNEUROSCI.2621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]