Abstract
Atherosclerosis is characterized by early endothelial dysfunction and altered vascular smooth muscle cells (VSMCs) contractility. The forming atheroma is a site of excessive production of cytokines and inflammatory ligands by various cell types that mediate inflammation and immune responses. Key factors contributing to early stages of plaque development are IFNγ and TLR4. This review provides insight in the differential STAT1-dependent signal integration between IFNγ and TLR4 signals in vascular cells and atheroma interacting immune cells. This results in increased leukocyte attraction and adhesion and VSMC proliferation and migration, which are important characteristics of EC dysfunction and early triggers of atherosclerosis.
Keywords: atherosclerosis, IFN-gamma, Toll-like receptors, STAT1, IRFs, signal integration
Introduction
Atherosclerosis and arteriosclerosis, the morphological correlates of vascular disease, are characterized by early endothelial dysfunction and altered contractility of vascular smooth muscle cells (VSMCs). In 1856 Rudolf Virchow presented a theory that inflammation is the driving force of atherosclerosis. However, scientific proof for this was discovered only 30 years ago.1 Inflammation participates importantly in host defenses against infectious agents and injury, but it also contributes to the pathophysiology of atherosclerosis. Various factors can injure vascular endothelium leading to the release of numerous inflammatory mediators resulting in recruitment of blood leukocytes, which inflict further inflammatory response. Thus, cells of both innate and adaptive immunity modulate the chronic inflammatory process initiating and acting in the atherosclerotic plaque development.2 The change in the milieu prompts vascular smooth muscle cells to undergo de-differentiation characterized by loss of contractility, increased cell motility and proliferation.3 These processes together with buildup of lipids, cholesterol, calcium and cellular debris within the intima of the vessel wall lead to the formation of advanced atherosclerotic plaque, vascular remodeling and acute and chronic luminal obstruction.4
A seminal signal transduction pathway operating at the frontier of innate and adaptive immunity and importantly contributing to inflammation is the JAK-STAT pathway. The involvement of this pathway in atherosclerosis has been long appreciated.5,6 However, until only recently the attention of the researchers was primarily focused on immune cells. As such it has become clear that for instance in macrophages, dendritic cells as well as lymphocytes, JAK-STAT-mediated signal integration exists between triggers of innate and adaptive immunity, which forms a basic aspect of the host defense against pathogens. Work of more recent nature, including that of our group, uncovered the unique role of STAT1 in cross-talk between the pro-inflammatory activators IFNγ and LPS. Moreover, this signal integration takes place not only in immune cells, but also in cells from the vasculature (ECs and VSMCs) and collectively leads to increased inflammation and progression of vascular damage.4,7 In this review we will summarize the molecular mechanisms of this phenomenon and highlight the biological impact of these findings that could potentially lead to discovery of novel pharmaceutical targets and diagnostic and prognostic assay development.
Atherosclerosis, Inflammation and Immunity
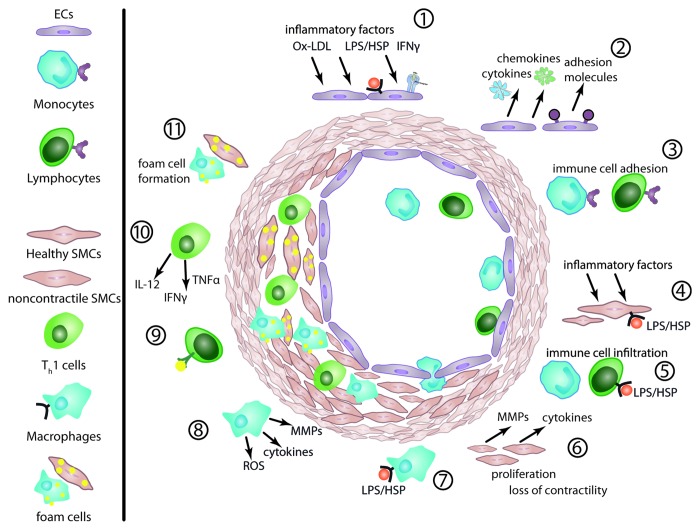
The function of healthy arterial endothelium is to maintain proper blood flow and provide a physical barrier between blood and surrounding tissue. This is achieved by the ability of endothelial cells to inhibit thrombosis, leukocyte adhesion, VSMC proliferation and to regulate vessel tone. The very first incident in the chain of events leading to the formation of atherosclerotic plaques is endothelial cell injury and the resulting endothelial cell dysfunction (Fig. 1).8 Although there is still some debate on this, factors inducing EC dysfunction include: pulsatile blood flow and shear stress,9,10 oxidized LDL particles,11 pathogens,12 endogenous damage associated molecules (HSPs,13 fragments of extracellular matrix14,15) and pro-inflammatory cytokines released elsewhere in the organism carried by blood. Many of the above mentioned ligands act by activating scavenger receptors and pattern recognition receptors (PRRs), which are key elements of the innate immunity and are expressed on the surface of ECs.16 In response to cytokines, ECs produce chemokines, pro-inflammatory cytokines and also express adhesion molecules. This attracts circulating leukocytes and allows them to adhere to ECs and translocate into the intima. There, monocytes differentiate into macrophages (reviewed by Hoeksema et al.17), which phagocytize oxidized LDL and become foam cells, releasing pro-inflammatory cytokines, chemokines and matrix metalloproteinases (MMPs). T lymphocytes differentiate into T helper subsets, including Th1, Th2, Th17 and Treg cells. Among the different lymphocyte subsets, Th1 cells are considered to be pro-atherogenic, Treg atheroprotective and the role of Th2 and Th17 cells is still debated with contradictory evidence being published (reviewed by Butcher and Galkina).18 The release of cytokines and MMPs by the activated immune cells and ECs triggers VSMCs to proliferate, migrate and form foam cells resulting in vessel occlusion and neointima formation (Fig. 1). As such, the forming atheroma is a site of excessive production of cytokines and other inflammatory ligands by various cell types that mediate inflammation and immune responses, and promote vascular remodeling and tissue damage.
Figure 1. Atherosclerotic plaque development. The innate and adaptive immunity co-operate in an inflammatory process that leads to vessel occlusion. (1) Inflammatory triggers induce EC dysfunction by activating the JAK-STAT pathway and PRRs. (2) ECs release inflammatory cytokines, chemokines, adhesion molecules. EC layer permeability increases. (3) Procoagulant surface of ECs promotes leukocyte adhesion and activation. (4) The inflammatory stimuli acts also on SMCs. (5) Leukocytes infiltrate the intima. (6) Activated SMCs produce cytokines and MMPs, which further change the microenvironment. The cells lose contractility and gain motility and proliferate. (7) The infiltrating monocytes transform into macrophages, (8) producing MMPs, further cytokines and ROS. (9) T-cells react to auto-antigens like ox-LDL and differentiate (10) into effector subsets, producing additional cytokines. (11) Macrophages and SMCs phagocytize lipid particles and become foam cells.
IFNγ in Atherosclerosis
Since its discovery in 197019 interferon (IFN)γ has been recognized to not only stimulate immune cells and protect against viral infection, but also play a key role in a number of inflammatory diseases, such as atherosclerosis. The innate and adaptive immunity responses are both affected by the pro-inflammatory IFNγ, which is produced mainly by Th1 cells. IFNγ acts by activating macrophages, natural killer cells and B cells, but also vascular cells: smooth muscle and endothelial. The key role of IFNγ in atherosclerotic plaque development was supported by evidence from mouse models of plaque formation. It was shown that serological neutralization or genetic absence of IFNγ significantly reduces atheroma formation.20,21 In addition, IFNγ was found to be highly expressed within atherosclerotic lesions, further proving its critical role in atherogenesis and modeling of cell behavior and cell-cell interactions of all cell types existing in the vessel wall.22 Until now, most reports on the role of IFNγ in atherosclerosis depicted this cytokine as pro-inflammatory with a role in the development and progression of the plaque. IFNγ dependent events include activation and differentiation of T-cells, as well as macrophage-mediated release of inflammatory cytokines, specifically TNFα and IL6, and pathological amounts of nitric oxide. In addition to that, IFNγ can induce adhesion, cell apoptosis and matrix deposition, all of which resemble endothelial cell dysfunction and were shown to contribute to atherosclerotic lesion development.22 Lastly, the formation of SMC foam cells and inhibition of SMC proliferation is promoted by IFNγ both in vitro and in vivo.23
IFNγ Signaling Pathways
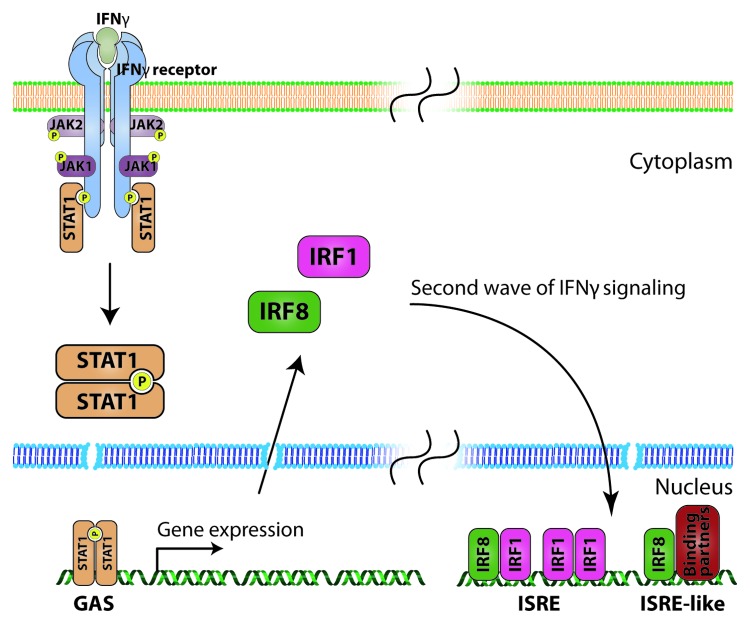
IFNγ is the sole member of the type II interferon class and as such mediates most of the cell responses through the now well-known JAK-STAT pathway (Fig. 2). Binding of homodimeric IFNγ leads to the activation of type II interferon receptor. The active receptor complex consists of two IFNGR1 chains and two IFNGR2 chains, which on the cytoplasmic side are associated with JAK1 and JAK2 kinases respectively. Bringing these kinases in close proximity allows them to cross-phosphorylate each other on specific tyrosine residues. Active JAK kinases can in turn phosphorylate the cytoplasmic domains of the IFNGR1 creating docking sites for the signal transducer and activator of transcription (STAT)-1 protein. STAT1 is a member of a family of transcription factors comprising in total seven proteins.24-26 STAT proteins share structural homology: N-terminal domain (ca. 140 a.a.), coiled-coil domain (ca. 180 a.a.), DNA binding domain (ca. 170 a.a.), linking domain (ca. 90 a.a.), SH2 domain (ca. 140 a.a.) and transcriptional activation domain. The N-terminal domain is involved in dimer complex formation and methylation, the coiled-coil region interacts with other transcription factors and the SH2 domain interacts with phosphorylated tyrosine motifs. Receptor bound STAT1 is phosphorylated, dissociates from the receptor and creates active homodimers that translocate to the nucleus where, by binding to interferon gamma activated sequence (GAS), they activate transcription. STAT1-target genes include chemokines (IP-10 and CCL5), adhesion molecules (ICAM-1) and transcription factors (IRF1 and IRF8).24,25,27
Figure 2. IFNγ signaling. Upon ligation, two IFNGR1 and two IFNGR2 chains create a tetrameric receptor. The cytoplasmic tails are bound by JAK1 and JAK2 kinases respectively. The close proximity allows the kinases to cross-phosphorylate each other. The activated kinases then phosphorylate specific tyrosine residues on the cytoplasmic domain of IFNGR1, creating docking sites for STAT1 transcription factor. Bound STAT1 is phosphorylated, dissociates from the receptor and creates active homodimers. These can translocate to the nucleus, where they bind the GAS element in the promoter region of interferon stimulated genes, which comprises the first, immediate response to IFNγ. Among these genes are IRF transcription factors, which act in a delayed manner, to sustain IFN induced response. IRF1 can act as a homodimer binding to the ISRE element. IRF8 cannot bind DNA by itself, and so acts as a heterodimer with IRF1 or other binding partners (e.g., PU.1) activating ISRE or ISRE-like elements.
IFNγ and IRFs
While the STAT1 homodimer is the primary and best characterized route to IFNγ-induced transcription, IFNγ additionally activates transcription factors of the interferon regulatory factor (IRF) family (Fig. 2). Currently nine members of this family have been identified in mammals (named IRF 1 to 9). All IRFs share a highly conserved N-terminal DNA-binding domain, which is ultrastructurally characterized by a helix-loop-helix motif with a signature tryptophan pentad. IRFs were identified as transcription factors that specifically bind to a highly conserved consensus site in the promoter region of type I IFN and interferon-inducible genes, named IFN-stimulated response element (ISRE).24,28 In this way IRFs were found to regulate the expression of many genes that play a pivotal role in a host of cellular functions such as proliferation, apoptosis, cell cycle regulation and regulation of innate and adaptive immune defense.28
In the context of IFNγ crucial role play IRF1 and IRF8, particularly by amplifying expression of IFNγ-responsive genes initiated by STAT1 (Fig. 2).26 In contrast to STAT1 and IRF1, which are ubiquitously expressed, IRF8 expression is thought to be restricted to lymphoid-cell lineages such as B, T and dendritic cells and macrophages. Accordingly, IRF8 was shown to take part in unique subsets of ISRE-mediated (named EIRE and EICE) but also in GAS-mediated transcription in co-operation with other IRFs (IRF1) or other transcription factors including PU.1 (also immune cell restricted) to drive the differentiation of these lineages. Thus, IRF8 may in part account for “immune cell-specific” STAT1-dependent functions of IFNγ.29 Interestingly, recently we obtained evidence for the first time that IRF-8 is highly expressed in EC and VSMC after IFNγ treatment30 (Chmielewski et al., manuscript in preparation), suggesting that it could also regulate “vasculo-specific” STAT1-dependent functions of IFNγ. The role of IRF-8 in EC and VSMC (dys)function has not been studied but, it is tempting to speculate that IRF-8 specifically regulates STAT1-dependent IFNγ-directed transcriptional responses in cell types involved in vascular dysfunction. In addition, IRF8 could potentially mediate IFNγ-directed cross-talk between EC, VSMC and atheroma-interacting immune cells.
TLR4 Signaling
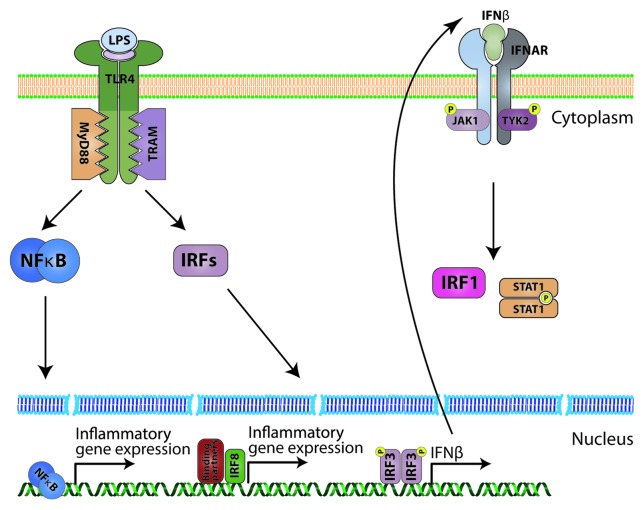
Toll-like receptors (TLRs) are a more recent discovery than interferons. The name of the family comes from the Drosophila Toll receptor, which was originally recognized to have a role in embryonic development.31 It was only in 1996 that its fundamental contribution to the fly’s innate immune response to fungi was revealed by a group of J. Hoffmann.32 The first human TLR was discovered in 1994,33 but its subsequent role in defense against pathogens was uncovered in 1997.34,35 Data obtained by many research groups since then showed that TLRs are important regulators of the innate immune system. To date, there have been at least 13 TLRs discovered in mammals, 10 of which are expressed in human cells.36 TLRs are responsible for detection and recognition of multiple exogenous pathogen associated molecular patterns (PAMPs) and endogenous damage associated molecular patterns (DAMPs). This function places them at the frontline of the host cell response to infection, inflammation and injury. Exogenous molecules recognized by TLRs include: bacterial lipoproteins and lipoteichoic acid (TLR2), double stranded RNA (TLR3) and lipopolysaccharide (TLR4). TLR2 and TLR4 can also be ligated by stress or injury-induced altered host-derived (“self”) structures, which include heat shock proteins and products of extracellular matrix degradation.36 Depending on the receptor and the nature of the ligand, the active receptor complex may form either a dimer or a monomer with the cytoplasmic domain of the receptor acting as a scaffold for multiple adaptor proteins which relay the signal downstream. This cytoplasmic domain, namely Toll/IL-1 resistance (TIR) domain, is present on all TLRs and their adapters and allows interactions between them.36 The adaptor protein myeloid differentiation protein-88 (MyD88) is involved in signaling of most TLRs, with the exception of TLR3.37 TLR4 (Fig. 3) was shown to utilize all four described TIR-containing adapters: MyD88 and MAL (MyD88-adaptor-like; also known as TIRAP) seem to act in pair to activate the MyD88-dependent pathway resulting in NFκB activation, whereas TIR domain-containing adaptor protein inducing IFNβ (TRIF; also known as TICAM1) and TRIF-related adaptor molecule (TRAM) in pair activate the interferon pathway.38 Together this leads to the induction of various target genes that include type I IFNs, chemokines and cell surface molecules. Since a pro-arteriosclerotic effect has been demonstrated for most of these, TLRs as the main endogenous contributor have also been investigated for their potential role in the development of arteriosclerosis.
Figure 3. TLR4 signaling (simplified). LPS binds the homodimeric TLR4 receptor with the help of MD2 molecule. Ligation of the receptor allows the cytoplasmic TIR domain to interact with accessory molecules. Generally, there are two pathways. One, through MyD88 leads to activation of the NFκB transcription factor, which induces expression of inflammatory cytokines. The second pathway, through TRAM, activates IRFs. IRF8 interacts with other transcription factors leading to inflammatory gene expression. IRF3 is phosphorylated, homodimerizes and induces IFNβ production, which in an auto- and paracrine way can stimulate cells to induce IRF1 and STAT1 in the JAK-STAT dependent manner.
TLR-Mediated Immune Responses in the Vessel
In particular, TLR4 is expressed in both human and mouse atherosclerotic lesions. Expression has mainly been located to endothelial cells and macrophages within the lesion. Also, patients with acute coronary syndromes or coronary arteriosclerotic lesions have an increased TLR4 expression on circulating monocytes as compared with control patients.39 Finally, there is evidence that increased TLR expression correlates with endothelial dysfunction in cardiac transplant recipients.40 Mice deficient in TLR4 have reduced atherosclerosis which establishes that Toll-like receptor dependent pathways contribute to disease development.41 Similarly, TLR4 has been implicated in vascular inflammation in an angiotensin II directed mouse model of vascular dysfunction.42 Moreover, TLR4−/− mice are protected against obesity.43 Further evidence that TLR signaling is important in ischemia-reperfusion injury comes from myocardial ischemia models in which TLR4 signaling is important for infarct size and subsequent left ventricular dysfunction.44 Thus, experimental and clinical evidence exists that TLR4 signaling at the very least participates in vascular damage.
STAT1 and IRF8 in TLR Signaling
TLR signaling leads to the induction of various target genes that include those encoding type I IFNs, pro-inflammatory cytokines, chemokines and cell surface molecules. Some of these genes are regulated secondary to LPS-induced IFNβ, which after secretion binds to the type I IFN receptor to activate gene expression (including IP-10, MCP-1, CCL5, ISG15 and iNOS) in a STAT1-dependent manner (Fig. 3). As such, STAT1 has been identified as an important mediator in the biological response to different TLRs, including TLR4.
TLRs have also been shown to utilize the IRF family. Specifically, IRF1, IRF3, IRF5, IRF7 and IRF8 were shown to contribute to TLR-mediated signaling.28 IRF1,45 IRF546 and IRF747 directly interact with MyD88 in TLR9 signaling. This interaction allows for their activation and subsequent translocation to the nucleus, where they can induce gene expression. IRF3 is constitutively expressed and upon TLR4 activation is phosphorylated in the Myd88 independent pathway leading to IFNβ expression, which in turn can induce IRF1 expression.48 IRF8 is at several levels connected to the TLRs. Independent of TLRs, IRF8 increases TLR gene expression in B-cells and myeloma cells.49 As a TLR signaling component, IRF8 interacts with TNF-receptor associated factor (TRAF) 6, a MyD88 recruited ubiquitin ligase, and regulates the production of type I IFNs and other inflammatory mediators. In this way, unmethylated CpG via TLR9 induces activation of IRF8 in dendritic cells that is mediated by NFκB. Consequently, IRF8-deficient mice reveal a signaling defect of TLR9-mediated induction of TNFα and interleukin-6.50 In dendritic cells IRF8 also facilitates TLR2 and TLR4 mediated induction of interleukins, NO synthase and TNFα that involves activation of several kinases like ERK, JNK and MAP kinase.51 IRF8-deficient mice are highly susceptible to several pathogens, including Listeria monocytogenes52 and lymphocytic choriomeningitis virus,53 due to defects in both innate and adaptive immunity. In addition, macrophages from IRF8−/− mice produce diminished levels of TNFα, IL1β and IL12p70 in response to LPS.
STAT1- and IRF8-Mediated Crosstalk between IFNγ and LPS
The synergy between IFNγ and TLR has been implicated in the host defense against pathogens. IFNγ produced by T cells and other cells is considered to enhance TLR signaling in DCs and macrophages for the efficient induction of inflammatory mediators to eliminate pathogens.54,55 Lately, evidence has been provided on the mechanistic insights of this cross-talk between IFNγ and TLR signaling pathways, with STAT1 being a critical mediator.7,56,57 In general it is believed that the transactivation ability of the macrophage STAT1 pool is super-activated upon stimulation with both IFNγ and LPS, relative to either agonist alone.7,54 The STAT1-targets IRF1 and IRF8 have also been shown to contribute to the signal integration between IFNγ and LPS. IRF1 is a major mediator of IFNγ signaling, and is regulated by both IFNγ and TLR agonists as a consequence of STAT1 and NFκB elements in the IRF1 promoter that mediate transcriptional induction.58-61 Zhao et al.51 reported that in macrophages IRF8 was upregulated in response to either IFNγ or LPS, and was super-induced by IFNγ and LPS co-administration. Correspondingly, IFNγ and LPS synergistically induced the expression of pro-inflammatory factors, including IL-1, IL-6, IL-12, NO and TNF-α, in an IRF8-dependent manner. Comparable synergism was observed between IFNγ and peptidoglycan (PGN; a TLR2 ligand) and poly (I:C) (a TLR3 ligand) in the induction of IL-12 promoter activity. Also in macrophages, IRF8 has been linked to the IFNγ and LPS-mediated transcriptional activation of CCL5 (RANTES),62 a known chemokine actively involved in leukocyte recruitment to the injured artery during vascular remodeling. These findings suggest that in immune cells STAT1 and IRF8 are unique points of convergence for the antimicrobial synergism between IFNγ and TLRs.
Our recent observations7 suggest that also in ECs and VSMCs STAT1 orchestrates a platform for cross-talk between IFNγ and LPS. Moreover, increased production of STAT1 protein in these cells, strictly dependent on IFNγ provides a potential mechanism resulting in augmented STAT1 phosphorylation when both IFNγ and LPS are present. This coincided with increased expression of the chemokine IP-10 and the adhesion molecule ICAM-1 as well as adhesion of U937 leukemia cells to ECs, in a STAT1- and TLR4-dependent manner.7 Interestingly, under the same conditions we also observed a significant increase in IRF8 gene expression as compared with both factors alone. More importantly, this correlated with a dramatic amplification of CCL5 (RANTES) gene and protein expression in a STAT1 as well as IRF8-dependent fashion. (Chmielewski et al., manuscript in preparation)
In addition to its known immune cell functions, this uncovers a novel role of IRF8 in ECs as well as VSMCs, facilitating signaling events initiated by both IFNγ and LPS, thus providing a platform for IRF8-dependent crosstalk between the two pathways and leading to increased expression of pro-inflammatory mediators.
Multiple Signal Convergence of STAT1 in Vascular Dysfunction
Integration of IFNγ and TLR signaling pathways occurs through synergy between TLR- and IFNγ-induced transcription factors, which is likely to be a global mechanism to allow the integration of multiple input signals for synergistic, coordinated regulation of gene expression. IRF1, IP-10, ICAM-1 and iNOS genes contain both STAT1 and NFκB binding sites in their promoter.59,60,63-68 For example, IFNγ signaling induced iNOS mRNA via STAT1, but induction was maximal only when the iNOS promoter NFκB sites were occupied following TLR ligation.64,68,69
More detailed investigation of STAT1-dependent transcriptional synergism between IFNγ and LPS in cells from the vasculature predicts the existence of different regulatory mechanisms through the additional involvement of IRF1 and IRF8 (Fig. 4). Our studies in ECs and VSMCs confirmed that IP-10 and ICAM-1 are IFNγ70,71 as well as LPS-inducible72,73 genes while their expression coincided with STAT1 phosphorylation, although in a different way. In case of IP-10, the dramatic increase in gene expression observed in ECs and VSMCs treated with IFNγ followed by LPS correlated with a predominant STAT1-dependent, but IRF8-independent (Chmielewski et al., manuscript in preparation) mechanism involved in the integration of both signals. Interestingly, the synergism between IFNγ and IL1β or TNFα in mouse embryonic fibroblasts and macrophages was shown to result in increased expression of IP-10 in a STAT1-dependent manner and requiring IRF1, but not NFκB.66,74,75 Since a protein synthesis-dependent mechanism was involved and IRF1 is upregulated by both IFNγ and LPS, it is tempting to speculate that a similar IRF1-mediated mechanism could play a role in IP-10 expression in IFNγ-primed vascular cells that are subsequently stimulated by LPS.7
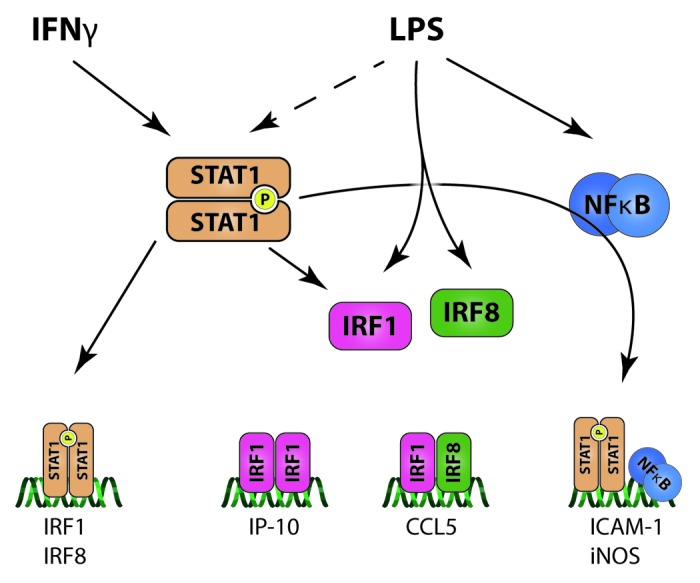
Figure 4. IFNγ and LPS signaling integration in vascular cells. IFNγ and LPS use the same transcription factor (STAT1) to elicit cell response. This cross-talk causes amplification of STAT1 activation. This pro-inflammatory factor can induce secondary transcription factor genes by itself (IRF1 and IRF8) and also interact with LPS-dependent NFκB leading to elevated expression of ICAM-1 and iNOS. The second wave of signaling includes IRF1 and IRF8, which are both induced by STAT1 and also directly by LPS. IRF1 can act as a homodimer to induce expression of IP-10 and also can interact with IRF8 to induce CCL5. This creates a multi-layer integration of signaling between IFNγ and LPS which leads to increased inflammation and vascular dysfunction.
In contrast, the increased expression of ICAM-1 RNA in ECs treated with IFNγ followed by LPS was weaker as compared with IP-10 and most likely involved an NFκB-dependent mechanism.7 IFNγ-induced ICAM-1 expression was shown to be STAT1-dependent.71 Similar to the iNOS gene, the ICAM-1 promoter contains STAT1 and NFκB binding sites and maximal transcription requires both signals.59,63,76 This suggests that a mechanism involving the cooperation between STAT1 (IFNγ and LPS-mediated) and NFκB (strictly LPS-mediated) is responsible for ICAM-1 expression in IFNγ-primed vascular cells that are subsequently stimulated by LPS.
The transcriptional regulation of the CCL5 gene in macrophages in response to IFNγ and LPS uncovered a novel role of IRF8 and required both the IRF1 and NFκB binding sites.62 Indeed, IRF8 complexed with IRF1 at the ISRE was responsible for the IFNγ signal, while IRF8 interacting with NFκB and PU.1 at the NFκB site in the IFNγ and LPS response.62 PU.1 is a member of the large Ets family and is an important regulator of myeloid and lymphoid cell differentiation.77 Since PU.1 expression is restricted to immune cells and IRF1 and IRF8 are upregulated by both IFNγ and LPS, the IRF8-dependent expression of CCL5 in IFNγ-primed vascular cells that are subsequently stimulated by LPS (Chmielewski et al., manuscript in preparation) is highly likely to involve an IRF1/IRF8-mediated mechanism and not an IRF8/NFκB.
Future Perspectives
Together with the established roles of IFNγ and TLRs in atherosclerotic pathology, the synergism between IFNγ and TLRs in ECs and VSMCs and atheroma-interacting immune cells in response to exogenous and endogenous atherogenic ligands could result in amplification of STAT1-mediated pro-inflammatory responses in the damaged vessel. In co-operation, the mechanisms as proposed in Figure 4 could offer an explanation for the differential STAT1-dependent signal integration between IFNγ and TLR4 signals in vascular cells, with the novel role of IRF8 providing an additional layer to the overall complexity. As a consequence, in the presence of IFNγ and LPS (or any other exogenous or endogenous TLR4 ligands), pro-inflammatory mediators like IP-10, CCL5 and ICAM-1 can be over-produced in ECs and VSMCs and may in turn function on leukocyte attraction and adhesion and VSMC proliferation and migration,78-81 which are important characteristics of EC dysfunction and early triggers of atherosclerosis.
Translational implications
Despite the tremendous progress made in atherosclerosis management, it is still a common health problem and a major financial load on healthcare system. Until now, therapies focused on stabilizing patients with moderate and advanced pathologies, partially because clinicians are lacking diagnostic assays able to detect early changes and partially because of the lack of information about the molecular basis of early plaque development. Novel experimental background summarized in this review brings promise of new intervention and diagnostic tools that could act in the early stages of atherosclerotic plaque development.
STAT1 represents an interesting novel target of therapeutic intervention that has a crucial role in mediating the interplay between damaged vessels and host immunity to control atherosclerosis mediated by IFNγ and TLR-directed crosstalk. With its immune cell specificity and novel vascular specific functions, we uncovered IRF8 as an attractive novel therapeutical target that could provide a way to control early plaque formation in a cell type specific manner and with greater specificity.
Finally, crosstalk between IFNγ and TLR4 relying on STAT1 and IRF8 results in amplification of expression of inflammatory mediators, such as IP-10, ICAM-1 and CCL5. These mediators could potentially be quantified in the serum of patients and used as a measure of disease initiation and progression. Although the concept of serum markers of atherosclerosis has been pursued already for some time without much success, the new experimental findings summarized in this review show new promise that such specific markers could be selected and used to monitor and diagnose subclinical atherosclerotic changes.
Acknowledgments
The work in this paper was supported in part by Polish Ministry of Science and Higher Education (Iuventus Plus 0493/IP1/2011/71), Polish National Center for Science (NN302 016 339 and NN301 073 140) and Foundation for Polish Science (FOCUS 3/2009 and MPD/2010/3).
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/22469
References
- 1.Poston RN, Davies DF. Immunity and inflammation in the pathogenesis of atherosclerosis. A review. Atherosclerosis. 1974;19:353–67. doi: 10.1016/S0021-9150(74)80001-8. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 3.Orr AW, Hastings NE, Blackman BR, Wamhoff BR. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. 2010;47:168–80. doi: 10.1159/000250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikorski K, Czerwoniec A, Bujnicki JM, Wesoly J, Bluyssen HA. STAT1 as a novel therapeutical target in pro-atherogenic signal integration of IFNγ, TLR4 and IL-6 in vascular disease. Cytokine Growth Factor Rev. 2011;22:211–9. doi: 10.1016/j.cytogfr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Marrero MB, Schieffer B, Li B, Sun J, Harp JB, Ling BN. Role of Janus kinase/signal transducer and activator of transcription and mitogen-activated protein kinase cascades in angiotensin II- and platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J Biol Chem. 1997;272:24684–90. doi: 10.1074/jbc.272.39.24684. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–61. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikorski K, Chmielewski S, Przybyl L, Heemann U, Wesoly J, Baumann M, et al. STAT1-mediated signal integration between IFNγ and LPS leads to increased EC and SMC activation and monocyte adhesion. Am J Physiol Cell Physiol. 2011;300:C1337–44. doi: 10.1152/ajpcell.00276.2010. [DOI] [PubMed] [Google Scholar]
- 8.Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, et al. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9:830–4. doi: 10.1016/j.autrev.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008;117:1082–9. doi: 10.1161/CIRCULATIONAHA.107.720730. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Ando J. New molecular mechanisms for cardiovascular disease:blood flow sensing mechanism in vascular endothelial cells. J Pharmacol Sci. 2011;116:323–31. doi: 10.1254/jphs.10R29FM. [DOI] [PubMed] [Google Scholar]
- 11.Sevanian A, Hodis HN, Hwang J, McLeod LL, Peterson H. Characterization of endothelial cell injury by cholesterol oxidation products found in oxidized LDL. J Lipid Res. 1995;36:1971–86. [PubMed] [Google Scholar]
- 12.Prasad A, Zhu J, Halcox JP, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections: role of endothelial dysfunction. Circulation. 2002;106:184–90. doi: 10.1161/01.CIR.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- 13.Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:1547–59. doi: 10.1161/01.ATV.0000029720.59649.50. [DOI] [PubMed] [Google Scholar]
- 14.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81:173–82. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–84. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 17.Hoeksema MA, Stöger JL, de Winther MP. Molecular pathways regulating macrophage polarization: implications for atherosclerosis. Curr Atheroscler Rep. 2012;14:254–63. doi: 10.1007/s11883-012-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butcher M, Galkina E. Current views on the functions of interleukin-17A-producing cells in atherosclerosis. Thromb Haemost. 2011;106:787–95. doi: 10.1160/TH11-05-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milstone LM, Waksman BH. Release of virus inhibitor from tuberculin-sensitized peritoneal cells stimulated by antigen. J Immunol. 1970;105:1068–71. [PubMed] [Google Scholar]
- 20.Hidalgo LG, Halloran PF. Role of IFN-gamma in allograft rejection. Crit Rev Immunol. 2002;22:317–49. doi: 10.1615/CritRevImmunol.v22.i4.50. [DOI] [PubMed] [Google Scholar]
- 21.Leon ML, Zuckerman SH. Gamma interferon: a central mediator in atherosclerosis. Inflamm Res. 2005;54:395–411. doi: 10.1007/s00011-005-1377-2. [DOI] [PubMed] [Google Scholar]
- 22.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, et al. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–11. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 23.McLaren JE, Ramji DP. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009;20:125–35. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Wesoly J, Szweykowska-Kulinska Z, Bluyssen HA. STAT activation and differential complex formation dictate selectivity of interferon responses. Acta Biochim Pol. 2007;54:27–38. [PubMed] [Google Scholar]
- 25.Levy DE, Darnell JE., Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 26.Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev. 2008;19:383–94. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Bluyssen AR, Durbin JE, Levy DE. ISGF3 gamma p48, a specificity switch for interferon activated transcription factors. Cytokine Growth Factor Rev. 1996;7:11–7. doi: 10.1016/1359-6101(96)00005-6. [DOI] [PubMed] [Google Scholar]
- 28.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–84. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 29.Kanno Y, Levi BZ, Tamura T, Ozato K. Immune cell-specific amplification of interferon signaling by the IRF-4/8-PU.1 complex. J Interferon Cytokine Res. 2005;25:770–9. doi: 10.1089/jir.2005.25.770. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–79. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 32.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 33.Nomura N, Miyajima N, Sazuka T, Tanaka A, Kawarabayasi Y, Sato S, et al. Prediction of the coding sequences of unidentified human genes. I. The coding sequences of 40 new genes (KIAA0001-KIAA0040) deduced by analysis of randomly sampled cDNA clones from human immature myeloid cell line KG-1. DNA Res. 1994;1:27–35. doi: 10.1093/dnares/1.1.27. [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 35.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 36.Keogh B, Parker AE. Toll-like receptors as targets for immune disorders. Trends Pharmacol Sci. 2011;32:435–42. doi: 10.1016/j.tips.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–7. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004;6:1361–7. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Methe H, Kim JO, Kofler S, Weis M, Nabauer M, Koglin J. Expansion of circulating Toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation. 2005;111:2654–61. doi: 10.1161/CIRCULATIONAHA.104.498865. [DOI] [PubMed] [Google Scholar]
- 40.Methe H, Zimmer E, Grimm C, Nabauer M, Koglin J. Evidence for a role of toll-like receptor 4 in development of chronic allograft rejection after cardiac transplantation. Transplantation. 2004;78:1324–31. doi: 10.1097/01.TP.0000137930.40597.03. [DOI] [PubMed] [Google Scholar]
- 41.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eissler R, Schmaderer C, Rusai K, Kühne L, Sollinger D, Lahmer T, et al. Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens Res. 2011;34:551–8. doi: 10.1038/hr.2010.270. [DOI] [PubMed] [Google Scholar]
- 43.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–98. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 44.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–64. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 45.Schmitz F, Heit A, Guggemoos S, Krug A, Mages J, Schiemann M, et al. Interferon-regulatory-factor 1 controls Toll-like receptor 9-mediated IFN-beta production in myeloid dendritic cells. Eur J Immunol. 2007;37:315–27. doi: 10.1002/eji.200636767. [DOI] [PubMed] [Google Scholar]
- 46.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–9. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 47.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–40. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 48.Sakaguchi S, Negishi H, Asagiri M, Nakajima C, Mizutani T, Takaoka A, et al. Essential role of IRF-3 in lipopolysaccharide-induced interferon-beta gene expression and endotoxin shock. Biochem Biophys Res Commun. 2003;306:860–6. doi: 10.1016/S0006-291X(03)01049-0. [DOI] [PubMed] [Google Scholar]
- 49.Shin DM, Lee CH, Morse HC., 3rd IRF8 governs expression of genes involved in innate and adaptive immunity in human and mouse germinal center B cells. PLoS One. 2011;6:e27384. doi: 10.1371/journal.pone.0027384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsujimura H, Tamura T, Kong HJ, Nishiyama A, Ishii KJ, Klinman DM, et al. Toll-like receptor 9 signaling activates NF-kappaB through IFN regulatory factor-8/IFN consensus sequence binding protein in dendritic cells. J Immunol. 2004;172:6820–7. doi: 10.4049/jimmunol.172.11.6820. [DOI] [PubMed] [Google Scholar]
- 51.Zhao J, Kong HJ, Li H, Huang B, Yang M, Zhu C, et al. IRF-8/interferon (IFN) consensus sequence-binding protein is involved in Toll-like receptor (TLR) signaling and contributes to the cross-talk between TLR and IFN-gamma signaling pathways. J Biol Chem. 2006;281:10073–80. doi: 10.1074/jbc.M507788200. [DOI] [PubMed] [Google Scholar]
- 52.Fehr T, Schoedon G, Odermatt B, Holtschke T, Schneemann M, Bachmann MF, et al. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J Exp Med. 1997;185:921–31. doi: 10.1084/jem.185.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holtschke T, Löhler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–17. doi: 10.1016/S0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 54.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–24. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Hu X, Chen J, Wang L, Ivashkiv LB. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J Leukoc Biol. 2007;82:237–43. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 56.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–50. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harada H, Takahashi E, Itoh S, Harada K, Hori TA, Taniguchi T. Structure and regulation of the human interferon regulatory factor 1 (IRF-1) and IRF-2 genes: implications for a gene network in the interferon system. Mol Cell Biol. 1994;14:1500–9. doi: 10.1128/mcb.14.2.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pine R. Convergence of TNFalpha and IFNgamma signalling pathways through synergistic induction of IRF-1/ISGF-2 is mediated by a composite GAS/kappaB promoter element. Nucleic Acids Res. 1997;25:4346–54. doi: 10.1093/nar/25.21.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sims SH, Cha Y, Romine MF, Gao PQ, Gottlieb K, Deisseroth AB. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol Cell Biol. 1993;13:690–702. doi: 10.1128/mcb.13.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sirén J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174:1932–7. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Ma X. Interferon regulatory factor 8 regulates RANTES gene transcription in cooperation with interferon regulatory factor-1, NF-kappaB, and PU.1. J Biol Chem. 2006;281:19188–95. doi: 10.1074/jbc.M602059200. [DOI] [PubMed] [Google Scholar]
- 63.Caldenhoven E, Coffer P, Yuan J, Van de Stolpe A, Horn F, Kruijer W, et al. Stimulation of the human intercellular adhesion molecule-1 promoter by interleukin-6 and interferon-gamma involves binding of distinct factors to a palindromic response element. J Biol Chem. 1994;269:21146–54. [PubMed] [Google Scholar]
- 64.Gao J, Morrison DC, Parmely TJ, Russell SW, Murphy WJ. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J Biol Chem. 1997;272:1226–30. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 65.Jahnke A, Johnson JP. Synergistic activation of intercellular adhesion molecule 1 (ICAM-1) by TNF-alpha and IFN-gamma is mediated by p65/p50 and p65/c-Rel and interferon-responsive factor Stat1 alpha (p91) that can be activated by both IFN-gamma and IFN-alpha. FEBS Lett. 1994;354:220–6. doi: 10.1016/0014-5793(94)01130-3. [DOI] [PubMed] [Google Scholar]
- 66.Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J Immunol. 1995;154:5235–44. [PubMed] [Google Scholar]
- 67.Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. 1997;272:14899–907. doi: 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- 68.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 69.Blanchette J, Jaramillo M, Olivier M. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology. 2003;108:513–22. doi: 10.1046/j.1365-2567.2003.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramana CV, Gil MP, Han Y, Ransohoff RM, Schreiber RD, Stark GR. Stat1-independent regulation of gene expression in response to IFN-gamma. Proc Natl Acad Sci U S A. 2001;98:6674–9. doi: 10.1073/pnas.111164198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walter MJ, Look DC, Tidwell RM, Roswit WT, Holtzman MJ. Targeted inhibition of interferon-gamma-dependent intercellular adhesion molecule-1 (ICAM-1) expression using dominant-negative Stat1. J Biol Chem. 1997;272:28582–9. doi: 10.1074/jbc.272.45.28582. [DOI] [PubMed] [Google Scholar]
- 72.Elkon R, Linhart C, Halperin Y, Shiloh Y, Shamir R. Functional genomic delineation of TLR-induced transcriptional networks. BMC Genomics. 2007;8:394. doi: 10.1186/1471-2164-8-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 74.Shultz DB, Fuller JD, Yang Y, Sizemore N, Rani MR, Stark GR. Activation of a subset of genes by IFN-gamma requires IKKbeta but not interferon-dependent activation of NF-kappaB. J Interferon Cytokine Res. 2007;27:875–84. doi: 10.1089/jir.2007.0031. [DOI] [PubMed] [Google Scholar]
- 75.Shultz DB, Rani MR, Fuller JD, Ransohoff RM, Stark GR. Roles of IKK-beta, IRF1, and p65 in the activation of chemokine genes by interferon-gamma. J Interferon Cytokine Res. 2009;29:817–24. doi: 10.1089/jir.2009.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tessitore A, Pastore L, Rispoli A, Cilenti L, Toniato E, Flati V, et al. Two gamma-interferon-activation sites (GAS) on the promoter of the human intercellular adhesion molecule (ICAM-1) gene are required for induction of transcription by IFN-gamma. Eur J Biochem. 1998;258:968–75. doi: 10.1046/j.1432-1327.1998.2580968.x. [DOI] [PubMed] [Google Scholar]
- 77.Yordy JS, Muise-Helmericks RC. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19:6503–13. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]
- 78.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–9. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 80.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–12. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 81.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041–50. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]