Abstract
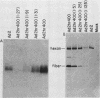
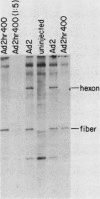
In monkey cells abortively infected with human adenovirus serotype 2, the synthesis of the fiber polypeptide of the virion capsid is reduced by at least a factor of 100 when compared with that in monkey cells productively infected with a host range mutant of adenovirus serotype 2 (Ad2hr400). However, the steady-state level of fiber-encoding mRNA present in abortively infected monkey cells is only reduced by a factor of 5 to 10. When mRNA isolated from abortively and productively infected monkey cells was microinjected into the cytoplasms of uninfected or abortively infected monkey cells, no differences in the efficiency of translation of the fiber messages from these two sources were observed. These results suggest that the block to synthesis of the fiber polypeptide in abortively infected monkey cells does not reside in the translational machinery of the abortively infected cells themselves but may involve compartmentalization of the fiber message within the cells or an altered processing of the fiber message which prevents correct presentation to the ribosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W. Spontaneous mutants of the adenovirus-simian virus 40 hybrid, Ad2+ND3, that grow efficiently in monkey cells. Virology. 1981 May;111(1):263–269. doi: 10.1016/0042-6822(81)90670-x. [DOI] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Altered mRNA splicing in monkey cells abortively infected with human adenovirus may be responsible for inefficient synthesis of the virion fiber polypeptide. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4023–4027. doi: 10.1073/pnas.81.13.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Posttranscriptional block to synthesis of a human adenovirus capsid protein in abortively infected monkey cells. J Mol Appl Genet. 1983;2(1):31–43. [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Miller M. R., Lehtomaki D. M., Pardee A. B. Comparison of DNA replication and repair enzymology using permeabilized baby hamster kidney cells. J Biol Chem. 1979 Aug 10;254(15):6904–6908. [PubMed] [Google Scholar]
- Flint S. J., Sharp P. A. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J Mol Biol. 1976 Sep 25;106(3):749–774. doi: 10.1016/0022-2836(76)90263-1. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Anderson C. W. Block to multiplication of adenovirus serotype 2 in monkey cells. J Virol. 1975 Dec;16(6):1650–1668. doi: 10.1128/jvi.16.6.1650-1668.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F., Chow L. T. Incomplete splicing and deficient accumulation of the fiber messenger RNA in monkey cells infected by human adenovirus type 2. J Mol Biol. 1980 May 15;139(2):221–242. doi: 10.1016/0022-2836(80)90306-x. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979 Aug;17(4):957–966. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Klessig D. F. Isolation of a variant of human adenovirus serotype 2 that multiplies efficiently on monkey cells. J Virol. 1977 Mar;21(3):1243–1246. doi: 10.1128/jvi.21.3.1243-1246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick J. J., Ju G., Skalka A. M., Stacey D. W. Biological activity of cloned retroviral DNA in microinjected cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4383–4387. doi: 10.1073/pnas.78.7.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Klessig D. F. Normal translation of human adenovirus mRNA in cell-free lysates prepared from abortively as well as productively infected monkey cells. J Virol. 1982 Oct;44(1):426–430. doi: 10.1128/jvi.44.1.426-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Anderson C. W. Translation of adenovirus 2 late mRNAs microinjected into cultured African green monkey kidney cells. J Virol. 1984 Aug;51(2):559–562. doi: 10.1128/jvi.51.2.559-562.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Westphal H. A cascade of adenovirus early functions is required for expression of adeno-associated virus. Cell. 1981 Nov;27(1 Pt 2):133–141. doi: 10.1016/0092-8674(81)90367-6. [DOI] [PubMed] [Google Scholar]
- Zorn G. A., Anderson C. W. Adenovirus type 2 expresses fiber in monkey-human hybrids and reconstructed cells. J Virol. 1981 Feb;37(2):759–769. doi: 10.1128/jvi.37.2.759-769.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]