Abstract
High density lipoprotein (HDL) cholesterol has beneficial effects beyond its atheroprotective function in reverse cholesterol transport, including cardioprotection against ischemia reperfusion (IR) injuries. Two major constituents of HDL, namely the structural protein apolipoprotein AI (apoAI) and the sphingolipid sphingosine-1-phosphate (S1P) appear to contribute to this cardioprotective effect via the activation of intrinsic prosurvival signaling pathways that still remain to be clarified.
Recently, a powerful prosurvival signaling pathway, termed the survivor activating factor enhancement (SAFE) pathway, which involves the activation of signal transducer and activator of transcription 3 (STAT3) and tumor necrosis factor α (TNF), has been shown to protect against ischemia-reperfusion injuries.
The present review summarizes the evidence for the roles of HDL and S1P in cardioprotection and discusses the signaling pathways that have been implicated. It thus provides support for our contention that S1P should be considered in potential formulations of reconstituted HDL (reHDL) that may be tested for cardioprotection against coronary artery disease via the activation of the SAFE pathway.
Keywords: HDL, SAFE pathway, TNF, ischemia, reperfusion injuries, sphingolipids
Introduction
Cardiovascular disease (CVD) is projected to be the leading cause of worldwide mortality by 2020, with patients mainly affected by ischemic heart disease.1,2
High density lipoprotein (HDL) is one of the three principal serum macromolecular protein-lipid complexes. Its quantitatively major components are phospholipids, cholesterol and the structural peptide, apolipoprotein (apo) AI, but there are numerous other lipids and peptides associated with the lipoprotein. Albeit of minor concentration, the latter appear to contribute to the functioning of HDL [a prime example being sphinogosine-1-phosphate (S1P), the focus of this review]. As HDL exist as discrete spherical particles, the heterogeneous distribution of these minor lipid and peptide components across the particles is suggested to underlie functional heterogeneity between the particles. For several decades, the principal clinical and vascular interest of HDL has been their well-established strong negative correlation with risk of atherosclerotic disease.3 It is thought to reflect their ability to remove cholesterol from the blood vessel wall and transport it to the liver for biliary excretion. With the growing realization of the compositional heterogeneity of HDL allied to demonstrations of other, beneficial influences on the vasculature [anti-apoptotic, anti-inflammatory, anti-oxidant, anti-thrombotic, protection against ischemia reperfusion injury (IR)4], it is presently thought that the lipoprotein plays a much more extensive role in cardioprotection.
New mechanisms involved in HDL-induced cardioprotection are presently a subject of particular interest, with recent studies suggesting that HDL are capable of influencing a number of intracellular prosurvival signaling pathways. Recently, a powerful prosurvival signaling pathway, named as the survivor activating factor enhancement (SAFE) pathway, has been demonstrated to protect the heart against stress situations.5 This pathway involves the activation of cytokine tumor necrosis factor α (TNF) and the transcription factor signal transducer and activator of transcription 3 (STAT3).6 The SAFE path was initially discovered as a protective signaling pathway activated by ischemic pre- and post-conditioning.7,8 Recent data strongly suggest that HDL, principally the constituent S1P, protect against injury during IR via the activation of the SAFE pathway.
The present review looks at the cardioprotective role of HDL with specific attention to its protective role against IR injury. The delineation of the main constituents of HDL involved in this effect (with particular emphasis on S1P) and the understanding of the prosurvival signaling pathways (in particular the SAFE pathway) activated by HDL and S1P may lead to the development of reconstituted HDL (reHDL) of defined composition as a novel therapy against ischemic heart disease.
HDL and Cardioprotection
The beneficial effect of HDL on IR was first reported in an isolated rat heart model where treatment with HDL, given during the ischemic period, reduced post-ischemic arrhythmias.9 Similarly, HDL perfused for 10 min immediately before the ischemic period improved left ventricular developed pressure (LVDP), decreased the coronary perfusion pressure (CPP) and creatine kinase (CK) release, concomitant with a decrease of the myocardial content of TNF and an increase in prostaglandins.10 In isolated cardiomyocytes, HDL limited the apoptosis of hypoxia-reoxygenation11 and doxorubicin-induced cardiotoxicity.12 HDL, given in vivo prior to the ischemic insult, improved the perfusion and reduced infarct size, neutrophil infiltration and apoptosis.13,14 In humans, HDL reduced the risk and extent of percutaneous coronary intervention (PCI)-related myocardial infarction and improved long-term outcome in patients undergoing elective PCI.15
Role of S1P and ApoA1 in HDL-Induced Cardioprotection
The complex role of HDL mirrors the complexity of its composition.4,16 Different components of HDL, including the structural peptide apoAI and the lipid component S1P, are thought to act as key players in HDL-induced cardioprotection4,17 after binding to their specific cell surface receptors, namely the receptor scavenger receptor B1 (apoAI) and the S1P receptor family (S1PR).
ApoAI and cardioprotection against IR
Several studies have reported that treatment with reHDL containing apoAI, apoAI Milano or apoAI mimetic protects the heart against IR injury.18-21
Perfusion of reHDL containing apoAI protects the isolated rat heart subjected to an IR insult, with a beneficial effect on myocardial function comparable to that of human HDL (increase in LVDP, decrease in CPP and CK release and modulation of TNF and prostaglandin release).18 Of note, post-ischemic treatment with reHDL containing apoAI showed lesser improvement in cardiac function than pre-ischemic treatment. This cardioprotective effect of apoAI was confirmed with reHDL containing apoAI mimetic peptide in both isolated rat and rabbit heart models of IR injury.19,21 In vivo, treatment with reHDL weekly for 4 weeks after permanent ligation of the left coronary artery prevented left ventricular remodeling and improved myocardial function after myocardial infarction.22 In addition to the protection of myocardial function, treatment with apoAI and apoAI mimetic peptide was associated with a reduction of the endothelial inflammatory response.21,23
An antiarrhythmogenic effect during IR was observed with reHDL in vivo, whereas apoAI alone did not significantly reduce the duration of ventricular tachycardia and ventricular fibrillation at reperfusion.24 This protective effect was inhibited by specific inhibitors of Akt, nitric oxide (NO) or extracellular signal-regulated kinase 1/2 (ERK1/2).24
The use of reHDL containing apoAI (15–80 mg/kg, but principally 80 mg/kg) has been extended to humans in limited pilot studies. Improvement in atherogenic parameters was observed. Thus, infusion of reHDL for a 5 week period provoked a change in atherosclerotic plaque morphology and/or reduction of plaque volume, as analyzed by intravascular ultrasound.25,26 A single injection of reHDL reduced the lipid content of the plaque after one week only, therefore suggesting that even a short treatment period with apoAI may improve plaque composition to limit plaque rupture and cardiovascular events.27 In addition to the direct effect of reHDL on the atherosclerotic plaque, an anti-inflammatory action was observed in diabetic patients.28
In patients with acute coronary syndrome, a single injection of reHDL (80 mg/kg) improved the blood lipid profile (increase in HDL and decrease in low density lipoprotein level), but failed to improve vascular function.29 However, the composition of reHDL was limited to apoAI alone; addition of S1P to reHDL may have enhanced a cardioprotective effect in these patients.
S1P and cardioprotection against IR
HDL acts as a major carrier for S1P in the plasma.30 This sphingolipid is particularly present in the subpopulation of HDL type 3 with a density between 1.12–1.21 g/ml.31 Recent data suggest that another HDL component, apolipoprotein M (ApoM), may regulate the content and the metabolism of S1P in HDL. Hence, the level of circulating S1P is strongly reduced in ApoM knockout mice and increased in ApoM overexpressing mice.32 Of note, S1P and apoM levels were significantly reduced in heterozygous carriers of mutations that lower HDL levels, but S1P and apoM levels were not affected in heterozygous carriers of mutations that increase HDL levels.33 Further investigations are required for the delineation of the exact impact of apoM on S1P metabolism in HDL particles.
The cardioprotective effect of S1P has been extensively studied over the last decade with the delineation of a cardioprotective role against IR for both extracellular and intracellular S1P. Extracellular S1P actions are mediated via five receptor subtypes (S1PR1–5) that belong to the family of G protein-coupled receptors. Only three are expressed in the heart (S1PR1–3).17,34 Although these receptors stimulate some pathways in common, they are not redundant.
Pre-incubation with S1P before an IR insult significantly reduced the infarct size of both in vitro and in vivo models.35-38 Similar findings were reported when S1P was given at the onset of reperfusion.39
The receptor subtypes S1P1 and S1P3 are thought to be involved in the protective effect of S1P as VPC23019 (S1P1 and S1P3 antagonist) abolished the protection with S1P.39 Surprisingly, infarct size induced by myocardial IR in vivo is not affected in S1P2 knockout mice or S1P3 knockout mice, but the infarct size doubles in S1P2 and S1P3 double receptor knockout mice.40
Intracellular S1P formation is mediated via two isoforms of sphingosine kinase (SK): SK1 and SK2. Of note, the intracellular formation of S1P may play a crucial role as a mediator of prosurvival signaling events. Both SK1 and SK2 seem to be required for cardioprotection by ischemic pre- and post-conditioning and infarct size is increased in SK2-deficient mice subjected to IR compared with their littermate controls.38,41-43 Correspondingly, activation of SK with the ganglioside GM-1 in mice enhanced cardioprotection against IR.38
There is some data from animal and human studies to suggest that S1P plays a role in the cardioprotective effect of HDL. Hence, HDL fails to protect in S1PR3-deficient mice subjected to left coronary artery ligation.13 In isolated cardiomyocytes, the actions of S1P receptor inhibitors indicate that protection of HDL against simulated oxidative injury is dependent on both S1PR1 and S1PR3.11 Similarly, HDL protects against apoptosis induced by doxorubicin via S1PR2.12 Compatible with these observations, an alteration of both S1P and HDL levels in plasma is observed in patients with coronary artery disease.15
In order to evaluate the precise role of apoAI and S1P in the protective effects of HDL, we used reHDL containing only apoAI or apoAI supplemented with S1P. Our data demonstrate that S1P is essential to protect against the cardiotoxic effects of doxorubicin in vitro.12 In agreement with these data we have also recently observed that addition of S1P to classical reHDL (containing apoAI) improves the protection against ischemia reperfusion injury in a murine isolated heart model.44 Moreover, the cardioprotective capacity of reHDL containing both apoAI and S1P is comparable to that of native HDL.
HDL/S1P and Cell Survival Signaling
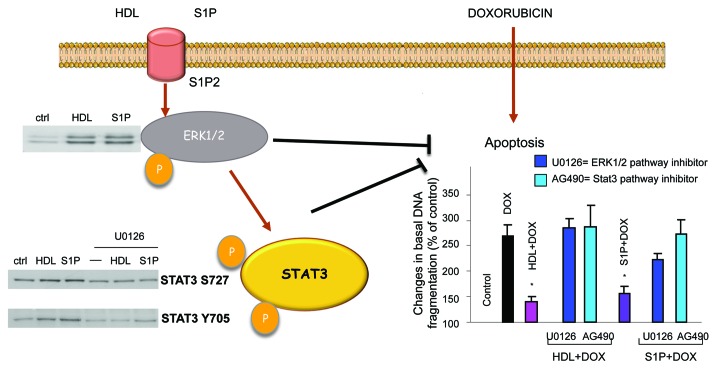
The direct actions of HDL on the signaling pathways in cardiac cells have been poorly investigated. One study showed that HDL protects cultured cardiomyocytes against hypoxia-reoxygenation damage via the activation of ERK1/2 and Akt.11 This protective effect occurred via S1PR1 and S1PR3, which are involved in the activation of ERK1/2 and Akt, respectively. No role was attributed to S1PR2 but this was not investigated.11 In neonatal cardiomyocytes, we have shown that HDL and S1P prevent apoptosis induced by doxorubicin via S1PR2 and subsequent activation of ERK1/2 and STAT3; p38 MAPK was not involved (Fig. 1).12 In neonatal cardiomyocytes, both HDL and S1P can induce the phosphorylation of connexin 43 (Cx43) via the protein kinase C (PKC), both being key players in cardioprotection.45
Figure 1. HDL and S1P induce ERK1/2 and STAT3 phosphorylation (serine 727 and tyrosine 705) via the S1P2 receptor subtype. HDL-induced STAT3 phosphorylation (both serine 727 and tyrosine 705) are abolished in the presence of U0126 (MEK1/2 inhibitor). HDL and S1P significantly reduce the apoptosis (measured by DNA fragmentation) induced by doxorubicin treatment in isolated cardiomyocytes. This protective effect is significantly inhibited when cardiomyocytes are pre-incubated with U0126 or AG490 (JAK2 inhibitor). Abbreviations as described in the text. Adapted from references 12 and 70.
In cardiac cells, S1P induces the phosphorylation of Akt and Bcl-2-associated death promoter (BAD) which are essential for its ability to enhance survival during hypoxia/reoxygenation in adult mouse cardiac myocytes.35 In neonatal rat cardiomyocytes, S1P and the SK activator GM-1 protects against hypoxia-associated cell death, whereas dimethylsphingosine, an inhibitor of sphingosine kinase (SK), enhanced cell death.46 Similarly S1P and GM-1 induce protection against ischemia-induced cardiac damage in mice. Protection is absent in the hearts of PKC-ε knockout mice.38 In the murine heart, Jin and colleagues also showed that PKC-ε is recruited by ischemic preconditioning with subsequent activation of SK1 that mediates cardioprotective effects.43
In endothelial cells, HDL suppresses apoptosis. This protection is mediated via S1P3 and subsequent activation of intracellular signaling pathways involving ERK1/2, Akt and eNOS.47,48 Interestingly, ERK1/2 and Akt are both required for eNOS upregulation, mediated via S1P3.49 Phosphorylation induces the release of NO, which leads to vasodilation and protection of the endothelium. Interestingly, one of the first applications of reHDL containing S1P showed activation of ERK1/2 via S1P2 and 3 and this activation played a role in endothelium tube formation.50
In conclusion, HDL and S1P can both activate intracellular signaling pathways that may be involved in cardioprotection. The precise mechanisms remain to be elucidated.
The SAFE Pathway and Cardioprotection against IR
An alternative protective signaling pathway activated by HDL/S1P is the survivor activating factor enhancement (SAFE) pathway. First described in 2009, the SAFE pathway involves the activation of the pro-inflammatory cytokine TNF and STAT3.5,6
TNF and cardioprotection
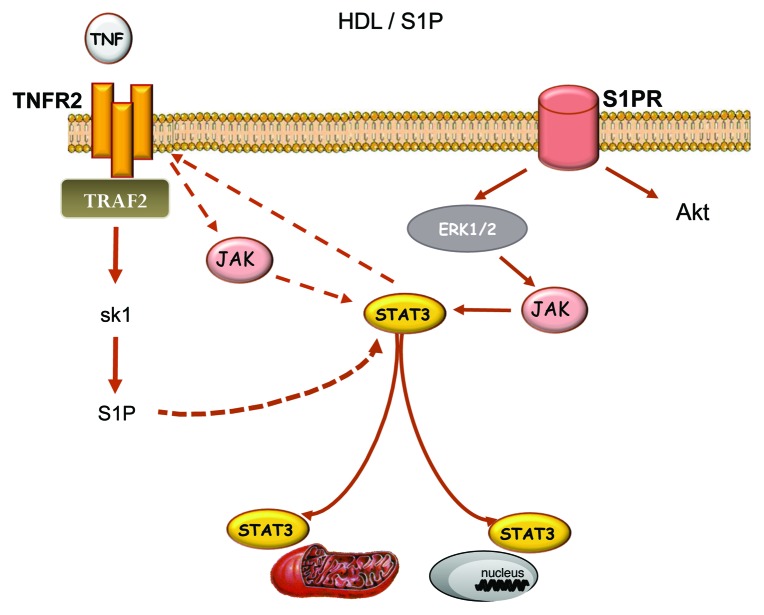
Generally considered cytotoxic, activation of TNF during IR has proved to be paradoxically cardioprotective in a dose- and time-dependent manner. Hence, TNF protects against IR in a dose-dependent manner. Small amounts of exogenous TNF (0.5 ng/ml, in vitro) given prior to IR enhanced cell survival while higher concentrations (10–20 ng/ml, in vitro) were cytotoxic.37,51,52 Also, TNF is an important endogenous cardioprotectant released by ischemic pre- and post-conditioning7,8 and many other pharmacological agents such as bradykinin, opioids, ethanolamine, melatonin and resveratrol (for a review see refs. 53 and 54). This cardioprotective effect appears to be mediated by TNF produced from the cardiomyocytes as TNF cardiomyocyte specific knockout mice were resistant to post-conditioning55 Low doses of exogenous TNF, given prior to the ischemic insult or at the onset of reperfusion, confer cardioprotection by modulation of free radical production and inactivation of pro-apoptotic proteins such as Bad, after binding to its specific receptors.7,52,56 Two TNF receptor isoforms have been identified in the heart, TNF receptor type 1 (TNFR1) and TNF receptor type 2 (TNFR2). Interestingly, exogenous TNF confers cardioprotection in TNFR1 knockout mice but fails to protect TNFR2 knockout mice, therefore suggesting that its cardioprotective effect is mediated via the activation of TNFR2.8 In addition, mice with cardiac-restricted overexpression of TNF receptor-associated factor 2 (TRAF2) are protected from IR, therefore suggesting that TNF confers cardioprotection via TRAF2 as a downstream target of TNFR2.57 TRAF2 is capable of activating the formation of S1P via SK158 (see Fig. 2). The protective effect of TNF is inhibited in the presence of the sphingolipid pathway inhibitor, N-oleoylethanolamine, therefore suggesting that S1P acts as a downstream target of TNF/TNFR2/TRAF2 for cardioprotection.37
Figure 2. HDL/S1P-induced activation of the SAFE pathway. Extracellular sphingosine-1 phosphate (S1P) activates the SAFE pathway that involves TNF and STAT3. The activation of STAT3 may occur after activation of ERK 1/2 following the binding to its specific receptor or via the activation of TNF receptor 2. Activation of STAT3 downstream of TNFR2 remains unclear but may involve the activation of intracellular S1P downstream of the activation of TRAF2 and sphingosine kinase 1. Abbreviations as described in the text.
JAK-STAT3 and cardioprotection
Once TNF binds to its specific receptors, another signaling path, the Janus kinase (JAK)-STAT3 pathway, can be activated. JAKs are a family of tyrosine kinases that are associated with the cytoplasmic domain of cytokine and growth factor receptors (including TNFR and gp130) and play a major role in transducing signals from the cytosol to the nucleus (for a review, see refs. 59 and 60). Upon activation of the receptors, JAK2 phosphorylates and creates a docking site for STAT3 proteins that, in turn, are activated by phosphorylation (Fig. 2). Tyrosine phosphorylation of STAT3 enables it to homodimerize and translocate to the nucleus. Serine phosphorylation of STAT3 is required for its translocation to the mitochondria where it regulates the electron transport chain.61-64
Neither TNF receptor contains protein tyrosine kinase activity or any motif suggesting a biochemical activity but TNF is paradoxically capable to promote induction of specific tyrosine phosphorylation.65 While TNFR1 can directly interact with and form signaling complexes with JAK kinases, the interaction between TNFR2 and JAK2 is still unclear.65 In fact, this interaction may involve the activation of SK1 and intracellular S1P. Following the binding of TNF to TNFR2, activation of TRAF2 can upregulate SK1,58 which in turn, may catalyze the formation of intracellular S1P and subsequent activation of JAK-STAT3.
Activation of the SAFE pathway with TNF-JAK-STAT3 signaling is required for the cardioprotective effect of ischemic pre- and post-conditioning as TNF knockout or cardiomyocyte STAT3 knockout abolishes protection with a conditioning stimulus.7,8,66,67 It should be noted that activation of the JAK-STAT3 pathway also occurs with many other cardioprotective agents such as melatonin, resveratrol, erythropoietin, cannabinoid agonists, insulin and prostaglandins.53,68 More recently, a link between HDL and JAK-STAT3 has also been unveiled.69
HDL/S1P and the SAFE Pathway for Cardioprotection
HDL/S1P and TNF signaling
Using an isolated heart model, our recent data suggest that TNF activation is required in HDL-induced cardioprotection as HDL failed to protect against ischemia-reperfusion in TNF knockout mice.69 If TNF seems to require the presence of intracellular S1P for cardioprotection, exogenous S1P paradoxically requires the activation of TNF signaling for cardioprotection as S1P failed to protect against IR in TNF-deficient mice (see Fig. 3). The mechanisms involved in S1P-induced activation of TNF signaling remain unclear but may involve STAT3 activation following S1P receptor binding.70 In cancer cells, very recent data suggest that STAT3 may also promote TNFR2 activation,71 therefore suggesting that exogenous activation of S1P may trigger the SAFE pathway via TNFR2, following the activation of STAT3 (see Fig. 2). Stimulation of TNFR2 will then mobilize TRAF2 that, in turn, activates SK1 and the endogenous sphingolipid pathway to promote JAK-STAT3 activation and downstream prosurvival signaling cascades.
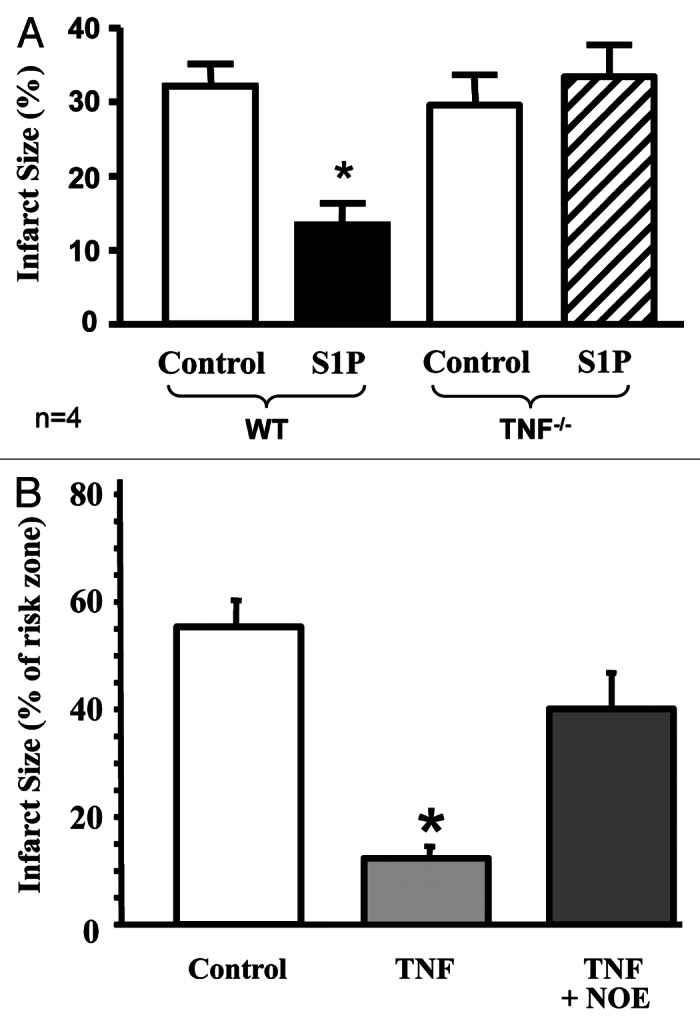
Figure 3. S1P and TNF for cardioprotection. In the isolated mouse heart model, exogenous S1P fails to protect TNF knockout mice against an IR insult (A), therefore suggesting that exogenous S1P requires TNF signaling for cardioprotection. In contrast, exogenous TNF fails to protect isolated rat heart subjected to IR in the presence of the inhibitor of the sphingolipid pathway (NOE, N-oleoylethanolamine) (B), therefore suggesting that intracellular formation of S1P is required in TNF-induced cardioprotection. Abbreviations as described in the text. *p < 0.05 vs. control group. Adapted from references 37 and 73.
HDL/S1P and JAK-STAT3 signaling
Our recent data support a link between HDL and JAK-STAT3 signaling as human HDL was not able to confer cardioprotection in the isolated STAT3-deficient mouse heart subjected to an IR insult.69 In neonatal rat ventricular cardiomyocytes, human HDL, S1P or reHDL containing S1P induced a time- and concentration-dependent serine and tyrosine phosphorylation of STAT3 as well as an increase in STAT3 binding to DNA.70 In contrast, reHDL without S1P (containing apoAI only) had a weaker effect on both STAT3 phosphorylation and DNA-binding. Both HDL and S1P induced STAT3 phosphorylation principally through the receptor S1P2, although, following treatment with HDL, a possible involvement of an additional receptor with a lesser impact may occur. Activation of STAT3 is also required in the cardioprotective effect of HDL and S1P against doxorubicin-induced apoptosis12 (see Fig. 1). Ethanolamine, a downstream product of S1P, confers cardioprotection via the activation of STAT3.72 In an ex vivo mouse model of global ischemia, post-conditioning with S1P (10 nM) failed to protect against IR in STAT3-deficient mice.73 Interestingly, S1P leads to a time-dependent increase in serine phosphorylation of STAT3 in both nucleus and mitochondria.73 The activation of STAT3 with exogenous S1P in rat intestinal smooth muscle cells exerts a local proinflammatory effect.74 Other studies suggest that STAT3 plays an important role in cancer and in prostate cancer cells. A link between STAT3 and HDL has been reported as both HDL and S1P increase serine phosphorylation of STAT3 (but not tyrosine) via S1P2 and S1P3 receptors.75 These data strongly support the role of STAT3 as a downstream target of HDL/S1P.
HDL/S1P and downstream targets of the SAFE pathway
In the context of cardioprotection, mitochondria appear to be the main downstream target of the SAFE pathway as mitochondrial activated STAT3 contributes to cardioprotection by stimulation of respiration and inhibition of mitochondrial permeability transition pore (mPTP) opening.64 In isolated cardiomyocytes, the protective effect of HDL or S1P against simulated ischemia was associated with an activation of mitochondrial STAT3 and the inhibition of mPTP opening.76 In contrast, STAT3-deficient mice were not protected with HDL or S1P with, likewise, failure to inhibit mPTP opening.76
Another important mediator that may be stimulated by HDL/S1P downstream of the SAFE pathway is Cx43. This major myocardial gap junction protein is responsible for rapid and synchronous transmission of the cardiac action potential. Mitochondrial Cx43 is known as a key element of the signal transduction cascade affording protection by ischemic preconditioning (IPC).77 In neonatal rat cardiomyocytes, short-term treatment with HDL or S1P induces phosphorylation of Cx43.45 Modulation of Cx43 could be dependent on the JAK-STAT3 pathway.78 Other targets of STAT3 have been identified including pro- and anti-apoptotic proteins. In a mouse ex vivo model of global ischemia, post-conditioning with S1P protected the heart and simultaneously induced nuclear phosphorylation of FOXO-1. This was not observed in STAT3-deficient mice.73 FOXO-1 is a pro-apoptotic protein known to enhance hypertrophy, oxidative stress and IR injury in its non-phosphorylated (active) form.79,80 Caspase-3, responsible for the cleavage of key cellular proteins leading to the typical morphological changes observed in cells undergoing apoptosis, could be a downstream target of HDL/S1P-induced STAT3 activation. Indeed, in a rat model of left anterior descending coronary artery ligation, inhibition of STAT3 results in an increase in caspase-3 activity while in HUVEC cells submitted to apoptosis induced by growth factor deprivation, HDL prevented activation of caspase-3.47,81 BAD and glycogen synthase kinase 3 β (GSK3β) are potential downstream targets of the SAFE pathway but their implication in HDL/S1P-induced cardioprotection needs to be clarified.8,52,82 Similarly, the pro-apoptotic protein Bax that promotes mitochondrial outer membrane permeabilization and release of cytochrome c into the cytosol may also be a downstream target of HDL. S1P is known to suppress cellular levels of Bax and STAT3 has been reported to regulate Bax protein levels.81 Nitric oxide (NO) is another possible downstream target. The JAK-STAT3 pathway can increase inducible NO synthase protein and activity.83,84 In an in vivo mouse model of coronary artery ligation, HDL- and S1P-mediated cardioprotection is dependent on NO.13 This effect may be mediated via SK1, which is known to alter the expression and production of NO.85
Interaction of the SAFE Pathway with Other Cell Survival Signaling
In ventricular cardiomyocytes ERK1/2, Rho kinase (ROCK), phospholipase C (PLC) and Src were involved in the STAT3 activation promoted by HDL or S1P. Neither p38 MAPK, nor phosphatidylinositol 3-kinase (PI3K) or PKC were implicated.70 In the isolated mouse heart model, ischemic post-conditioning with S1P induced STAT3 activation and this effect was decreased in presence of a PI3K/Akt inhibitor (wortmannin).73 Accordingly, STAT3 knockout mice failed to increase Akt phosphorylation.73
Several studies exploring the interaction between JAK-STAT3 and PI3K/Akt have provided inconsistent results that may, in part, be due to different models and experimental systems. Some studies report a dual interaction between JAK-STAT3 and PI3K/Akt: a reduction in Akt phosphorylation occurs in the presence of a JAK-STAT3 pathway inhibitor (AG490) and vice versa, STAT3 phosphorylation is diminished in the presence of a PI3K/Akt inhibitor (wortmannin).86-88 Other studies suggest a one way regulation with JAK-STAT3 being an upstream regulator of PI3K/Akt82,89,90 or vice versa.91,92 Finally JAK-STAT3 and PI3K/Akt can also act independently.8,87,89,93 The interaction of JAK-STAT3 pathway with GSK3β, a downstream target of Akt, is also controversial. Some studies suggest downregulation of GSK3β phosphorylation in the presence of an inhibitor of STAT382,86 while other studies do not support an involvement for GSK3β as a downstream target of STAT3.8 Additional studies are required to explore the exact interaction between Akt and STAT3 in the context of HDL-induced cardioprotection.
Conclusion
Strong evidence, both in clinical and in experimental settings, underlines the key role of S1P in HDL-induced cardioprotection against coronary artery disease.
Evidently it focuses attention on the HDL-S1P association, and how this could affect the cardioprotective influence of the lipoprotein. It is known that HDL is the principal carrier of the lipid,34 which, due to its hydrophobic nature, cannot circulate freely in serum. It has also been suggested that S1P is limited to a subfraction of HDL particles,31 reflecting the HDL functional heterogeneity mentioned earlier. Further study of the precise nature of this S1P-rich HDL would be of particular interest. There is little data on the regulation of S1P association with HDL, although recent studies indicate that binding occurs through a minor HDL apolipoprotein, apoM.32 Little is known about this peptide, which underlines the need to investigate the impact of apoM on HDL-S1P function. Another important point to address is whether the variations in the S1P content of HDL can influence risk of vascular disease. Studies are presently hampered by the analytical procedure, as quantification of S1P presently requires a relatively cumbersome methodology. Nevertheless, preliminary studies indicate that significantly lower levels of HDL-associated S1P are a feature of patients with coronary disease.15
This protective effect is mediated, at least in part, via the activation of the powerful prosurvival signaling pathway that involves TNF and STAT3. Further studies are still required in order to understand the exact signaling events involved in this protective signaling cascade. This review also provides persuasive evidence that modification of the basal composition of reHDL, by addition of S1P, would improve their therapeutic potential against coronary artery disease and other stress related pathologies.
Acknowledgments
The authors are funded, in part, by the Swiss South African Joint Research Programme (JRP 16) to S.L. and R.W.J., the Swiss National Science Foundation to R.W.J. (SNSF 310030-135221), to M.A.F. (SNSF PBGEP3-125930), the National Research Foundation South Africa, the South African Medical Research Council to S.L. and the University of Cape Town to S.P.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/19754
References
- 1.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 3.Barter PJ, Rye KA. High density lipoproteins and coronary heart disease. Atherosclerosis. 1996;121:1–12. doi: 10.1016/0021-9150(95)05675-0. [DOI] [PubMed] [Google Scholar]
- 4.Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/S0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 5.Lecour S. Multiple protective pathways against reperfusion injury: a SAFE path without Aktion? J Mol Cell Cardiol. 2009;46:607–9. doi: 10.1016/j.yjmcc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, et al. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase) Circulation. 2005;112:3911–8. doi: 10.1161/CIRCULATIONAHA.105.581058. [DOI] [PubMed] [Google Scholar]
- 8.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–8. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 9.Mochizuki S, Okumura M, Tanaka F, Sato T, Kagami A, Tada N, et al. Ischemia-reperfusion arrhythmias and lipids: effect of human high- and low-density lipoproteins on reperfusion arrhythmias. Cardiovasc Drugs Ther. 1991;5(Suppl 2):269–76. doi: 10.1007/BF00054748. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi L, Rossoni G, Gomaraschi M, Sisto F, Berti F, Franceschini G. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-alpha content and enhancing prostaglandin release. Circ Res. 2003;92:330–7. doi: 10.1161/01.RES.0000054201.60308.1A. [DOI] [PubMed] [Google Scholar]
- 11.Tao R, Hoover HE, Honbo N, Kalinowski M, Alano CC, Karliner JS, et al. High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia-reoxygenation through lipoprotein-associated sphingosine 1-phosphate. Am J Physiol Heart Circ Physiol. 2010;298:H1022–8. doi: 10.1152/ajpheart.00902.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frias MA, Lang U, Gerber-Wicht C, James RW. Native and reconstituted HDL protect cardiomyocytes from doxorubicin-induced apoptosis. Cardiovasc Res. 2010;85:118–26. doi: 10.1093/cvr/cvp289. [DOI] [PubMed] [Google Scholar]
- 13.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–9. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 14.Levkau B, Hermann S, Theilmeier G, van der Giet M, Chun J, Schober O, et al. High-density lipoprotein stimulates myocardial perfusion in vivo. Circulation. 2004;110:3355–9. doi: 10.1161/01.CIR.0000147827.43912.AE. [DOI] [PubMed] [Google Scholar]
- 15.Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Gräler MH, et al. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821–32. doi: 10.1007/s00395-010-0112-5. [DOI] [PubMed] [Google Scholar]
- 16.Genest J. Lipoprotein disorders and cardiovascular risk. J Inherit Metab Dis. 2003;26:267–87. doi: 10.1023/A:1024449603891. [DOI] [PubMed] [Google Scholar]
- 17.Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48:2325–33. doi: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Rossoni G, Gomaraschi M, Berti F, Sirtori CR, Franceschini G, Calabresi L. Synthetic high-density lipoproteins exert cardioprotective effects in myocardial ischemia/reperfusion injury. J Pharmacol Exp Ther. 2004;308:79–84. doi: 10.1124/jpet.103.057141. [DOI] [PubMed] [Google Scholar]
- 19.Marchesi M, Booth EA, Rossoni G, García RA, Hill KR, Sirtori CR, et al. Apolipoprotein A-IMilano/POPC complex attenuates post-ischemic ventricular dysfunction in the isolated rabbit heart. Atherosclerosis. 2008;197:572–8. doi: 10.1016/j.atherosclerosis.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Marchesi M, Booth EA, Davis T, Bisgaier CL, Lucchesi BR. Apolipoprotein A-IMilano and 1-palmitoyl-2-oleoyl phosphatidylcholine complex (ETC-216) protects the in vivo rabbit heart from regional ischemia-reperfusion injury. J Pharmacol Exp Ther. 2004;311:1023–31. doi: 10.1124/jpet.104.070789. [DOI] [PubMed] [Google Scholar]
- 21.Gomaraschi M, Calabresi L, Rossoni G, Iametti S, Franceschini G, Stonik JA, et al. Anti-inflammatory and cardioprotective activities of synthetic high-density lipoprotein containing apolipoprotein A-I mimetic peptides. J Pharmacol Exp Ther. 2008;324:776–83. doi: 10.1124/jpet.107.129411. [DOI] [PubMed] [Google Scholar]
- 22.Kiya Y, Miura S, Imaizumi S, Uehara Y, Matsuo Y, Abe S, et al. Reconstituted high-density lipoprotein attenuates postinfarction left ventricular remodeling in rats. Atherosclerosis. 2009;203:137–44. doi: 10.1016/j.atherosclerosis.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 23.Gu SS, Shi N, Wu MP. The protective effect of ApolipoproteinA-I on myocardial ischemia-reperfusion injury in rats. Life Sci. 2007;81:702–9. doi: 10.1016/j.lfs.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Imaizumi S, Miura S, Nakamura K, Kiya Y, Uehara Y, Zhang B, et al. Antiarrhythmogenic effect of reconstituted high-density lipoprotein against ischemia/reperfusion in rats. J Am Coll Cardiol. 2008;51:1604–12. doi: 10.1016/j.jacc.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Tardif JC, Grégoire J, L’Allier PL, Ibrahim R, Lespérance J, Heinonen TM, et al. Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–82. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 26.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 27.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–91. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 28.Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, et al. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol. 2009;53:962–71. doi: 10.1016/j.jacc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Chenevard R, Hurlimann D, Spieker L, Bechir M, Enseleit F, Hermann M, et al. Reconstituted HDL in Acute Coronary Syndromes. Cardiovasc Ther 2011 [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Tomura H, Kuwabara A, Kimura T, Miura S, Noda K, et al. Correlation of high density lipoprotein (HDL)-associated sphingosine 1-phosphate with serum levels of HDL-cholesterol and apolipoproteins. Atherosclerosis. 2005;178:199–205. doi: 10.1016/j.atherosclerosis.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Kontush A, Therond P, Zerrad A, Couturier M, Négre-Salvayre A, de Souza JA, et al. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler Thromb Vasc Biol. 2007;27:1843–9. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 32.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnström J, Sevvana M, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108:9613–8. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karuna R, Park R, Othman A, Holleboom AG, Motazacker MM, Sutter I, et al. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 2011;219:855–63. doi: 10.1016/j.atherosclerosis.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 34.Keul P, Sattler K, Levkau B. HDL and its sphingosine-1-phosphate content in cardioprotection. Heart Fail Rev. 2007;12:301–6. doi: 10.1007/s10741-007-9038-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–8. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 36.Vessey DA, Li L, Kelley M, Zhang J, Karliner JS. Sphingosine can pre- and post-condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Mol Toxicol. 2008;22:113–8. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 37.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–18. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 38.Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–7. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 39.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297:H1429–35. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–51. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 41.Vessey DA, Li L, Jin ZQ, Kelley M, Honbo N, Zhang J, et al. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:961059. doi: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin ZQ, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–40. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 43.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–9. doi: 10.1161/01.CIR.0000143632.06471.93. [DOI] [PubMed] [Google Scholar]
- 44.Frias MA, Somers S, Moren X, Lacerda L, Opie LH, James RW, et al. The presence of sphingosine-1 phosphate in reconstituted HDL is required for better protection against lethal reperfusion injuries. Eur Heart J. 2011;32:687. [Google Scholar]
- 45.Morel S, Frias MA, Rosker C, James RW, Rohr S, Kwak BR. The natural cardioprotective particle HDL modulates connexin43 gap junction channels. Cardiovasc Res. 2012;93:41–9. doi: 10.1093/cvr/cvr257. [DOI] [PubMed] [Google Scholar]
- 46.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713–7. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 47.Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem. 2001;276:34480–5. doi: 10.1074/jbc.M103782200. [DOI] [PubMed] [Google Scholar]
- 48.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, et al. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–5. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 49.Nofer JR, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–81. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuo Y, Miura S, Kawamura A, Uehara Y, Rye KA, Saku K. Newly developed reconstituted high-density lipoprotein containing sphingosine-1-phosphate induces endothelial tube formation. Atherosclerosis. 2007;194:159–68. doi: 10.1016/j.atherosclerosis.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Lacerda L, McCarthy J, Mungly SF, Lynn EG, Sack MN, Opie LH, et al. TNFα protects cardiac mitochondria independently of its cell surface receptors. Basic Res Cardiol. 2010;105:751–62. doi: 10.1007/s00395-010-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deuchar GA, Opie LH, Lecour S. TNFalpha is required to confer protection in an in vivo model of classical ischaemic preconditioning. Life Sci. 2007;80:1686–91. doi: 10.1016/j.lfs.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 53.Lecour S, James RW. When are pro-inflammatory cytokines SAFE in heart failure? Eur Heart J. 2011;32:680–5. doi: 10.1093/eurheartj/ehq484. [DOI] [PubMed] [Google Scholar]
- 54.Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011;14:893–907. doi: 10.1089/ars.2010.3360. [DOI] [PubMed] [Google Scholar]
- 55.Lacerda L, Jacobs M, Opie LH, Lecour S. Ischaemic postconditioning confer protection via TNFalpha:identifying the cellular origin of TNF. Eur Heart J. 2011;32:662. [Google Scholar]
- 56.Lecour S, Rochette L, Opie L. Free radicals trigger TNF alpha-induced cardioprotection. Cardiovasc Res. 2005;65:239–43. doi: 10.1016/j.cardiores.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Burchfield JS, Dong JW, Sakata Y, Gao F, Tzeng HP, Topkara VK, et al. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circ Heart Fail. 2010;3:157–64. doi: 10.1161/CIRCHEARTFAILURE.109.899732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–8. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurdi M, Booz GW. JAK redux: a second look at the regulation and role of JAKs in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1545–56. doi: 10.1152/ajpheart.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–85. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, et al. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011;286:29610–20. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–8. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 64.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–85. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo D, Dunbar JD, Yang CH, Pfeffer LM, Donner DB. Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J Immunol. 1998;160:2742–50. [PubMed] [Google Scholar]
- 66.Smith RM, Suleman N, McCarthy J, Sack MN. Classic ischemic but not pharmacologic preconditioning is abrogated following genetic ablation of the TNFalpha gene. Cardiovasc Res. 2002;55:553–60. doi: 10.1016/S0008-6363(02)00283-3. [DOI] [PubMed] [Google Scholar]
- 67.Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, et al. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res. 2004;63:611–6. doi: 10.1016/j.cardiores.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 68.Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S. Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J Pineal Res. 2011;50:374–80. doi: 10.1111/j.1600-079X.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- 69.Frias MA, Somers S, Lacerda L, James RW, Lecour S. HDL protects against lethal reperfusion injury via the SAFE pathway. SA Heart J. 2010;7:202. [Google Scholar]
- 70.Frias MA, James RW, Gerber-Wicht C, Lang U. Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: role of sphingosine-1-phosphate. Cardiovasc Res. 2009;82:313–23. doi: 10.1093/cvr/cvp024. [DOI] [PubMed] [Google Scholar]
- 71.Hamilton KE, Simmons JG, Ding S, Van Landeghem L, Lund PK. Cytokine induction of tumor necrosis factor receptor 2 is mediated by STAT3 in colon cancer cells. Mol Cancer Res. 2011;9:1718–31. doi: 10.1158/1541-7786.MCR-10-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly RF, Lamont KT, Somers S, Hacking D, Lacerda L, Thomas P, et al. Ethanolamine is a novel STAT-3 dependent cardioprotective agent. Basic Res Cardiol. 2010;105:763–70. doi: 10.1007/s00395-010-0125-0. [DOI] [PubMed] [Google Scholar]
- 73.Somers S, Frias M, Lacerda L, Opie L, Lecour S. Interplay between SAFE and RISK pathways in sphingosine-1-phosphate-induced cardioprotection. Cardiovasc Drugs Ther. 2012;26:227–37. doi: 10.1007/s10557-012-6376-2. [DOI] [PubMed] [Google Scholar]
- 74.Gurgui M, Broere R, Kalff JC, van Echten-Deckert G. Dual action of sphingosine 1-phosphate in eliciting proinflammatory responses in primary cultured rat intestinal smooth muscle cells. Cell Signal. 2010;22:1727–33. doi: 10.1016/j.cellsig.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 75.Sekine Y, Suzuki K, Remaley AT. HDL and sphingosine-1-phosphate activate stat3 in prostate cancer DU145 cells via ERK1/2 and S1P receptors, and promote cell migration and invasion. Prostate. 2011;71:690–9. doi: 10.1002/pros.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hacking D, Kelly RF, Yellon DM, Opie LH, Hausenloy DJ, Lecour S. Sphingosine-1 phosphate mediates cardioprotection by modulation. SA Heart J. 2010;7:203. [Google Scholar]
- 77.Schulz R, Boengler K, Totzeck A, Luo Y, Garcia-Dorado D, Heusch G. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007;12:261–6. doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- 78.Ozog MA, Bernier SM, Bates DC, Chatterjee B, Lo CW, Naus CC. The complex of ciliary neurotrophic factor-ciliary neurotrophic factor receptor alpha up-regulates connexin43 and intercellular coupling in astrocytes via the Janus tyrosine kinase/signal transducer and activator of transcription pathway. Mol Biol Cell. 2004;15:4761–74. doi: 10.1091/mbc.E04-03-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–78. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–68. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, et al. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res. 2000;47:797–805. doi: 10.1016/S0008-6363(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 82.Pedretti S, Raddatz E. STAT3α interacts with nuclear GSK3beta and cytoplasmic RISK pathway and stabilizes rhythm in the anoxic-reoxygenated embryonic heart. Basic Res Cardiol. 2011;106:355–69. doi: 10.1007/s00395-011-0152-5. [DOI] [PubMed] [Google Scholar]
- 83.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci U S A. 2001;98:9050–5. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nayak D, Huo Y, Kwang WX, Pushparaj PN, Kumar SD, Ling EA, et al. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience. 2010;166:132–44. doi: 10.1016/j.neuroscience.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 86.Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croué A, Henrion D, et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011;106:1329–39. doi: 10.1007/s00395-011-0210-z. [DOI] [PubMed] [Google Scholar]
- 87.Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008;79:127–33. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 88.Lu Y, Zhou J, Xu C, Lin H, Xiao J, Wang Z, et al. JAK/STAT and PI3K/AKT pathways form a mutual transactivation loop and afford resistance to oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem. 2008;21:305–14. doi: 10.1159/000129389. [DOI] [PubMed] [Google Scholar]
- 89.Goodman MD, Koch SE, Fuller-Bicer GA, Butler KL. Regulating RISK: a role for JAK-STAT signaling in postconditioning? Am J Physiol Heart Circ Physiol. 2008;295:H1649–56. doi: 10.1152/ajpheart.00692.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fuglesteg BN, Suleman N, Tiron C, Kanhema T, Lacerda L, Andreasen TV, et al. Signal transducer and activator of transcription 3 is involved in the cardioprotective signalling pathway activated by insulin therapy at reperfusion. Basic Res Cardiol. 2008;103:444–53. doi: 10.1007/s00395-008-0728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3 beta. Am J Physiol Heart Circ Physiol. 2006;291:H827–34. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 92.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–21. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 93.Smith CC, Dixon RA, Wynne AM, Theodorou L, Ong SG, Subrayan S, et al. Leptin-induced cardioprotection involves JAK/STAT signaling that may be linked to the mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2010;299:H1265–70. doi: 10.1152/ajpheart.00092.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]