Abstract
Th17 cells are important mediators of autoimmunity, yet the mechanisms by which they are controlled are not fully understood. Studies in mice, including a recent article in Nature Immunology by Yang et al., show that IL-2 is an important inhibitory factor for the differentiation of Th17 cells, inducing phosphorylation of STAT5, which outcompetes STAT3 binding at the IL-17 locus. In humans however, IL-2 appears to be crucial for Th17 differentiation, yet inhibits the expansion of antigen-specific Th17 clones, again via a STAT5 mechanism. Here we discuss how the article by Yang et al. offers a novel mechanism to explain how changes in the balance of different cytokines in the inflammatory environment may alter the stability or phenotype of regulatory T cells and T helper cell subsets.
Keywords: Foxp3, IL-17, STAT3, STAT5, Th17
It is widely accepted that T helper (Th) 1 cells and perhaps more so, Th17 cells are the key mediators of T cell pathogenesis in autoimmune disorders including multiple sclerosis, rheumatoid arthritis and colitis. When helper T cell subsets were first described, they were considered to be mutually exclusive. In the presence of IL-12, naïve T cells were shown to express STAT4 and T-bet and produce IFNγ. Th2 cells could be induced in the presence of IL-4 via the upregulation of GATA-3 and STAT6.1 Moreover, the ectopic expression of T-bet in polarized Th2 cells resulted in the production of IFNγ and the suppression of Th2 cytokines.2 Later, the discovery of Th17 cells expressing STAT3 and RORγt also appeared to demonstrate a phenotype distinct from Th1 or Th2 cells.3
The first studies to question this distinct irreversible fate involved CD4+ regulatory T cells (Tregs). Production of TGF-β in the absence of inflammation led to the development of Tregs; however in the presence of inflammatory cytokines such as IL-6 and IL-1β the differentiation of Th17 cells dominated.4 This suggested that the Th17 phenotype was particularly sensitive to environmental cues. Indeed recent literature has identified cells producing both IL-17 and cytokines associated with other T helper cell subsets. These Th17 “dual producers” are seen most frequently in inflammatory disease settings, such as autoimmunity5 or allergy.6 Moreover, the adoptive transfer of Th17 cells to lymphopenic mice drives the conversion of Th17 cells to IFNγ producers and triggers an IFNγ-mediated diabetes.7
For many years, it has been recognized that IL-2 plays a key role, not only in the expansion and proliferation of T cells, but also in the development and function of Tregs. In 2007, a new role for IL-2 was reported: its ability to inhibit Th17 differentiation.8 A more recent publication by Yang et al. investigated the mechanism of this inhibition.9
Mice deficient in IL-2 develop inflammatory colitis associated with an increase in IL-17 and IL-22 production by CD4+ T cells. IL-6 and STAT3 signals are key for the induction of Th17 cells, therefore Yang et al. bred IL-2 deficient mice that lack STAT3 expression in the CD4+ T cell compartment. The lack of IL-2 combined with the loss of STAT3 led to a significantly prolonged lifespan and reduced colitis in these mice, which was associated with a decrease in the proportions of IL-17 and IL-22 producing CD4+ T cells. Interestingly, this control of Th17 differentiation was not dependent on the expression of Foxp3 as CD4+Foxp3+ regulatory T cells were reduced in IL-2 deficient mice, but their numbers were not restored in the IL-2/STAT3 deficient mice. Furthermore, the in vitro differentiation of Th17 cells was reduced by addition of IL-2 to CD4+ T cells derived from WT mice or scurfy mice that carry a mutated Foxp3 gene. This may not be a surprising result as despite the pathogenicity of Th17 cells, several groups have reported that they cannot be restrained by naturally occurring CD4+Foxp3+ Tregs.10-14
Although IL-2 inhibits the production of IL-17 and IL-22 by CD4+ T cells, the expression of the Th17 transcription factor RORγt was only slightly reduced. This suggests IL-2 does not mediate its suppressive effect through the inhibition of RORγt expression, and indeed, overexpression of RORγt by CD4+ T cells did not prevent the capacity of IL-2 to suppress Th17 differentiation.
STAT3 promotes the production of IL-17 by binding to specific sites within the IL-17 a-f locus. Yang et al. show that STAT5 can compete for these STAT3 binding sites. This means that in the presence of IL-2, STAT5 binding to the IL-17 locus prevents the binding of STAT3 and its enhancer elements, inhibiting production IL-17. In addition, STAT5 can modify the IL-17 locus, altering the acetylation of specific histones, partly through the recruitment of NCOR2, a histone deacetylator adaptor protein.
The ability of IL-2 to inhibit Th17 differentiation through the STAT5-mediated displacement of STAT3 from the IL-17 locus is an exciting finding. Recently, STAT5 has also been shown to negatively regulate the development of T follicular helper cells (TFH). TFH cells are crucial for the development of germinal centers and expansion of their numbers has been associated with the development of autoantibodies in a lupus model.15 Similar to Th17 cells, TFH cell development is dependent on IL-6 and STAT3, but also requires IL-21 and expression of Bcl-6. Two studies now reveal that in response to IL-2, STAT5 signals result in the upregulation of Blimp-1, leading to a repression of Bcl-6 and a reduced development of TFH cells.16,17 It would be interesting to discover whether STAT3 binds regions of the Bcl-6 locus and determine whether repression of TFH cell development is only via the active production of Blimp-1 or due to competition between STAT3 and STAT5 for binding sites in the Bcl-6 gene locus.
Many genes are now known to be regulated by more than one STAT. It is interesting however that STATs can act, not only as promoters of gene expression, but also as repressors via the displacement of activating STATs and the recruitment of histone deacetylases. As Yang et al. describe, the cytokine environment and the specific gene locus both contribute to whether the gene is transcribed or repressed. For example, when there is an environment of low IL-2 but high IL-6, STAT3 signals dominate and IL-17A and IL-17F are produced. However in an environment of high IL-2 and high IL-6, STAT5 maintains the capacity to act as a repressor for IL-17A. In contrast, under these conditions the displacement of STAT3 by STAT5 at the IL-17F locus is prevented.
This novel idea, that one STAT can displace another, raises the question of whether this is a specific property of STAT3 and STAT5 competition, or if other STATs also possess this function? As previously discussed, it has been known for many years that the cytokine environment can influence the development of T helper cell subsets. There is however, increasing interest in the propensity of T cells to switch from one lineage to another and this is particularly relevant to regulatory T cell stability. One might propose that STATs play a role in the switching of Tregs into Th17 cells or Th17 to Th1 cells if the balance of cytokines change in the environment, such as that during inflammatory disease (Fig. 1). It has been known for many years that the control of Th17 differentiation can be dependent on Th1 cells and the production of IFNγ. Indeed, IFNγ can suppress the production of IL-17 by the activation of STAT1 and the upregulation of SOCS1 and SOCS3.18 Furthermore, in an inflammatory environment with the balance of IL-2 and pro-inflammatory cytokines altered, regulatory T cells could convert to Th17 cells (Fig. 1).
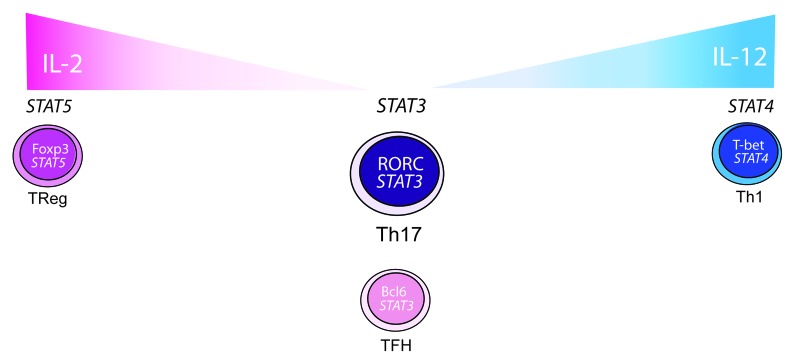
Figure 1. Do STATs control T cell plasticity? In murine and human cells, STAT3 binds to the IL-17 locus in T cells promoting the production of IL-17 and the differentiation of the Th17 subtype. STAT3 is also important for the differentiation and sustained phenotype of TFH cells, characterized by their expression of Bcl6. The cytokine environment can however influence the differentiation of T cells. For example, in the mouse, increasing amounts of IL-2 induces production of STAT5 that can displace STAT3 from the IL-17 locus, inhibiting IL-17 production and Th17 cell differentiation. TFH cells are also susceptible to increasing amounts of IL-2, resulting in the induction of STAT5, downregulation of Bcl6 and inhibition of TFH cell differentiation. IL-2 and STAT5 signals are required for the Treg phenotype; however, Tregs have been reported to produce IL-17 in some inflammatory diseases. It may therefore be possible that in highly inflammatory environments, Tregs become unstable and STAT5 can no longer actively displace STAT3 allowing Tregs to make IL-17. In humans, IL-2 can have positive effects on the differentiation of Th17 cells and the switching of Treg cells into Th17 cells. Production of IL-17 by re-stimulated Th17 cells can be inhibited by IL-2 and STAT5 signals. Using the same model, it is possible that the ability of Th17 cells to covert to IFNγ producers could stem from IL-12 driven STAT4 expression. If STAT4 can prevent the binding of STAT3 to the IL-17 locus, it may inhibit production of IL-17, while promoting production of IFNγ allowing the Th17 cell to switch to a Th1 phenotype.
In humans however, the role of IL-2 in Th17 differentiation may differ to that in mice. IL-2 in combination with IL-1β, IL-23 and TGF-β differentiates human Th17 cells from naïve CD4+ T cells19 and addition of IL-2 to healthy PBMC can enhance IL-17 production by memory CD4+ T cells18. These studies suggest a positive role for IL-2 in the differentiation and expansion of human Th17 cells. In contrast, IL-2 driven phosphorylation of STAT5 was shown to mediate a reduction in IL-17 production and RORγt expression in re-stimulated human Th17 clones differentiated in the presence of microbial antigens.20
T cell activation in the presence of IL-2 is key however for IL-1β, IL-23 and TGF-β to mediate the switching of a small proportion of human naïve Tregs into IL-17 producing T cells.21 The role of IL-2 in Th17 differentiation and expansion is not completely understood, but whether IL-2 is simply required as an early signal to stimulate naïve T cells and thus permit responsiveness to cytokines that drive a Th17 phenotype, or whether the mechanism of Th17 lineage decision is indeed different between mice and humans will be interesting to explore in the future. What is becoming apparent from recent studies is that T helper cell differentiation and T cell switching is dependent on a balance between different cytokines in the environment, be it IL-2 and IL-6 or IL-1β and IL-12, rather than the presence or absence of particular cytokines and the signals they induce.
In conclusion, identifying the role of STATs in the activation and repression of specific genes in humans could be vital to understanding how inflammatory cell populations can be regulated and how the stability of different T helper and Treg populations can be controlled and manipulated in a disease setting.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/20409
References
- 1.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 2.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 3.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 5.Suryani S, Sutton I. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J Neuroimmunol. 2007;183:96–103. doi: 10.1016/j.jneuroim.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–30, e1-4. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Bending D, De la Peña H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–72. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Yang X-P, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–54. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105:19396–401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106:8635–40. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104:17034–9. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–65. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 16.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–50. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, et al. STAT5 negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. 2012;287:11234–9. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amadi-Obi A, Yu C-R, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 19.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human T(H)17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–8. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 21.Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORγt+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010;107:19402–7. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]


