Abstract
In cardiac and many other systems, chronic stress activates avfamily of structurally and functionally conserved receptors and their downstream signaling molecules that entail tyrosine, serine or threonine phosphorylation to transfer the messages to the genetic machinery. However, the activation of the Janus kinases (JAKs) and their downstream signal transducer and activator of transcription (STATs) proteins is both characteristic of and unique to cytokine and growth factor signaling which plays a central role in heart physiology. Dysregulation of JAK-STAT signaling is associated with various cardiovascular diseases. The molecular signaling and specificity of the JAK-STAT pathway are modulated at many levels by distinct regulatory proteins. Here, we review recent studies on the regulation of the STAT signaling pathway that will enhance our ability to design rational therapeutic strategies for stress-induced heart failure.
Keywords: JAK-STAT signaling, cardio-protection, heart, heart failure, inflammation
Introduction
Initially uncovered by experiments aimed at understanding IFNα and IFN-γ induced transcriptional activation, a new pathway of signal transduction from the cell surface to genes in the nucleus has been recognized in last decades of 19th century.1 The pathway is called the JAK-STAT pathway. Similar to other pathways, association of ligands to their receptors leads to activation of one of the JAK family of tyrosine kinases associated with a trans-membrane receptor, and subsequently leads to the phosphorylation on tyrosine of one or more of a family of latent cytoplasmic transcription factors called STATs.2,3 These latter proteins perform a dual role, first as signal transducers by acting as substrates of the JAKs, and after phosphorylation and nuclear translocation, by acting as transcriptional activators.4,5 While IFNα and IFNγ were the first polypeptide ligands described that trigger this pathway, it is now known that many other ligands such as IL-6 family cytokines, IL-10 and neurohormones can also activate proteins in the pathway.5 The details of early experiments in hematopoietic and other systems with the IFNs and cytokines have been summarized in many decent reviews;6,7 however, its cardiovascular responses are still limited in literature. In current review, we will mainly discuss the critical role of STAT pathway in cardiovascular diseases.
Structure and function of STAT3 protein
Seven STATs (STATs 1, 2, 3, 4, 5A, 5B and 6) have been identified in mammals and range in size from 750 to 900 amino acids.8 All of these STAT proteins share a common feature. The structural and functional analysis of these proteins suggests that they have six conserved domains (Fig. 1). This includes the N-terminal domain (NH2), the coiled-coiled domain (CCD), the DNA binding domain (DBD), the linker domain and the SH2/tyrosine activation domain. In contrast, the carboxyl-terminal transcriptional activation domain (TAD) is quite divergent and contributes to STAT specificity (Fig. 1). The four core domains contact each other by large inter-domain interfaces, suggesting that structural changes induced in one domain may also affect other domains. The identification of STAT homologs in simpler eukaryotes suggests that this family arose from a single gene.

Figure 1. Schematic illustrations of STATs domains and structural features. N-terminal domain; coiled–coil domain (CCD); DNA binding domain (DBD); Linker domain; Src homology domain 2 (SH2) and the tyrosine residue (Y) phosphorylation sites.
The N-terminal half of the protein (~125 amino acids) consists of two relatively poorly characterized domains. This domain is well conserved between these families of protein and is reported to promote cooperativity in DNA binding and to regulate nuclear translocation. It represents an independently folded and stable moiety, which can be cleaved from the full-length molecule by limited proteolysis.9 Several studies suggest that N-terminal dimerization promotes cooperativity of binding to tandem GAS (IFNγ activated sequences) elements.9-11 Other studies have suggested that the N-terminal STAT domain promotes interaction with the other proteins or receptors and that it regulates nuclear translocation.12 The coiled-coil domain (amino acids ~135 to ~315) consists of a four-helix bundle that protrudes about 80 Å laterally from the core structure. Many studies have also implicated the coiled-coil domain in receptor binding and tyrosine phosphorylation in addition to nuclear export.13,14 The domains that constitute the carboxyl-terminus are well understood. The DNA-binding domain (DBD; amino acids ~320 to ~500) recognizes members of the γ-activated sequence (GAS) family of enhancers and (like the upstream coiled-coil domain) appears to regulate nuclear export. The adjacent linker domain (amino acids ~500 to ~600) is important in assuring the appropriate structure of the DNA-binding motif and also appears to regulate nuclear export in resting cells. This domain also shows the structural similarity to calcium-binding domain. Not surprisingly, the SH2 domain (amino acids ~600 to ~700) is the most highly conserved motif and mediates both receptor-specific recruitment and STAT dimerization. The number of direct contact sites between amino acid residues and DNA are modest, accounting for a dissociation constant in the nanomolar range. Thus, cooperativity in DNA binding is likely to be important in effective transcriptional activity. All of these proteins (STAT1–6) except STAT2 are known to homodimerize in vivo. Dimerization requires the binding of a phosphorylated tyrosine activation motif on one STAT subunit to the SH2 domain of the other subunit.15 Finally, the carboxyl-terminus carries a transcriptional activation domain (TAD), which is conserved between homologs (e.g., murine and human). However, the carboxyl-terminus varies considerably in both length and sequence between different STAT family members. Once again, STAT2 is an exception, since its TAD sequence diverges considerably between the murine and human homologs.15
JAK-STAT activation
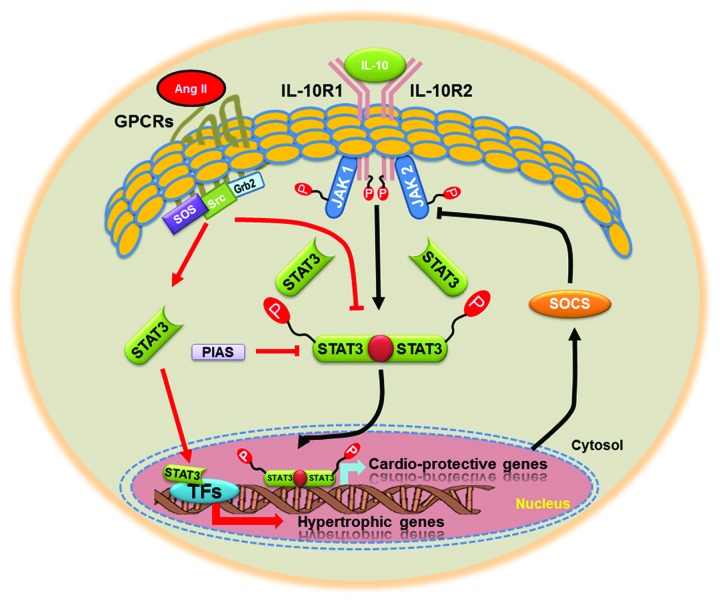
As a part of normal cytokine signaling cascade, the JAK-STAT pathway is also initiated by binding of a ligand to its receptor in the plasma membrane and the subsequent homo- or heterodimerization of the receptor. In the heart, IL-6, IL-11, leukemia inhibitory factor (LIF), oncostatin M, ciliary neurotrophic factor (CNTF) and cardiotrophin-like cytokine (CT-1) are the major cytokines that transduce their signals via glycoprotein 130 (gp130) predominantly to STAT3 and 5.16 The receptor dimerization, in turn, induces phosphorylation and activation of JAK proteins, which are associated with the intracellular domain of the receptor. JAK proteins phosphorylate the receptor, thereby creating docking sites for cytosolic STAT proteins via their SH2 domains. Subsequently, STAT proteins become phosphorylated on a specific tyrosine residue (for example, Tyr705 for STAT3) by activated JAK kinases, and undergo homo- or heterodimerization by interaction of the phosphotyrosine residue of one STAT monomer and the SH2 domain of the other monomer. Once dimerized, these proteins dissociate from the receptor and translocate into the nucleus, where they bind to specific DNA sequences and regulate the expression of target genes (Fig. 2). In the heart, STAT proteins regulate the expression of genes encoding proteins mainly involved in inflammation, cellular signaling, apoptosis, angiogenesis and extracellular matrix composition.17,18 The phosphorylation of an additional specific serine residue in the transactivation domain of STAT proteins (Ser727 for STAT3) generally promotes transcriptional activity. The activation and nuclear translocation of STATs usually occurs within 15 min, but STAT proteins are also rapidly inactivated, resulting in a half-life of nuclear phosphorylated STAT between 15 and 30 min.19 Upon de-phosphorylation by nuclear phosphatases, STAT proteins shuttle back into the cytosol via the nuclear pore. The JAK-STAT pathway is not only controlled via phosphorylation of the signaling proteins, but also by negative regulators. Upon activation, STATs bind to the promoter region of SOCS genes (suppressors of cytokine signaling) and upregulate the transcription of these target genes (Fig. 2). SOCS proteins (mainly SOCS 1 and 3) negatively regulate the JAK-STAT pathway by either directly binding to JAK, by binding to the receptor and to JAK, or by competing with STATs for the docking sites at the receptor.20 Another negative feedback mechanism of the JAK/STAT pathway comprises SHP-2 proteins (src homology 2 domain containing protein tyrosine phosphatase), which dephosphorylate the receptor, JAK or STAT proteins.21 The details of the canonical JAK-STAT signaling pathway have been extensively reviewed elsewhere.16 The full activation of different STAT subtypes is determined by many factors. These include animal’s species, cell type differences and its intra-cellular distribution.22,23
Figure 2. A putative model for JAK-STAT signaling pathway. Upon binding ligand (IL-10), receptor-associated JAKs become activated and mediate phosphorylation of specific receptor tyrosine residues. This leads to the recruitment and phosphorylation of STAT3. Activated STAT3 is released from the receptor, dimerize, translocate to the nucleus, and bind with transcription factors to regulate expression of many cardio-protective, anti-inflammatory or growth related genes. As a feedback loop it also regulates expression of suppressor of cytokines signaling genes (SOCS). Many protein inhibitors (PIAS) can also regulate the transcriptional activity of STAT3. In addition to JAKs, PTKs and MAPK, activated by Ang II, may participate in phosphorylation of STAT3. Activated STAT3 translocate to the nucleus to activate genes, such as c-fos, c-myc, α2-macroglobulin and tissue inhibitor metalloproteinase-1 (TIMP-1) by binding to the sis-inducible element (SIE) of the promoter.
STAT Signaling in Cardiovascular Diseases
All seven STAT family members have been reported to be expressed in the heart and/or cultured cardiac myocytes, fibroblasts and endothelial cells.24,25 At cellular compartment level, STAT localization is not restricted to the cytosol and the nucleus but recently, STAT proteins have also been identified in mitochondrial fraction.26,27 As for the exact role of JAK-STAT signaling in cardiac function and diseases, most of the available information relates to STAT1 and 3 family members. Various stimuli which activate hypertrophic growth of cardiac myocytes and/or provide cardio-protection have been demonstrated to activate JAK-STAT signaling in the heart. Importantly, studies have established that these stimuli also enhance cardiac STAT functional activity, as assessed by electrophoretic mobility shift assays of DNA binding activity, or promoter-reporter assays of transcriptional activity.28 Mechanical stretch and pressure over-load,28,29 myocardial infarction30 and ANG II treatment31 has been shown to activate cardiac JAK-STAT signaling. In addition, ischemia and ischemia/reoxygenation also activates JAK-STAT signaling in cultured ventricular myocytes, isolated heart preparations or the in situ hearts.28 It has been well documented that monocytes and macrophages produce inflammatory cytokine to repair the injury during myocardial infarction and hypertrophy.5 Angiotensin II also mimics the action of cytokines by activation of tissue inhibitor of metalloproteinase-1 (TIMP-1), an important factor associated with cardiac remodeling.5 The mechanisms whereby G-protein receptors couple to tyrosine phosphorylation in general and more specifically to the JAK-STAT pathway, are not yet clearly elucidated. Previously, it was suggested that YXXQ regions of AT1 receptors could serve as a docking sites to facilitate STAT3 phosphorylation.31 Other studies reported that association of JAK2 with AT1 receptor facilitates STATs activation.5,32 Alteration in JAK-STAT signaling pathways were also reported in patients with end-stage dilated cardiomyopathy.33 The early activation of STAT3 during diseased stage could be the protective response of system to reduce the cardiac death and remodeling.
Recent reports have shown that transgenic mice with global deletion of STATs are embryonically lethal further suggesting its role in development and growth.34 To sidestep the embryonic lethal phenotype of global STAT3 knockout mice and to better understand the rle of STAT3 in the heart cardiac myocytes-specific STAT3 KO mice have been developed. These mice are significantly more susceptible to cardiac injury under the influence of various stress signals suggesting a protective role of STAT3 in heart.35 However, a major disadvantage of these tissue specific STAT3-KO mice is the pathological phenotype, which develops with age and thus limits the direct assessment of the role of STAT3 in cardio-protection. Recently, Bolli and coworkers generated inducible, cardiac myocyte-specific STAT3-deficient mice, which are of great value since they overcome the problems of embryonic lethality and the consequences of chronic alterations in STAT3-dependent gene expression.36 These mice excluded the possibility of age-dependent alterations in apoptosis, fibrosis, capillary density, and cardiac function, and importantly these mice did not show age dependent cardiac hypertrophy or dilatation. Therefore, the inducible cardiac myocyte-specific STAT3-KO mouse represents a novel and attractive model to study the role of STAT3 in the cardio-protection by ischemic pre- and post-conditioning without the confounding effects associated with chronic STAT3 deletion.36
Mechanism for cardio-protective action of STAT3 is not well studied in cardiovascular diseases. Mostly, the protective effect of STAT3 in the heart is linked to the reduction in inflammation. Evidence for this notion comes from the studies in which STAT3-deficient mice treated with lipopolysaccharide (LPS) demonstrated significantly more apoptosis and fibrosis than their WT counterparts.37 Furthermore, STAT3 KO cardiac myocytes secrete significantly more tumor necrosis factor α (TNFα) in response to LPS than those with WT STAT3.35 The anti-inflammatory and anti-fibrotic effects of STAT3 could be due to direct transcriptional inhibition of nuclear factor kappa-B (NFκB).35,37 Indirectly, we have also shown that use of IL-10, an anti-inflammatory pleotropic cytokines, markedly activated STAT3 phosphorylation after myocardial infarction injury and thus improve heart function.30
Many recent studies indicated that STAT3 induces the expression of pro-angiogenic factors from resident cardiac cells.38,39 It has been shown that overexpression or activation of STAT3 in cardiac myocytes enhances VEGF expression, which, in turn, promotes myocardial capillary formation.38,40 Cardiac myocyte-specific deletion of VEGF reduces coronary microvascularization,41 suggesting that VEGF may be an important target gene mediating pro-angiogenic effects of STAT3. A recent interesting study reported that STAT3 plays a critical role during pregnancy related adaptive cardiac hypertrophy by activation of angiogenesis (increased VEGF) and by inhibition of oxidative stress (increased MnSOD level).42 In our previous report we documented that IL-10 markedly increase the VEGF and capillary beds in infarct zone after myocardial infarction.30 This effect of IL-10 on microvasculature is STAT3-dependent as STAT3 inhibitor markedly eliminated the IL-10 responses.30 In contrast, other groups have shown STAT3 does not affect the expression of VEGF.17 Using STAT3 KO animals they reported that VEGF expression is unaffected by STAT3 deletion although these animals showed compromised heart function and reduced angiogenesis after myocardial infarction. Interestingly, STAT3 KO animals showed increased expression of connective tissue growth factor (CTGF), plasminogen activator inhibitor 1 (PAI-1), tissue inhibitor of matrix metalloproteinase 1 (TIMP1) and thrombospondin 1 (TSP1) compared with WT mice after MI.17 All these factors are potent suppressor of angiogenesis as well as activator of fibroblast proliferation.17 In addition, the increased fibrosis and myocyte cell loss might be due to reduced blood and thus oxygen supply to the heart cells in this model.17
Recently we found that endothelial progenitor cells (EPCs) can also play critical role in neovascularization in heart.43 During ischemic condition (in MI model) both homing and survival of EPCs in the heart was markedly reduced. In this fascinating study we have shown that during myocardial infarction the mobilization of EPCs from bone marrow (BM) to heart is impaired and contributes to diminished angiogenesis and markedly reduced left ventricular functions.43 We also found that modulation of inflammation IL-10 therapy markedly improves EPCs survival and neovascularization in the border zone of the infarct. Interestingly, IL-10 dependent increase in EPCs survival and function is partially dependent on STAT3 signaling, at least in in vitro system.43 In addition, we also showed that differentiation of functional cardiac myocytes from pluripotent embryonic stem cells (ES) under the influence of cytokines and growth factors requires STAT3 activity. In this study use of selective STAT3 inhibitor, markedly reduced the leukemia inducing factor (LIF) and bone morphogenic protein 2 (BMP-2) induced cardiac myocytes differentiation from murine ES cells.44 In addition, SDF1/CXCR signaling is critical for the homing of progenitor cells from bone marrow to the heart for cardiac repair during heart failure.45 Recently it has been shown that inhibition of STAT3 (both pharmacological and genetic knockdown) significantly abolished the SDF1/CXCR1 mediated cardiac protection during ischemia/reperfusion injury.46 Another pertinent mechanism for cardio-protective action of STAT3 is by reducing stress-induced cell death in heart as apoptosis is one of the hallmarks of heart failure. Hydrogen peroxide (a potent cell death activator) treated neonatal rat ventricular myocytes (NRCM) showed reduced expression of phosphorylated STAT3. In isolated rat hearts, perfused with AG-490 (potent JAK1/STAT3 inhibitor) enhanced the number of apoptotic cardiac myocytes.47 The anti-apoptotic function of STAT3 was also confirmed in mice with a cardiac myocyte-specific deletion of STAT3. These mice showed higher ischemia/reperfusion-induced apoptosis compared with wild type control.17 Furthermore, the STAT3-dependent transcriptional upregulation of anti-apoptotic and cyto-protective proteins (Bcl-xL, Hsp70 and MnSOD) clearly demonstrated the critical role of STAT3 in cardiac myocytes survival.48,49
Although early activation of STAT3 is well established during ischemia/reperfusion (I/R), its transcriptional regulation is probably too slow a process to provide the immediate rescue from cell death during early minutes of reperfusion. Mitochondria plays significant role in the progression of apoptotic signals. Many recent studies have suggested that STAT3 activation is required to inhibit mitochondrial malfunctioning.26,50 Recently, STAT3 has been identified in cardiac myocyte mitochondria, and its pharmacological inhibition or genetic ablation impaired complex I respiration27,51 and calcium retention capacity.52 Conversely, a mitochondrial-targeted STAT3 overexpression in mice preserved complex I respiration during simulated ex vivo ischemia and reduced the formation of reactive oxygen species. These studies suggest that a pool of STAT3 resides in the mitochondria and try to inhibit ROS production by inhibition of complex I and II activity. Evidence for this notion comes from the study where cardiac myocytes specific overexpression of transcriptionally inactive mitochondrial STAT3 in transgenic mice hindered the ischemia-induced mitochondrial complete complex I and II activity, cytochrome C release and ROS production.50 Recent studies, using pharmacological inhibitors, have demonstrated that ischemic post-conditioning preserve mitochondrial complex 1 respiration both in vivo and in vitro.23 The exact phosphorylation site that is important for the improvement in mitochondrial function by STAT3 is not clear but may vary with species: In mouse cardiac myocyte mitochondria serine727 site,27,51 whereas both the serine727 and the tyrosine705 site in rat cardiac myocyte mitochondria52 and only the tyrosine705 site in pigs23 is deemed important. The increased STAT3 phosphorylation at tyrosine705 was associated not only with better preservation of complex 1 respiration but also with improved calcium retention capacity as a measure of mitochondrial permeability transition pore inhibition.53 The cardio-protective action of STAT3 is also partially dependent on the age of animals. In a recent study it has been demonstrated that the beneficial effects of ischemic post-conditioning is lost in aged animals and suggested that reduced STAT3 levels may be cause of this effect.54
Interestingly, in contrast to the cardio-protective nature of active STAT3, some recent studies also suggested that the un-phosphorylated STAT3 can induce adverse cardiac remodeling.55-57 During chronic stress the signals from AT1R leads to unregulated expression of STAT3 resulting in excessive accumulation of un-phosphorylated STAT3 into the nucleus where they can bind with target gene (such as p300/CBP) promoters thereby inducing genes that are involved in adverse heart function.55 It is believed that the normal mechanisms of STAT clearance from nucleus is impaired during chronic stress pathology and thus contribute toward the accumulation of U-STAT3. Moreover, U-STATs have been shown to partner with NFκB p65 and IRF1 in binding to different hybrid DNA elements. Hence, U-STAT3 may partner with different factors depending on the cell type and sequence context, thereby regulating constitutive gene expression both positively and negatively.56,57
JAK-STAT as Therapeutic Targets for Heart Diseases
There is significant discrepancy with regards to the role of STATs in the heart. Many investigators believe that STATs signaling might be detrimental and induce distractive signaling in heart, however, we and others published data indicating that STAT3 mediates cardio-protective signaling.30,36,50 STAT3 activation is important for facilitating protective effects in the heart such as compensatory hypertrophy or a reduction in apoptosis. Additionally, as mentioned above STAT3 activation is central for the cardio-protection by ischemic pre- and post-conditioning. Stimulation of STAT3 activity is expected to be beneficial during the transition from compensated hypertrophy to heart failure. Agonist induced activation of STAT signaling in the myocardium could be a potential strategy to induce cardio-protective signaling. However, STATs as transcriptional activators or co-activators control the transcription of many target gene and excessive activation of some genes might be detrimental. For example, IL-6 mediated excessive activation of iNOS gene increased STAT3-dependent nitric oxide synthesis and decreases cardiac contractility.58 In addition a consistent STAT3 activation could lead to malignant transformation.59,60 Therefore, the STAT3 activation in cardiovascular disease must be carefully controlled and well defined treatment strategies aiming for a balanced STAT3 signaling should be developed in order to protect the heart from pathophysiological stress.
In conclusion, STAT signaling is an important mechanism in the protection of cardiac cells from different stress. To understand the mechanisms and inhibit the transition of adaptive hypertrophy to maladaptive hypertrophy, STAT signaling could play an important role, as it gets activated during physiological hypertrophy and inhibited in chronic pathological conditions. Basic and translational studies should be focused toward understanding the factors/stimuli that regulate STAT activity at late stage of diseases. Another area of research that should make a significant stride in the next few years is to generate safer agonists and carrier molecules that can be given to patients with an end-stage disease condition to improve heart function.
Acknowledgments
Work described in this manuscript was in part supported by National Institute of Health grants HL091983, HL105597, HL095874, HL053354 and HL108795 to R.K.
Glossary
Abbreviations:
- STAT
signal transducer and activator of transcription
- JAK
Janus kinase
- SOCS
suppressors of cytokine signaling
- PIAS
protein inhibitor of STAT
- CNTF
ciliary neurotrophic factor
- LIF
leukemia inhibitory factor
- CT-1
cardiotrophin-like cytokine
- TAD
trans-activation domain
- TIMP-1
tissue inhibitor of metalloproteinase-1
- SDF1
stromal cell-derived factor-1
- CXCR
C-X-C chemokine receptor
- BMP
bone morphogenic protein
- CTGF
connective tissue growth factor
- VEGF
vascular endothelial growth factor
- EPCs
endothelial progenitor cells
- ES
endothelial cells
- TNF
tumor necrosis factor
- LPS
lipopolysaccharides
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/20115
References
- 1.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 2.Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zürcher G, Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991;11:2057–65. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilks AF. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989;86:1603–7. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heim MH. The Jak-STAT pathway: specific signal transduction from the cell membrane to the nucleus. Eur J Clin Invest. 1996;26:1–12. doi: 10.1046/j.1365-2362.1996.103248.x. [DOI] [PubMed] [Google Scholar]
- 5.Dostal DE, Hunt RA, Kule CE, Bhat GJ, Karoor V, McWhinney CD, et al. Molecular mechanisms of angiotensin II in modulating cardiac function: intracardiac effects and signal transduction pathways. J Mol Cell Cardiol. 1997;29:2893–902. doi: 10.1006/jmcc.1997.0524. [DOI] [PubMed] [Google Scholar]
- 6.Richard AJ, Stephens JM. Emerging roles of JAK-STAT signaling pathways in adipocytes. Trends Endocrinol Metab. 2011;22:325–32. doi: 10.1016/j.tem.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gough DJ, Levy DE, Johnstone RW, Clarke CJ. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev. 2008;19:383–94. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/S0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 9.Vinkemeier U, Cohen SL, Moarefi I, Chait BT, Kuriyan J, Darnell JE., Jr. DNA binding of in vitro activated Stat1 alpha, Stat1 beta and truncated Stat1: interaction between NH2-terminal domains stabilizes binding of two dimers to tandem DNA sites. EMBO J. 1996;15:5616–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Vinkemeier U, Moarefi I, Darnell JE, Jr., Kuriyan J. Structure of the amino-terminal protein interaction domain of STAT-4. Science. 1998;279:1048–52. doi: 10.1126/science.279.5353.1048. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Sun YL, Hoey T. Cooperative DNA binding and sequence-selective recognition conferred by the STAT amino-terminal domain. Science. 1996;273:794–7. doi: 10.1126/science.273.5276.794. [DOI] [PubMed] [Google Scholar]
- 12.Strehlow I, Schindler C. Amino-terminal signal transducer and activator of transcription (STAT) domains regulate nuclear translocation and STAT deactivation. J Biol Chem. 1998;273:28049–56. doi: 10.1074/jbc.273.43.28049. [DOI] [PubMed] [Google Scholar]
- 13.Zhang T, Kee WH, Seow KT, Fung W, Cao X. The coiled-coil domain of Stat3 is essential for its SH2 domain-mediated receptor binding and subsequent activation induced by epidermal growth factor and interleukin-6. Mol Cell Biol. 2000;20:7132–9. doi: 10.1128/MCB.20.19.7132-7139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begitt A, Meyer T, van Rossum M, Vinkemeier U. Nucleocytoplasmic translocation of Stat1 is regulated by a leucine-rich export signal in the coiled-coil domain. Proc Natl Acad Sci U S A. 2000;97:10418–23. doi: 10.1073/pnas.190318397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler C, Darnell JE., Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–51. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 16.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT axis. Basic Res Cardiol. 2007;102:393–411. doi: 10.1007/s00395-007-0674-z. [DOI] [PubMed] [Google Scholar]
- 17.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–95. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 18.Snyder M, Huang XY, Zhang JJ. Identification of novel direct Stat3 target genes for control of growth and differentiation. J Biol Chem. 2008;283:3791–8. doi: 10.1074/jbc.M706976200. [DOI] [PubMed] [Google Scholar]
- 19.Haspel RL, Salditt-Georgieff M, Darnell JE., Jr. The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase. EMBO J. 1996;15:6262–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Cooney RN. Suppressors of cytokine signaling (SOCS): inhibitors of the JAK/STAT pathway. Shock. 2002;17:83–90. doi: 10.1097/00024382-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008;79:127–33. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–8. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 24.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci U S A. 2001;98:9050–5. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–85. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Szczepanek K, Chen Q, Larner AC, Lesnefsky EJ. Cytoprotection by the modulation of mitochondrial electron transport chain: The emerging role of mitochondrial STAT3. Mitochondrion. 2012;12:180–9. doi: 10.1016/j.mito.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Booz GW, Day JN, Baker KM. Interplay between the cardiac renin angiotensin system and JAK-STAT signaling: role in cardiac hypertrophy, ischemia/reperfusion dysfunction, and heart failure. J Mol Cell Cardiol. 2002;34:1443–53. doi: 10.1006/jmcc.2002.2076. [DOI] [PubMed] [Google Scholar]
- 29.Pan J, Fukuda K, Kodama H, Makino S, Takahashi T, Sano M, et al. Role of angiotensin II in activation of the JAK/STAT pathway induced by acute pressure overload in the rat heart. Circ Res. 1997;81:611–7. doi: 10.1161/01.res.81.4.611. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. 2009;104:e9–18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peeler TC, Conrad KM, Baker KM. Endothelin stimulates sis-inducing factor-like DNA binding activity in CHO-K1 cells expressing ETA receptors. Biochem Biophys Res Commun. 1996;221:62–6. doi: 10.1006/bbrc.1996.0545. [DOI] [PubMed] [Google Scholar]
- 32.Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–50. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 33.Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, et al. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.CIR.0000057545.82749.FF. [DOI] [PubMed] [Google Scholar]
- 34.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A. 1997;94:3801–4. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci U S A. 2003;100:12929–34. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolli R, Stein AB, Guo Y, Wang OL, Rokosh G, Dawn B, et al. A murine model of inducible, cardiac-specific deletion of STAT3: its use to determine the role of STAT3 in the upregulation of cardioprotective proteins by ischemic preconditioning. J Mol Cell Cardiol. 2011;50:589–97. doi: 10.1016/j.yjmcc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367:97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wincewicz A, Sulkowska M, Rutkowski R, Sulkowski S, Musiatowicz B, Hirnle T, et al. STAT1 and STAT3 as intracellular regulators of vascular remodeling. Eur J Intern Med. 2007;18:267–71. doi: 10.1016/j.ejim.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Demyanets S, Kaun C, Rychli K, Pfaffenberger S, Kastl SP, Hohensinner PJ, et al. Oncostatin M-enhanced vascular endothelial growth factor expression in human vascular smooth muscle cells involves PI3K-, p38 MAPK-, Erk1/2- and STAT1/STAT3-dependent pathways and is attenuated by interferon-γ. Basic Res Cardiol. 2011;106:217–31. doi: 10.1007/s00395-010-0141-0. [DOI] [PubMed] [Google Scholar]
- 40.Osugi T, Oshima Y, Fujio Y, Funamoto M, Yamashita A, Negoro S, et al. Cardiac-specific activation of signal transducer and activator of transcription 3 promotes vascular formation in the heart. J Biol Chem. 2002;277:6676–81. doi: 10.1074/jbc.M108246200. [DOI] [PubMed] [Google Scholar]
- 41.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, et al. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109:1280–9. doi: 10.1161/CIRCRESAHA.111.248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajasingh J, Bord E, Hamada H, Lambers E, Qin G, Losordo DW, et al. STAT3-dependent mouse embryonic stem cell differentiation into cardiomyocytes: analysis of molecular signaling and therapeutic efficacy of cardiomyocyte precommitted mES transplantation in a mouse model of myocardial infarction. Circ Res. 2007;101:910–8. doi: 10.1161/CIRCRESAHA.107.156786. [DOI] [PubMed] [Google Scholar]
- 45.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang C, Gu H, Zhang W, Manukyan MC, Shou W, Wang M. SDF-1/CXCR4 mediates acute protection of cardiac function through myocardial STAT3 signaling following global ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;301:H1496–505. doi: 10.1152/ajpheart.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hattori R, Maulik N, Otani H, Zhu L, Cordis G, Engelman RM, et al. Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol. 2001;33:1929–36. doi: 10.1006/jmcc.2001.1456. [DOI] [PubMed] [Google Scholar]
- 48.Hilfiker-Kleiner D, Hilfiker A, Drexler H. Many good reasons to have STAT3 in the heart. Pharmacol Ther. 2005;107:131–7. doi: 10.1016/j.pharmthera.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi-Takihara K, Kishimoto T. A novel role for STAT3 in cardiac remodeling. Trends Cardiovasc Med. 2000;10:298–303. doi: 10.1016/S1050-1738(01)00066-4. [DOI] [PubMed] [Google Scholar]
- 50.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, et al. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011;286:29610–20. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu H, Lizano P, Laure L, Sui X, Rashed E, Park JY, et al. H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload. Circulation. 2011;124:406–15. doi: 10.1161/CIRCULATIONAHA.110.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–85. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151–4. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 54.Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, et al. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–5. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 55.Yue H, Li W, Desnoyer R, Karnik SS. Role of nuclear unphosphorylated STAT3 in angiotensin II type 1 receptor-induced cardiac hypertrophy. Cardiovasc Res. 2010;85:90–9. doi: 10.1093/cvr/cvp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida Y, Kumar A, Koyama Y, Peng H, Arman A, Boch JA, et al. Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique TRAF6- and p65-dependent mechanism. J Biol Chem. 2004;279:1768–76. doi: 10.1074/jbc.M311498200. [DOI] [PubMed] [Google Scholar]
- 58.Yu X, Kennedy RH, Liu SJ. JAK2/STAT3, not ERK1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes. J Biol Chem. 2003;278:16304–9. doi: 10.1074/jbc.M212321200. [DOI] [PubMed] [Google Scholar]
- 59.Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–22. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- 60.Madoux F, Koenig M, Nelson E, Chowdhury S, Cameron M, Mercer B, et al. Modulators of STAT Transcription Factors for the Targeted Therapy of Cancer (STAT3 Activators). Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD), 2010. [PubMed] [Google Scholar]