Abstract
How wonderful would it be if there were a simple, cheap, safe, non-invasive treatment that could be administered to a patient to protect their organs from ischemia and reperfusion? Such a treatment might be used to protect the organs during temporary loss of blood flow, as occurs for example during a heart attack or stroke. As unlikely as this may sound, such a treatment has indeed been discovered, although research into the mechanism is only just beginning. A recent paper by Heusch et al. in Circulation Research has taken the first step in this direction, as explained below.
Keywords: STAT5, ischemia, preconditioning, remote, reperfusion
The story begins in 1986 with studies of dogs undergoing experimental myocardial infarction (40 min of coronary artery occlusion to induce lethal myocardial ischemia, followed by reperfusion for 4 d). Murry et al. discovered by serendipity that the dogs’ myocardium could be protected if the same coronary artery was subjected beforehand to four 5 min cycles of nonlethal ischemia and reperfusion. This protocol, called “ischemic preconditioning” (IPC), paradoxically reduced the subsequent infarct size to 25% of that observed in untreated control hearts.1 Unfortunately, despite its robustness and its efficacy, surgical IPC is completely impractical for routine administration as a cardioprotective prophylactic. Consequently, a great deal of scientific effort has been expended in delineating the myocardial signaling pathways that are activated by IPC, in the hope of identifying a pharmacological mimetic of the phenomenon. Yellon and colleagues identified a centrally important kinase signaling network, commonly referred to as the reperfusion injury salvage kinase (or “RISK”) pathway2 that appears to be at the heart of the observed protection. This definition encompasses all kinases which are specifically activated at reperfusion and improve cardiomyocyte survival (Fig. 1). Although originally described solely in terms of the ERK1/2 MAPK and Akt/PI3K kinase pathways, the JAK-STAT signaling pathway has recently been shown to form a critical “third arm” of the RISK pathway (sometimes referred to as the SAFE pathway).3,4
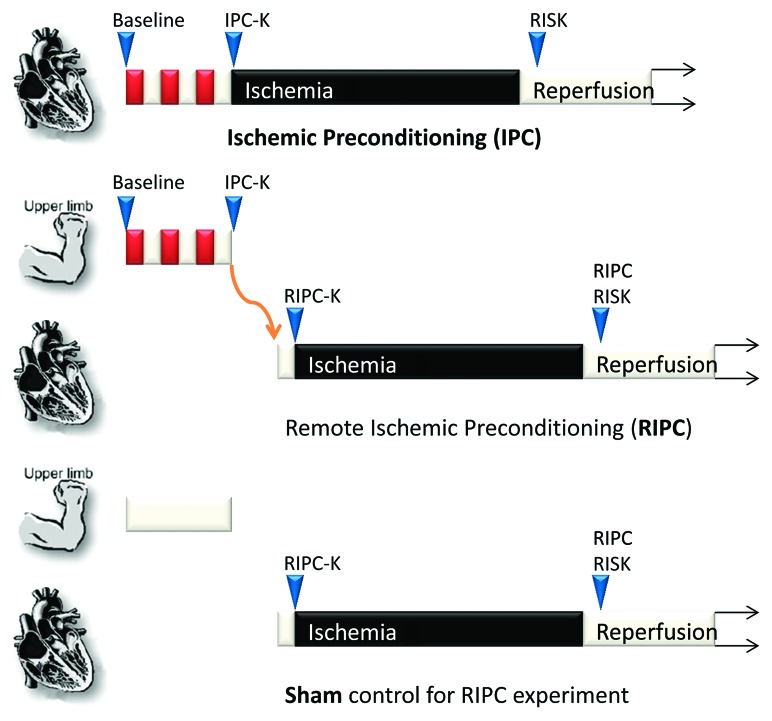
Figure 1. Ischemic preconditioning (IPC) involves repeated cycles of ischemia (red) and reperfusion (white) being applied directly to the target organ such as the heart, in order to protect it from an extended period of ischemia and reperfusion. A distinction is made between those kinases activated after IPC (IPC-K) and early during reperfusion (RISK). Remote ischemic preconditioning (RIPC) involves an additional step whereby protection is transmitted via a neuro-hormonal mechanism from the preconditioned limb to the heart. Here, a further distinction must be made between those kinases activated directly in the target organ by RIPC (RIPC-K), and the RISK kinases (RIPC RISK). In sham controls, no RIPC stimulus is applied.
It has proven relatively straightforward to identify compounds which activate these kinases in isolated perfused heart preparation and which induce cardioprotection, even in animal models. For example, insulin the canonical activator of Akt/PI3K, is a highly effective IPC mimetic,5 as is leptin, via JAK-STAT.6 However translation of experimental cardioprotective pharmacological agents into clinically detectable benefits in humans has been disappointing, with one of the limitations being the undesirable side-effects of the drugs (e.g., the blood glucose lowering effect of insulin), but also questions about efficacy. There is also a lingering suspicion that the polypharmaceutical nature of IPC will always be more effective than single pharmacological agents.
Then, in 1997 the remarkable discovery was made that instead of it being necessary to apply the preconditioning stimulus directly to the target organ, it could be applied to a “remote” organ, and the conditioned status would also be conferred upon the heart. The first demonstration of “IPC at a distance” was in rabbits.7 The technique was subsequently refined with the simple application of a tourniquet to the hind limb of a rat shown to reduce reperfusion arrhythmias.8 In 2002, the effectiveness of remote ischemic preconditioning (RIPC) in human volunteers was demonstrated using a blood pressure cuff placed on the upper arm to apply three 5 min cycles of alternating ischemia and reperfusion to the skeletal muscle of the forearm.9 This was sufficient to attenuate endothelial dysfunction due to ischemia and reperfusion injury in the contralateral limb.9 In a pig model, a similar RIPC protocol decreased the injury of experimental myocardial infarction by 51%.9
It is more problematic to find an opportunity to study cardioprotection in human hearts, but there are rare occasions when a heart must be deliberately stopped, which could allow for the testing of protective strategies and further allow a glimpse into their biochemical mechanisms. One such occasion is during coronary artery bypass (CABG) surgery, when the heart may be deliberately arrested for several hours before normal blood-flow is resumed. Despite all the protective measures in place during CABG surgery there is always an amount of what is termed peri-procedural (or peri-operative) injury that occurs and which can be quantified by measuring myocardial proteins released from dead cardiomyocytes into the circulation, e.g., troponin T or I. Several small, randomized trials have demonstrated that RIPC effectively decreases the extent of cardiac injury during cardiopulmonary bypass,10-12 and these positive results have been replicated in the recent paper by Heusch et al.13 To date, only secondary markers of cardiac injury have been evaluated, but larger clinical outcome studies are underway, and the results are awaited with great anticipation.14
With regular IPC, kinase activation is required at two time-points: during the actual IPC stimulus and during reperfusion (the “RISK” pathway) (Fig. 1). Activation of these kinases at reperfusion is believed to be the most relevant to conditioning, since their inhibition eliminates IPC.2 In the case of RIPC, analysis is further complicated since signaling initiates in the source organ, and is transmitted to the recipient organ. Transmission appears to involve both a neurally transmitted mechanism, as well as a peptide factor that is transmitted via the circulation.2,15 Given the superficial similarity between RIPC and IPC procedures, they might be expected to activate the same reperfusion injury kinases. On the other hand, it would be exciting if RIPC was found to activate a different kinase pathway from IPC, as this might reveal a new pharmacological target for cardioprotection.
The recent study by Heusch et al.13 investigated a large panel of kinase pathways in order to identify one which fit the RIPC “RISK profile,” i.e., that the pathway’s activity should be increased at reperfusion after prior RIPC, but not activated after a sham control procedure. The authors took biopsies of human hearts immediately after the control or RIPC procedure (point “RIPC-K” in Fig. 1) and from the same patients after ischemia and 10 min into reperfusion (point “RIPC RISK”). Twelve of the 24 patients received prior RIPC stimulus. The researchers then examined a panel of phosphorylation sites of candidate kinases and kinase targets previously implicated in IPC, including PKCα, PKCε, Src, JNK1, JNK2/3, H11/HSP22, VASP, eNOSthr, eNOSser, Akt, Erk1/2, p70S6K, GSK3β, JAK2, STAT1tyr, STAT3tyr, STAT3ser and STAT5tyr. All of these phospho sites are known to be strongly correlated with kinase activity. The first thing to note is that there were no differences in phosphorylation at baseline13 (i.e., at point “RIPC-K” in Fig. 1), suggesting that the factor mediating RIPC transmission does not activate these kinases in the target organ directly, (or alternatively that activation is transient and has returned to baseline in the intervening time between RIPC administration and tissue biopsy). Similar results have been obtained in the rat.16,17 More importantly, a significant increase in phosphorylated Akt, Erk, p70S6K, STAT1 and STAT3 was seen at reperfusion vs baseline, but surprisingly, the increase was the same in those patients to whom no RIPC protocol had been applied13 (Fig. 2). This suggests that, though they may be required for protection, they are certainly not sufficient.

Figure 2. A summary of the proteins with increased phosphorylation levels detected by Heusch et al. in biopsies of human hearts that had been subjected to sham or RIPC procedures before coronary artery bypass operations. Only STAT5A and STAT5B fit the profile of differential activation.
In contrast, among nearly 20 phosphorylation sites, the only one matching the “risk profile” (i.e., activated at reperfusion after prior RIPC, but not after the sham control procedure), was STAT513 (Fig. 2). Consequently, STAT5 phosphorylation may be part of the RISK pathway after RIPC in humans. Conclusive demonstration would require specific inhibition of STAT5, experiments that are not possible to perform in humans. For these, we must rely instead on animal experiments, and to date the role of the JAK-STAT pathway has been investigated only in direct IPC and not in RIPC. As it turns out, IPC is prevented by perfusion of rat or mouse hearts with the JAK-STAT inhibitor, AG490.18,19 Although AG490 is quite specific, it inhibits both JAK2 and JAK320 leading to inhibition of all cardiac isoforms of STAT21 and therefore cannot be used to examine STAT5 alone. Indeed, there is evidence for opposing effects of different STAT isoforms, with STAT3 activation protecting against cardiac ischemia and reperfusion injury while STAT1 activating cell death pathways.22 Experiments with knockout mice allow a more nuanced investigation of the role of specific STAT isoforms. These have suggested that hearts from STAT5A knockouts are unable to be preconditioned,21 although neither are hearts lacking STAT3.23,24 In contrast, hearts of STAT6 knockout mice remained amenable to IPC.21
Interestingly, the Akt inhibitor wortmanin when administered to pigs eliminated RIPC-induced cardioprotection against ischemia and reperfusion injury.25 Furthermore, increased Akt phosphorylation was measured in myocardial biopsies taken after cardiac reperfusion.25 This suggests that there may be differences in which kinase pathways are recruited by RIPC in different species, as has been suggested to be the case for a variant form of IPC called “postconditioning” (which is administered during early reperfusion).26 Other experimenters have administered the MAPK inhibitors SB203580 (p38), PD98059 (ERK1/2) or SP600125 (JNK1/2) to rats, but as they were injected prior to the application of RIPC they may have acted on both the remote organ (the limb) and the target organ (the heart) at any point in Figure 1, and results are more difficult to interpret.16 It is highly likely that there is a degree of cross-talk between the PI3K/Akt and JAK-STAT pathways. In fact, when RIPC is replicated in vitro by the transfer of coronary effluent from preconditioned rat hearts to naïve hearts, both PI3K/Akt and JAK-STAT pathways are involved.27,28
What might the mechanism of JAK-STAT cardioprotection be? Given the timescale, gene transcription is not likely to be involved, thereby implying a role for STAT independent of transcriptional regulation. A primary end-target of RISK pathways appears to be the mitochondria,29,30 and STAT3 has recently been detected in the mitochondria.31 Heusch et al. have previously shown that in pigs, STAT3 is activated in hearts subject to postconditioning, and is required for preservation of mitochondrial function.32 Intracellular changes in STAT3 localization would have been missed in the current study which looked at total cellular levels. As mentioned in their discussion, other proteins such as PKC and Src are also regulated by intracellular localization and would have escaped notice. Other caveats to keep in mind are that the sample size (12 per group) was relatively small, and may not have been sufficiently powered to detect small differences in a heterogeneous patient population. Furthermore, only one time-point was examined, so transient changes in phosphorylation may have been missed.
The revelation of STAT5 as an important target for human cardioprotection suggests further work should focus on ways to specifically and safely target STAT5 pharmacologically. Intriguingly, several GPCR ligands are known to preferentially activate STAT5 over other STATs, including erythropoietin,33 prolactin, IL-3, IL-5 and GM-CSF, and a number of these have been shown to be cardioprotective. STAT5 inhibitors would also be useful for investigating the role of STAT5 in cardioprotection, but transgenic mice with cardiac-restricted deletion or overexpression will be even more useful. The other approach that will be interesting to take is to “reverse engineer” RIPC, by trying to identify the factor that activates STAT5 in humans. If this factor can be purified then we may finally find the wanted peptide matching the newly identified RISK profile.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/20072
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–86. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 3.Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croué A, Henrion D, et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011;106:1329–39. doi: 10.1007/s00395-011-0210-z. [DOI] [PubMed] [Google Scholar]
- 5.Jonassen AK, Sack MN, Mjøs OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–8. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 6.Smith CC, Dixon RA, Wynne AM, Theodorou L, Ong SG, Subrayan S, et al. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2010;299:H1265–70. doi: 10.1152/ajpheart.00092.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–6. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 8.Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol. 1997;273:H1707–12. doi: 10.1152/ajpheart.1997.273.4.H1707. [DOI] [PubMed] [Google Scholar]
- 9.Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–3. doi: 10.1161/01.CIR.0000043806.51912.9B. [DOI] [PubMed] [Google Scholar]
- 10.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–9. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 11.Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–71. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- 12.Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–64. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- 13.Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res. 2012;110:111–5. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy DJ, Candilio L, Laing C, Kunst G, Pepper J, Kolvekar S, et al. The ERICCA Trial Investigators Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol. 2012;101:339–48. doi: 10.1007/s00392-011-0397-x. [DOI] [PubMed] [Google Scholar]
- 15.Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–5. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- 16.Heidbreder M, Naumann A, Tempel K, Dominiak P, Dendorfer A. Remote vs. ischaemic preconditioning: the differential role of mitogen-activated protein kinase pathways. Cardiovasc Res. 2008;78:108–15. doi: 10.1093/cvr/cvm114. [DOI] [PubMed] [Google Scholar]
- 17.Heinen NM, Pütz VE, Görgens JI, Huhn R, Grüber Y, Barthuber C, et al. Cardioprotection by remote ischemic preconditioning exhibits a signaling pattern different from local ischemic preconditioning. Shock. 2011;36:45–53. doi: 10.1097/SHK.0b013e31821d8e77. [DOI] [PubMed] [Google Scholar]
- 18.Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008;79:127–33. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 19.Hattori R, Maulik N, Otani H, Zhu L, Cordis G, Engelman RM, et al. Role of STAT3 in ischemic preconditioning. J Mol Cell Cardiol. 2001;33:1929–36. doi: 10.1006/jmcc.2001.1456. [DOI] [PubMed] [Google Scholar]
- 20.Wang LH, Kirken RA, Erwin RA, Yu CR, Farrar WL. JAK3, STAT, and MAPK signaling pathways as novel molecular targets for the tyrphostin AG-490 regulation of IL-2-mediated T cell response. J Immunol. 1999;162:3897–904. [PubMed] [Google Scholar]
- 21.Yamaura G, Turoczi T, Yamamoto F, Siddqui MA, Maulik N, Das DK. STAT signaling in ischemic heart: a role of STAT5A in ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H476–82. doi: 10.1152/ajpheart.00079.2003. [DOI] [PubMed] [Google Scholar]
- 22.Stephanou A. Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med. 2004;8:519–25. doi: 10.1111/j.1582-4934.2004.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, et al. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res. 2004;63:611–6. doi: 10.1016/j.cardiores.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Goodman MD, Koch SE, Afzal MR, Butler KL. STAT subtype specificity and ischemic preconditioning in mice: is STAT-3 enough? Am J Physiol Heart Circ Physiol. 2011;300:H522–6. doi: 10.1152/ajpheart.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, et al. Investigating the Signal Transduction Pathways Underlying Remote Ischemic Conditioning in the Porcine Heart. Cardiovasc Drugs Ther. 2012;26:87–93. doi: 10.1007/s10557-011-6364-y. [DOI] [PubMed] [Google Scholar]
- 26.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, et al. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–8. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 27.Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135–45. doi: 10.1007/s00395-010-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huffman LC, Koch SE, Butler KL. Coronary effluent from a preconditioned heart activates the JAK-STAT pathway and induces cardioprotection in a donor heart. Am J Physiol Heart Circ Physiol. 2008;294:H257–62. doi: 10.1152/ajpheart.00769.2007. [DOI] [PubMed] [Google Scholar]
- 29.Davidson SM, Hausenloy D, Duchen MR, Yellon DM. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol. 2006;38:414–9. doi: 10.1016/j.biocel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–85. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–8. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 33.Marzo F, Lavorgna A, Coluzzi G, Santucci E, Tarantino F, Rio T, et al. Erythropoietin in heart and vessels: focus on transcription and signalling pathways. J Thromb Thrombolysis. 2008;26:183–7. doi: 10.1007/s11239-008-0212-3. [DOI] [PubMed] [Google Scholar]