Abstract
Cell communication is well known to rely on direct contacts or on secreted factors that bind to receptors located on the surface of their target cells. In addition to this classical pathway, recent results have shown that cells produce microvesicles that contain functional DNA, RNA and proteins that can be directly transferred to recipient cells. This induces proliferation, differentiation or cell death to the same extent as classical soluble factors. New data obtained from the laboratory of Napoleone Ferrara show that these microvesicles also contain miRNAs that can induce angiogenic activities in neighboring endothelial cells. When secreted from cancer cells, these miRNA-loaded vesicles penetrate recipient cells where they activate the JAK-STAT pathway. This represents a new type of intercellular signaling and a new way of activating the STAT transcription factors that could be of interest for the design of cancer treatments.
Keywords: cell communication, microvesicle, miRNA, JAK kinases, STAT3, cancer, angiogenesis
Cell communication is mediated by direct contact between neighboring cells or by secreted factors. Soluble mediators such as cytokines or growth factors bind to receptors located on the surface of responsive cells to induce intracellular signaling pathways and gene transcription. This extracellular communication dictates the fate of target cells through the regulation of cell survival, proliferation and differentiation. Besides these normal functions, these signaling pathways play an essential role in cell transformation, tumor escape and drug resistance when they are deregulated.1 Consequently, many of these are recognized as essential targets of cancer treatment, assuming that their inactivation induces cell cycle arrest or cell death of addicted cancer cells. This approach has proven its efficiency; for instance, monoclonal antibodies such as trastuzumab, cetuximab or bevacizumab are effective in breast and colorectal cancers through the inactivation of the VEGF and EGFR signaling pathways.2-4 Considerable fundamental and clinical data strongly argue that the inactivation of circulating mediators involved in cancer cell survival is a major goal of cancer treatment. Although essential, this conceptual approach should not assume that soluble growth factors are the only pathway the cells use to communicate.
Recent results have shown that cell communication is also mediated by microvesicles which are produced by the cells to transmit information to their environment.5 These microvesicles induce cell proliferation, differentiation or cell death probably to the same extent as classical soluble factors. Three types of vesicles have been defined, depending on their size and origin. Exosomes (30–100 nm) originate from intracellular endosomes, ectosomes (100 nM–1 µM) also known as shedding vesicles are produced from membrane blebbing and apoptotic bodies (> 1 µM) are released from dying cells. Considerable interest stems from the discovery that these vesicles contain RNA and DNA molecules as well as proteins that are functional when transferred to recipient cells.6,7 Hence, in addition to the classical interaction between soluble factors and membrane receptors, a direct information is transferred to distant cells by circulating vesicles that directly modify the content and behavior of target cells.
The mechanisms by which these vesicles are produced and how they recognize and activate recipient cells are poorly understood. Their biological effect seems to depend on the cell of origin, for instance microvesicles produced from dendritic cells can regulate T cell activation whereas those produced from cancer cells affect tumor growth, angiogenesis and metastasis. This suggests that RNA, DNA or proteins are selectively packaged into microvesicles to allow the transmission of specific signals to target cells. In line with this hypothesis, fascinating results have recently detected elevated levels of Myc RNA and DNA sequences in vesicles isolated from medulloblastoma and in the serum of tumor-bearing mice.8 Medulloblastoma is a common tumor in children which presents a frequent amplification of c-Myc. These observations lead to the essential hypothesis that oncogenic sequences can be transferred by microvesicles and that a transforming activity can be transferred from a tumor cell to a less tranformed cell in the microenvironment. Since Myc is well known to induce genomic instability and the production of micronuclei,9 the transfer of these sequences might be the result of sustained DNA damage and mitotic catastrophy.10 If a transforming activity is effectively transferred by vesicles, it will be important to determine if receptors present on the surface of oncogenic exosomes induce the targeting of specific cells in the microenvironment. This also means that microvesicles, and the pathways that allow their generation, should be considered as new therapeutic targets.
Besides nucleic sequences, microvesicles can also directly transfer functional oncogenes as demonstrated with the VIII mutant form of the EGFR receptor.11 In glioblastoma cells expressing this mutant receptor, exosomes production increased significantly as compared with normal cells. These vesicles contain and transfer the mutated receptor in recipient cells that normally do not express it. This transferred oncogene is functional since its presence induced a significant increase in Erk and Akt phosphorylations in recipient cells. Most importantly, these cells then grow more efficiently in soft agar, indicating that they have acquired enhanced transformation capacities through exosomes uptake (which in this case were named “oncosomes” by the authors). Besides its mutated form, the wild-type EGFR receptor has also been detected in exosomes produced by cancer cell lines. Importantly, when normal endothelial cells were incubated with these EGFR-expressing vesicles, the receptor became detectable on their surface. This transfer then leads to a new and active pathway in recipient cells where VEGF and angiogenic activities are upregulated. Consequently, the authors have proposed that the production of EGFR-expressing oncosomes from tumor cells acts as angiogenic stimuli for neighboring endothelial cells. This relation was already known but expected to rely essentially on the production of VEGF or soluble angiogenic factors.
In addition to DNA sequences and proteins, recent results have shown that miRNAs are also packaged within microvesicles and transferred to recipient cells to modify their behavior. Associated with the Argonaute-2 (AGO-2) protein, they form a stable and protected complex12 within a circulating microvesicle. For instance, miRNAs produced by endothelial cells control the fate of smooth muscle cells.13 In producing cells, the KLF2 transcription factor activates miR-143/145 which are then packaged in microvesicles and transferred to muscle cells where they control gene expression and cell fate. Recent work performed by the laboratory of N. Ferrara has extended this initial observation to the JAK-STAT field.14 Using coculture assays, they have shown that tumor cells induce the upregulation of several miRNAs in endothelial cells. This ability to induce miRNA production was restricted to transformed cells since normal melanocytes or fibroblasts did not modify the target cells. This effect was also not mediated by soluble factors classically secreted by cancer cells such as VEGF, bFGF, TNFa, HGF or PDGF or angiopoietin-1. Surprisingly, Drosha knockdown in cancer cells prevented miRNA induction, indicating that the vast majority of the miRNAs detected in endothelial cells were produced in tumor cells. Accordingly, the authors found that these cells produced oncosomes that are internalized within endothelial cells to deliver the corresponding miRNAs. The authors focused on miR-9 and showed that its secretion promotes endothelial migration and favor tumor angiogenesis in vivo. The implantation of colorectal tumor cells in mice induced an increase in soluble miR-9 levels in the plasma and its inhibition by antagomirs resulted in tumor regression. Interestingly, the authors also found that miR-9 targets the SOCS5 gene in endothelial cells, resulting in the activation of the JAK1/2 kinase and of the STAT1 and STAT3 transcription factors. This result is important since the STAT3 oncogene is well known to play an important role in cell transformation or during chemotherapy escape but it also stimulates angiogenesis as a direct transcriptional activator of the VEGF gene.15-17
To our knowledge, this activation of STAT3 by vesicles-derived miRNAs is rather new since this transcription factor is essentially known to be activated by cytokine or growth factors such as IL-6 or by oncogenic kinases such as the Src or JAK kinases. These results further describe the importance of secreted miRNAs in the communication between cancer cells and their neighboring cells. In addition to well-known growth factors present in the stroma, oncosomes appear as new secreted activators of the JAK-STAT pathway. These results also extend the importance of STAT3 regulation by miRNAs. Important results by the laboratory of Kevin Struhl have described an epigenetic switch that induces a constitutive activation of the STAT3 and NFκB oncogenes in cancer cells.18,19 At the very early step of cell transformation, NFκB activates the expression of several miRNAs that induce the production of IL-6. This results in the activation of an autocrine loop that maintains a constant activation of the STAT3 transcription factor. Since several miRNAs are induced by STAT3 and NFκB, it will be important to determine if some are also secreted and involved in this feedback loop. A cooperative activity of secreted miRNAs and IL-6 could reactivate STAT3 and NFκB, maintain the oncogenic activation within tumor cells and induce a constant angiogenic switch in endothelial cells (Fig. 1). If this hypothesis is true, this means that soluble factors and miRNAs might also cooperate to induce an abnormal activation of STAT3 in neighboring cells. As stated above, it is tempting to speculate that this would modify the tumor microenvironment to allow tumor progression, eventually favor the transformation of less transformed cells or allow tumor escape in response to chemotherapy.15,17,20,21
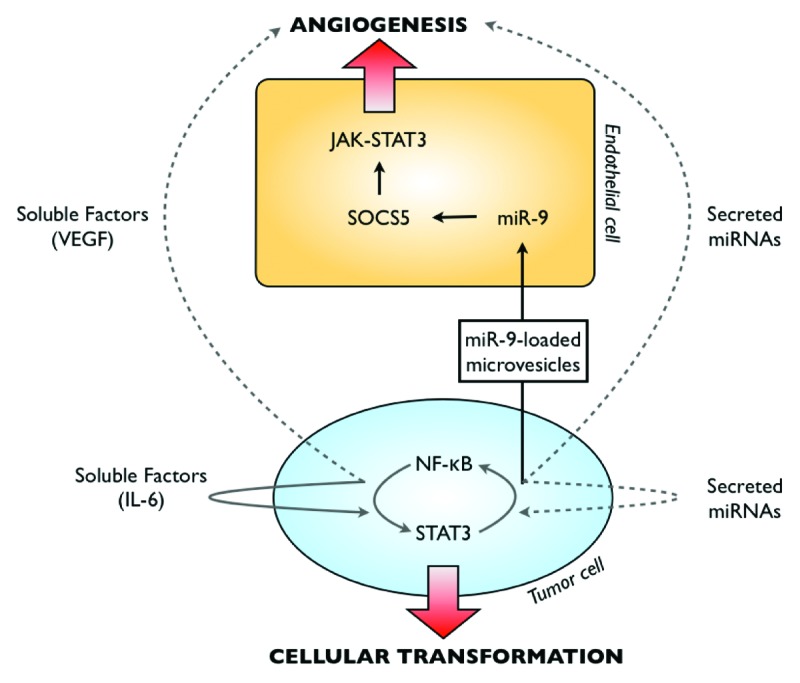
Figure 1. Hypothetical overview of cellular communications originating from cancer cells. Cell communication is mediated by direct contact between neighboring cells or by secreted factors. Growth factors produced by cancer cells are well known to enhance cell survival and abnormal proliferation. These soluble factors modify the microenvironment to favor tumor escape, for instance by the activation of endothelial cells and through the upregulation of angiogenic activities. Besides these secreted factors, it appears that microvesicles that contain miRNAs are also produced by cancer cells, to modify the microenvironment and probably to allow tumor progression. Recent studies have demonstrated the importance of the STAT3-NFκB pathway in the establishment of autocrine survival loop mediated by secreted factors. It will be important to determine if these secreted miRNAs are also involved in the constitutive activation of oncogenic pathways.
From all these results, it appears that microvesicles and miRNAs should be considered as new secreted activators of the STAT3 pathway and more generally as important mediators of the communication between tumor cells and their microenvironment. Besides growth factors and receptors inactivation, this signaling pathway represents new opportunities for innovative cancer treatments.
Acknowledgments
Work in our laboratory is supported by grants from the Ligue Contre le Cancer (comité du Maine et Loire), the Canceropole Grand Ouest, the Region Pays de la Loire and the Agence Nationale de la Recherche.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/22996
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Grothey A, Lenz HJ. Explaining the unexplainable: EGFR antibodies in colorectal cancer. J Clin Oncol. 2012;30:1735–7. doi: 10.1200/JCO.2011.40.4194. [DOI] [PubMed] [Google Scholar]
- 3.Bernards R. It’s diagnostics, stupid. Cell. 2010;141:13–7. doi: 10.1016/j.cell.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–9. doi: 10.1038/nature09339. [DOI] [PubMed] [Google Scholar]
- 5.Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–35. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–99. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22:125–32. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–44. doi: 10.1016/S1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 10.Vigneron A, Roninson IB, Gamelin E, Coqueret O. Src inhibits adriamycin-induced senescence and G2 checkpoint arrest by blocking the induction of p21waf1. Cancer Res. 2005;65:8927–35. doi: 10.1158/0008-5472.CAN-05-0461. [DOI] [PubMed] [Google Scholar]
- 11.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Zhu D, Huang L, Zhang J, Bian Z, Chen X, et al. Argonaute 2 Complexes Selectively Protect the Circulating MicroRNAs in Cell-Secreted Microvesicles. PLoS One. 2012;7:e46957. doi: 10.1371/journal.pone.0046957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–23. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30:1005–14. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barré B, Vigneron A, Perkins N, Roninson IB, Gamelin E, Coqueret O. The STAT3 oncogene as a predictive marker of drug resistance. Trends Mol Med. 2007;13:4–11. doi: 10.1016/j.molmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courapied S, Sellier H, de Carné Trécesson S, Vigneron A, Bernard AC, Gamelin E, et al. The cdk5 kinase regulates the STAT3 transcription factor to prevent DNA damage upon topoisomerase I inhibition. J Biol Chem. 2010;285:26765–78. doi: 10.1074/jbc.M109.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigneron A, Gamelin E, Coqueret O. The EGFR-STAT3 oncogenic pathway up-regulates the Eme1 endonuclease to reduce DNA damage after topoisomerase I inhibition. Cancer Res. 2008;68:815–25. doi: 10.1158/0008-5472.CAN-07-5115. [DOI] [PubMed] [Google Scholar]


