Abstract
Negative-stranded RNA viruses cover their genome with nucleoprotein (N) to protect it from the human innate immune system. Abrogation of the function of N offers a unique opportunity to combat the spread of the viruses. Here, we describe a unique fold of N from Leanyer virus (LEAV, Orthobunyavirus genus, Bunyaviridae family) in complex with single-stranded RNA refined to 2.78 Å resolution as well as a 2.68 Å resolution structure of LEAV N–ssDNA complex. LEAV N is made up of an N- and a C-terminal lobe, with the RNA binding site located at the junction of these lobes. The LEAV N tetramer binds a 44-nucleotide-long single-stranded RNA chain. Hence, oligomerization of N is essential for encapsidation of the entire genome and is accomplished by using extensions at the N and C terminus. Molecular details of the oligomerization of N are illustrated in the structure where a circular ring-like tertiary assembly of a tetramer of LEAV N is observed tethering the RNA in a positively charged cavity running along the inner edge. Hydrogen bonds between N and the C2 hydroxyl group of ribose sugar explain the specificity of LEAV N for RNA over DNA. In addition, base-specific hydrogen bonds suggest that some regions of RNA bind N more tightly than others. Hinge movements around F20 and V125 assist in the reversal of capsidation during transcription and replication of the virus. Electron microscopic images of the ribonucleoprotein complexes of LEAV N reveal a filamentous assembly similar to those found in phleboviruses.
Keywords: crystal structure, nucleoprotein complex with ssRNA, RNP
Although considerable success has been achieved in developing drugs for containing global viral killers such as HIV and influenza virus, inadequate treatments for reemerging and neglected viral infections pose a serious socioeconomic threat. Therefore, it is essential to study and develop interventions for containing outbreaks of these viruses.
Viruses have evolved intricate strategies to protect their genomes from destruction by host defenses. For example, encapsidation of the entire genome of negative-sense single-stranded RNA (ssRNA) viruses by nucleoprotein (N) molecules prevents attacks by the host. Recently, N proteins have also been shown to possess secondary functions such as the exoribonuclease activity of the Lassa virus N protein that helps the virus evade host immune surveillance (1, 2) or the endonuclease activity of the Crimean–Congo hemorrhagic fever virus (CCHFV) nucleoprotein (3). These additional roles for N protein were explained with the elucidation of the structure of the Lassa virus N protein that surprisingly revealed the presence of an exonuclease fold at the C terminus. Consequently, abrogation of the function of N is detrimental to the virus and therefore N can be targeted for therapeutic interventions. So far, structures of only five binary complexes of N with RNA are known. These include N proteins from rabies virus (RV) (4), vesicular stomatitis virus (VSV) (5), respiratory syncytial virus (RSV) (6), Lassa virus (1, 7) and Rift Valley fever virus (RVFV) (8). The structures reveal a ring-like oligomeric assembly of N with the RNA threading the cavity running along the inner or outer edge of the ring such that it cannot be accessed by the host’s defense machinery.
There are five genera in the Bunyaviridae family—Orthobunyavirus, Phlebovirus, Hantavirus, Nairovirus, and Tospovirus— of negative-sense ssRNA viruses that infect humans, animals, and plants (9, 10). Although the N protein essential for the propagation of the virus adopts a highly conserved structure within a genus, N proteins from different genera differ in their primary sequences and 3D architecture markedly. For example, the Hantaan virus N protein is reported to form trimeric structures using homotypic N–N protein interactions (11–13). The interaction sites have been mapped principally on the N and C terminals (14). The N protein of RVFV, a member of the Phlebovirus genus, has been proposed to oligomerize into a tetrameric, pentameric, or hexameric ring-like ribonucleoprotein (RNP) complex (8, 15). In contrast, CCHFV N (Nairovirus genus) exists as a monomer when expressed as a recombinant protein (3, 16, 17). Thus, the differences in the structure and preferred oligomeric states of bunyaviruses’ N proteins may reflect significantly different mechanism of RNP formation. Therefore, it is essential to determine representative structures of N proteins from each genus to understand their function and design inhibitors.
Orthobunyavirus is the largest genus with 170 viruses distributed across 48 species. Viruses belonging to this genus are responsible for serious human diseases such as pediatric encephalitis caused by La Crosse virus (LACV), an influenza-like syndrome caused by Tahyna virus, and a debilitating febrile illness caused by Oropouche virus across different geographic locations (18). In addition, recently, the first case of a human infected with a Bunyamwera virus (BUNV) (19) showing symptoms of febrile syndrome was reported from Argentina. More recently, using a metagenomic approach, a novel orthobunyavirus, Schmallenberg virus, isolated from ruminants, was identified as the causative agent of a widespread epidemic in Europe (20, 21). Currently, there is no structural information available on any viral N protein belonging to this genus. Therefore, we selected a prototype , Leanyer virus (LEAV), to study a representative N protein from this genus. The strain of the LEAV used in this study was originally isolated in northern Australia in 1974 and initial characterization of the virus showed that it neighbors the Simbu serogroup of viruses (9, 22). The LEAV genome is composed of three segments of single-stranded RNA: large (L), medium (M), and small (S). The L segment encodes the RNA-dependent RNA polymerase (RdRp), the M segment encodes two glycoproteins (Gn/Gc) of the envelope and a nonstructural protein (NSm), and the S segment encodes the nucleoprotein (N) and the nonstructural S protein (NSs). All of the three RNA segments are encapsidated by N, resulting in the formation of RNP complexes. These RNPs protect the genome and serve as a template for the viral RdRp during transcription and replication.
To study the nature of the N protein from LEAV and shed light on the mechanism of encapsidation of RNA, we solved the crystal structures of the binary complexes of oligomeric LEAV N with a 44-nt ssRNA or two segments of 24-nt ssDNA. The structures together with mutagenesis studies provide molecular details of the mode of RNA binding by LEAV N and identify regions essential for oligomerization and formation of RNP complexes. Electron microscopic image of the LEAV RNP complexes suggests a filamentous packing of the viral genome.
Results
Preparation and Characterization of the N Proteins.
Initially N proteins from four closely related species of the Orthobunyavirus genus—LEAV, BUNV (two isolates), and LACV—were selected for expression in Escherichia coli (Fig. S1). All four N proteins could be expressed as soluble proteins. Each N protein eluted in two separate peaks during size-exclusion chromatography (SEC) (Fig. S2A). The first peak contained highly oligomeric LEAV N protein, whereas the N protein that eluted in the second peak was homogenous and had a longer retention time (Fig. S2 A and B). Sedimentation velocity experiments using an analytical ultracentrifuge revealed that the LEAV N protein from the second peak formed tetramer (Fig. S2C). All of the N proteins bound bacterial nucleic acids (A260/280 absorbance ratio ≥ 1.1) that could not be completely removed by an RNase treatment. Addition of 3 M urea and 1 M NaCl to lysis buffer during the recovery of the protein removed nucleic acids completely from the N proteins (A260/280 absorbance ratio ∼0.6) (Fig. S2D). Removal of the RNAs did not disrupt the tertiary assembly of the N protein (Fig. S2D), suggesting that the oligomeric state of the LEAV N could be maintained in the absence of RNA. Such a feature is also exhibited by BUNV N (23, 24) and other negative-strand RNA viruses, such as VSV (25). RNA-free N could bind ssRNA (Kd = 0.166 μM) or ssDNA (Kd = 0.505 μM) with high affinity, as suggested by isothermal titration calorimetry (ITC) assays (Fig. S3 A and B). The binding was further confirmed by performing thermal shift assays in the presence of nucleic acids (Fig. S3C) (26).
The RNA-free LEAV N could be reassembled into RNPs by mixing the N with RNA (Fig. S3D). Such reconstituted N–ssRNA/ssDNA complexes seemed resistant to exhaustive RNase treatment. Therefore, the reconstituted complexes support a model of transcription and replication where the N protein can reversibly alternate between encapsidation and its nucleic acid-free form. LEAV N proteins with or without RNA of bacterial origin, as well as the reconstituted LEAV N with synthesized ssDNA (24-nt) or synthesized ssRNA (24-nt) complexes were screened for crystallization. Only LEAV N with RNA of bacterial origin and the reconstituted LEAV N with synthesized ssDNA complex crystallized and diffracted X-rays beyond 3.0 Å. The structure of the binary complex of LEAV N with ssRNA, crystallized in P1 space group, was solved by the Se–single wavelength anomalous dispersion (Se–SAD) method (27) using X2DF HT structure determination pipeline (28, 29) and refined to 2.78 Å resolution with an R factor of 23.33 (Rfree = 27.40). The LEAV N–ssDNA complex, crystallized in P1 space group as well, was solved by molecular replacement (30) using the LEAV N–ssRNA structure as a template and refined to 2.68 Å resolution with an R factor of 22.27 (Rfree = 24.30) (Table S1).
Overall Structure of LEAV N Bound with ssRNA/ssDNA.
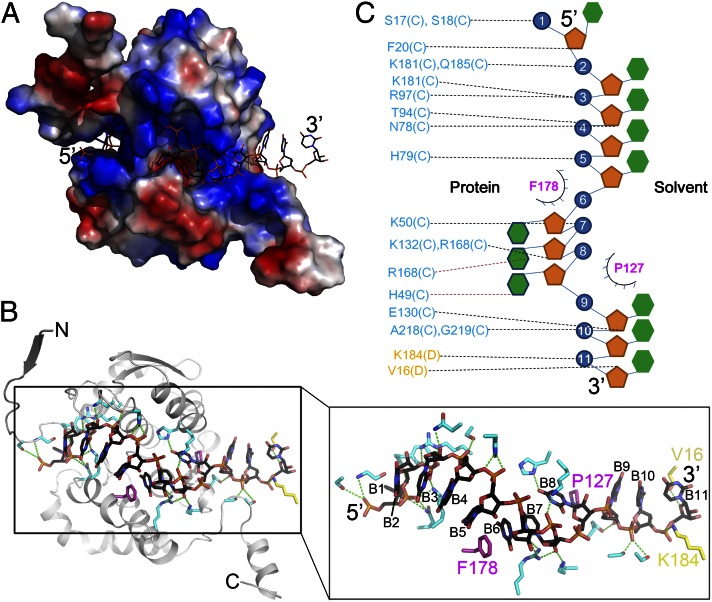
In the structure of the LEAV N–ssRNA complex, one tetramer of the protein is observed forming a ring-like assembly in the crystallographic asymmetric unit (ASU) with the RNA bound along the inner edge. The ring has an outer diameter of 100 Å, an inner diameter of 26 Å, and a width of 42 Å (Fig. 1A). Each protomer contains residues 6–232 and one tetramer of N encapsidates 44 nt of ssRNA. Each protomer contains 11 nt of ssRNA; this number is one nucleotide less than the number previously reported for BUNV N proteins based on biochemical studies (23, 24). Incidentally, the RNA extracted from the bacterially expressed tetrameric LEAV N–ssRNA complex exhibited a 40- to 50-nt size during electrophoresis, which is consistent with previous reports (23, 24) (Fig. S3E). The N protomer consists of two domains, an N lobe (1–125 aa) and a C lobe (starting at residue 126) containing 10 α-helices (four at the N lobe and six at the C lobe) and three β-sheets (all at the N lobe). Each protomer contacts two other protomers, one each on either side using the N- and C-terminal extensions, respectively (Fig. 1 B and C). The N lobe is preceded by an extension arm (1–20 aa) contacting an adjacent protomer. The C lobe has an extended tail (211–235 aa) that forms a firm link with the next protomer. These two interactions hold the protomers together in the tertiary assembly (Fig. 1A). The maximal interaction area between the interfaces of neighboring N protein is 1,095 Å2 calculated by PDBePISA software (31). Although the overall structure of LEAV N is novel, a DALI (32) analysis retrieved a low structural match with a small portion of an RNA or nucleotide binding protein. For example, a ribosomal protein S15 (Protein Data Bank ID code 1A32; rmsd of 2.65 Å over 51 overlapping Cα atoms as shown in Fig. S4A) and MazG nucleotide pyrophosphohydrolase (Protein Data Bank ID code 1VMG; rmsd of 3.38 Å for 62 matching Cα atoms shown in Fig. S4A) could be partially superimposed over the LEAV N structure.
Fig. 1.
Overall structure of the LEAV N–ssRNA/ssDNA complex. (A) Ribbon diagram (Left) and surface representation (Right) of the tetrameric, ring-like assembly of the LEAV N–ssRNA complex. Each N protomer is colored differently. The RNA is colored black. (B) Stereo view of LEAV N with ssRNA. Electron density of a 2Fo-Fc simulated annealing (SA) omit map for ssRNA bound in the cavity between N and C lobes contoured at 1.0 σ is shown. (C) Topology drawing of the structure of LEAV N. The N lobe is shown in blue, and the C lobe is colored red. (D) Ribbon diagram of the tetramer of LEAV N–ssDNA.
Similar to the LEAV N–ssRNA structure, the LEAV N–ssDNA complex also forms a ring-like tertiary assembly. The ASU contains a tetramer of N protein bound with two 17-nt-long chains of ssDNA (Fig. 1D). Comparison of the two kinds of binary complexes reveals that the N protomers are almost identical in the core domain with a rmsd value of 0.46 Å but differ slightly in the positions of the N-terminal arm (1–20 aa, with a rmsd value of 1.68 Å) and the helical C-terminal tail (211–235 aa, with a rmsd value of 1.94 Å) (Fig. S4 B and C). Likewise, examination of all four segments of RNA bound by protomers of the tetramer reveals asymmetry in the conformation of RNA. Superimposition of the four ssRNA segments shows that the nucleotides located near the interface between two adjacent protomers are more deviated geometrically than those located at the center of the protein subunits. The arm-to-core interactions essential for oligomerization are identical in all protomers. In addition, the mode of binding of RNA and DNA to the N is similar; both nucleic acids assume a nonstandard right-handed helical structure in the binary complexes (Fig. S4D).
Mode of Interaction of N Protein with ssRNA/ssDNA.
RNA binds in a positively charged cavity at the junction of the N and C lobes (Fig. 2A). The cavities of adjacent protomers are lined up sequentially, giving the appearance of a continuous circular channel running along the inner edge of the ring-like assembly. This RNA binding channel is dominated by positively charged amino acids such as lysines, arginines, and histidines. The dimensions of the cavity can accommodate ssRNA or ssDNA only, but not dsRNA or dsDNA. Each LEAV N binds 11 nt of ssRNA (Fig. S4E), eight bases at the center and three bases at the interface of the protomers (Fig. 2B). Although all of the bases were modeled as uracils, extra densities around some of the uracils suggest that purines could be modeled without introducing any steric obstruction. A series of positively charged side chains twist the sugar–phosphate backbone from one end of the protomer to another such that base 9 has rotated almost 225° with respect to base 2 along the horizontal axis of the RNA-binding channel. Of the eight RNA nucleotides that contact LEAV N protein, the 5′ base 1 interacts with F20, N21, P22, Q185, and R186. Base 2 stacks up against base 3, which is held in position by a hydrogen bond between the backbone phosphate and the backbone amide N of S18. Bases 2, 3, 4, and 5 stack against each other and are in vicinity of positively charged side chains such as K50, K53, K57, R84, R180, K184, and R186. The aromatic ring of F178 intercalates and stacks against bases 5 and 6, resulting in a twist of the sugar–phosphate backbone such that bases 6–8 are inserted deep into the RNA binding cavity. Similar to F178, P127 further twists the RNA backbone, giving rise to a pseudo-right-handed helical structure (Fig. 2 B and C). Nucleotides 9, 10, and 11 have hardly any contact with the protein, and the RNA could potentially exit the ring-like assembly from this gap at the N–N interface and enter into the next ring-like assembly of LEAV N protomers. Alternatively, base 11 stacks up against base 13 (corresponding to base 2 of the next protomer), resulting in a sharp turn of the RNA backbone that keeps the RNA inside the ring-like tertiary assembly. Base 12 (corresponding to base 1 of next protomer) starts the next repeat (Fig. S4E). Thus, the viral genome is protected from cellular nucleases by covering it with N using primarily electrostatic and hydrophobic interactions.
Fig. 2.
Interactions of LEAV N with RNA. (A) Surface electrostatic potential representation of LEAV N bound with ssRNA. A positively charged cavity between the N and C lobes tethers ssRNA. (B) Drawing depicting details of the interactions between LEAV N and RNA. The residues donating hydrogen bonds are colored in cyan (C chain) and yellow (D chain). F178 and P127, shown in purple, induce a pseudohelical turn of the RNA. B1–B11 are the bases of ssRNA from 5′ to 3′. (C) Schematic drawing highlighting LEAV N– ssRNA interactions. The coloring scheme is same as in B.
Inspection of the binary complex of LEAV N with ssDNA revealed an identical mode of binding of nucleic acids. The structure independently confirms a role for P127 and F178 in interaction with the bases. As observed in the ssRNA-bound structure, the aromatic rings of P127 and F178 are inserted between the bases, resulting in twisting of the ssDNA chain such that three bases get buried into the protein while the rest are either oriented toward the solvent or are facing the interface (Fig. S4F). The nature of the interactions tethering the ssDNA to LEAV N is similar. Thus, the N protein binds ssRNA and ssDNA in a similar fashion. However, the structures differ in the number of nucleotides bound by the N. Electron density along the inner edge of the tetramer permitted modeling of only 34 out of two 24-nt-long chains of DNA used for crystallization. Density for other nucleotides of DNA was missing. In contrast, clear electron density for a similar tetramer of LEAV N bound with RNA suggested that the tetramer of LEAV N bound 44 nt of RNA. This difference could be attributed to the differences in affinity of the N for DNA and RNA. Structures of the binary complexes clearly explain the higher affinity of the N protein for RNA versus DNA. The 2′OH group of the ribose sugar of nucleotides 1, 3, 7, and 9 is involved in hydrogen bonding (Fig. 2C), increasing the affinity of RNA for LEAV N. This is in agreement with the ITC results, in which the LEAV N showed higher affinity for ssRNA over ssDNA.
Using the structures of the binary complexes of LEAV N as a guide, we carried out mutagenesis studies to identify residues important for binding nucleic acids. Initially, 17 single- and multiple-point mutations of amino acids in vicinity of the RNA were constructed and expressed in E. coli. After purification, the A260/280 ratios for all of the single-point mutants were more than 1.10, indicating that N proteins harboring single mutations retained their ability to bind RNA. However, the double mutants K50E/K53E, K181E/K184E, and K53E/K184E exhibited markedly lower A260/280 ratios ranging from 0.55 to 0.86, which was similar to that of WT LEAV N without RNA. Interestingly, positions of K50, K53, K181, and K184 are highly conserved across viruses from the Orthobunyavirus genus, suggesting a conserved role for these amino acids in binding RNA. A complete list of mutations and their A260/280 ratios can be found in Table S2.
N- and C-Terminal Extensions Are Essential for Oligomerization.
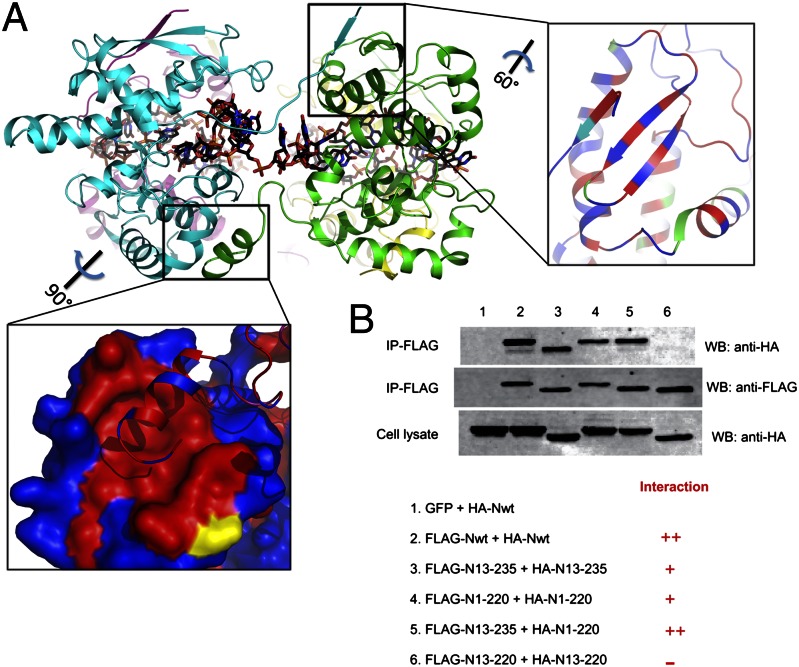
In the binary complex of LEAV N with ssRNA, each protomer of N is observed to interact with two other protomers, one on each side. Extensions at the N and C terminus protruding out of the protein to the left and right, respectively, carry out oligomerization (Fig. 3A). Amino acids 1–12 of a long loop (1–20) protruding out of the core domain of one protomer sit snugly into a hydrophobic cavity (L47, I44, S55, V64, and F68) surrounded by charged residues (R43, N48, K51, Q67, and D65) formed on the surface of the N lobe of a protomer placed to its left side (Fig. 3A). The intermolecular interactions of this region are characterized by three hydrogen bonds and several van der Waal’s interactions. Similarly, the C-terminal aa 221–235 located on the opposite face of the protein protrude out and sit into a cavity formed by three α-helices of the C lobe of the protomer to its right side. The interaction is primarily hydrophobic with F227, F231, and L228 from the extension thrust into the cavity formed by V200, I203, A163, I166, Q167, V169, V170, I176, L183, M179, Q204, V207, and M196 (Fig. 3A). Other than the intermolecular interactions of these two N- and C-terminal extension regions there are no additional contacts between the protomers.
Fig. 3.
N- and C-terminal extensions play a critical role in oligomerization of LEAV N. (A) Ribbon diagram of the LEAV N–ssRNA ring as viewed from the sides. The N-terminal extension arm stacks up a strand on the surface of the neighboring protomer, and the helix from the C-terminal extension sits in a hydrophobic cavity formed by three α-helices of the neighboring protomer. Red represents hydrophobic surface; blue represents hydrophilic surface. (B) The N- and C-terminal extension arm’s role in mediating the homotypic interactions between LEAV N protomers was examined in a cell-based coimmunoprecipitation assay.
The role of the N- and C-terminal extensions in mediating the homotypic interaction between LEAV N protomers was examined in a cell-based coimmunoprecipitation assay (Fig. 3B). Removal of either N- or C-terminal extension (N13–235 and N1–220, respectively) diminished N–N interaction. However, truncations of LEAV N lacking both the N- and C-terminal extensions (N13–220) lost their ability to interact with other protomers completely. Thus, the N- and C-terminal extensions play a vital role in the oligomerization of LEAV N, which is essential for viral genome encapsidation.
RNPs of LEAV N Are Filamentous.
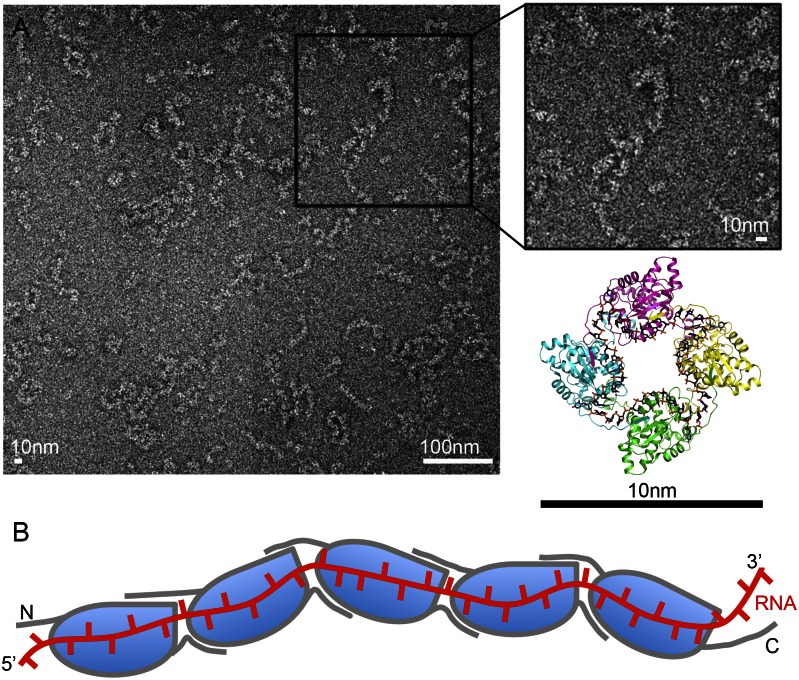
To gain insights into the nature of the packing of the LEAV genomic RNA, we obtained cryogenic EM images of LEAV RNPs that eluted in two separate peaks during SEC of LEAV N (Fig. S2A). LEAV N–RNA complexes from peak 2 had crystallized as a tetramer forming a circular ring-like assembly. EM analysis of this sample of LEAV N revealed a circular ring-like assembly with a diameter of about 10 nm consistent with the crystal structure (Fig. S5). To find out whether the genomic RNA is packed using these ring-like assemblies, we obtained EM images of highly polymeric RNPs of LEAV N from the peak 1 of SEC. Interestingly, the images showed a filamentous assembly of genomic RNA similar to phleboviruses (Fig. 4A). The N protein seems to thread viral RNA similarly to beads threading a string.
Fig. 4.
RNPs of LEAV N are filamentous. (A) Electron microscopic image of negative-stained LEAV N–ssRNA complexes (peak 1) show that the bacterially expressed, recombinant RNPs are filamentous. The diameter of the LEAV N–ssRNA complex is about 10 nm. (B) A plausible model of the RNP of LEAV N. The N and C terminals mediate oligomerization of LEAV N, with the RNA running along the positively charged cavity between the N and C lobes of successive protomers.
Discussion
The nature of the N protein has been shown to differ markedly between viruses from different genera. Here, we have described a representative 3D structure of N protein from the Orthobunyavirus genus. Our structural studies unveil a unique fold of N protein used by viruses to encapsidate genomic RNA. The binary complex of LEAV N with ssRNA of bacterial origin neatly captures the interaction of the ssRNA with the N protein and clearly explains the protection offered by the LEAV N to genomic RNA. Binding of N protein prevents access of viral RNA by the host defense machinery. Alignment of 43 unique sequences of LEAV N and its homologs from the Orthobunyavirus genus is depicted in Fig. S6 (33, 34). Mapping of the secondary structural elements of LEAV N over the alignment reveals that the RNA binding cavity is conserved across the homologs examined and is formed by highly conserved residues (Fig. S6). Further, comparison of the binary complexes of N proteins with ssRNA from five different ring-like negative ssRNA viruses reveals that the overall mode of sequestration of ssRNA by N proteins might be conserved. All N proteins are bimodular with the RNA binding site located in the deep chasm at the junction of the two lobes. Oligomerization is accomplished by using N- and C-terminal extensions (with the exception of RVFV, which uses only an N-terminal extension) that fit snugly into cavities of adjacent protomers, resulting in a ring-like circular tertiary assembly and helps bridge the RNA binding sites between adjacent N proteins, forming a contiguous channel. The RNA binding site runs along the inner edge (VSV, RV, RVFV, and LEAV) (4, 5, 8) or outer edge (RSV) (6) of the circular assembly (Fig. S7). RNA is bound by tethering the backbone phosphates and contacting at least three bases in the positively charged cavity. For example, N from VSV, RV, RSV, and LEAV contacts three stacked-up bases. Except for these three bases that are embedded deep into the cavity, all other bases are either facing the protein or pointing toward the solvent side. This mode of RNA binding is in sharp contrast to the ssRNA binding by RVFV, where all of the bases are oriented into the cavity. One would expect hydrogen bonding and some sort of sequence specificity for such a mode of binding RNA. However, RVFV N avoids hydrogen bonding with bases by using a hydrophobic environment to engage the π-electrons of the bases. For example, the side chains of I180, F176, T195, P182, P199, V179, P127, A203, and F33 make up the pocket that interacts with the bases. The RNA backbone is tethered using electrostatic interactions of the phosphates with the side chains of K67, R70, D96, and R106. Such a mode of binding RNA prevents formation of hydrogen bonds with the bases. However, the binding pocket of LEAV N is lined up with positively charged side chains on either side of the pocket that probably enhance the electropositive environment for binding RNA. However, barring only a few exceptions, the density for most of the side chains surrounding the bases is missing. Electron density for side chains of N21, R168, and R186 (B chain) reveals that these side chains donate hydrogen bonds to bases. Thus, LEAV N binds ssRNA by hydrogen bonding with the backbone and possibly with the bases as well. Interestingly, the number and length of the hydrogen bonds seem to vary among the four chains of the tetramer (Fig. 2C and Table S3). Previous studies based on extraction and sequencing of bacterial RNA bound by LEAV N’s homolog, BUNV N, during expression in bacterial cells seem to rule out sequence specific binding of RNA by BUNV N (23, 24). Perhaps the specificity could be limited to weak or tight binding, giving rise to regions of RNA that could be preferentially or easily uncapsidated for initiation of events such as replication or transcription (35–37).
Viral RNA is encapsidated by N proteins resulting in the formation of RNP complexes. EM images reveal that the RNP complexes of LEAV N assume a filamentous form (Fig. 4A), similar to those observed for phleboviral N proteins (8, 15). Thus, although the structure of the LEAV N is novel, the overall mode of tethering the viral RNA is similar to phleboviruses. The structure of LEAV N in complex with ssRNA and the EM images explain the mode of encapsidation by LEAV N (Fig. 4B). Furthermore, such encapsidation needs to be reversible so that polymerases can gain access to RNA for replication or transcription. This would require parts of the LEAV N to be flexible. We subjected LEAV N to Hinge-Prot (38) analysis to find out whether any secondary structural elements of LEAV N could move. The analysis predicted a hinge movement for the N-terminal 1–19 amino acids around hinge residue F20. Amino acids 1–19 are part of the N-terminal extension region that lies above the gap between two monomers. The RNA makes a sharp turn at the junction of monomers. Thus, a hinge movement of residues 1–19 could result in exposure of the RNA for assembly of the initiation complex. Assembly of the complex probably triggers further uncapsidation via a second hinge movement around V125. Hinge residue V125 is located between the N and C lobes. A movement around this residue opens the RNA binding site wide, exposing the RNA and making it available as a template for the polymerases. Elucidation of molecular events leading to the hinge movement could help explain precisely the triggers for uncapsidation. The knowledge of such events offers a unique opportunity for therapeutic interventions.
The structure of LEAV N in complex with ssRNA provides important information for designing antiviral drugs. The structure reveals that the ssRNA contacts a large area of the N protein with numerous interactions tethering the RNA to the protein. Clearly, it is hard to envisage a small molecule disrupting these interactions and dislodging the RNA completely from the protein. Instead, the results of our structure–function studies suggest that peptides or small molecules that interfere or obstruct the oligomerization of N proteins could be more effective in stalling the life cycle of the virus. In particular, the N- and C-terminal extension regions that mediate oligomerization of N protein could be targeted for development of antiviral drugs.
While the manuscript was under review, the structure of BUNV N belonging to the same Orthobunyavirus genus as LEAV N of the present study (the sequence identity between BUNV N and LEAV N is 39.8%) was solved by Li et al. (39). The overall structure of BUNV N is similar to that of LEAV N. Superimposition studies reveal that the two structures can be superimposed with an rmsd of 1.4 Å over 204 matching Cα atoms. These results further illustrate the fact that viruses belonging to the same genus use a highly conserved fold to tether viral genomic nucleic acids, whereas N proteins of viruses belonging to different genera differ markedly. Similar to LEAV N, the recombinant BUNV N assembles into a tetramer using a similar mode of oligomerization. The RNA binding site is located along the inner edge of the ring-like tetrameric assembly of N. Interestingly, the tetramer of BUNV N has been modeled with 36-nt RNA instead of the 44-nt RNA in this study of LEAV N.
In summary, the structure of the LEAV N–ssRNA complex unveils a unique fold used by viruses to cover their genome to evade host defense machinery. The structure described here is a unique representative structure for the nucleoproteins from Orthobunyavirus genus. Our crystallographic studies on the binary complex of LEAV N with ssRNA in combination with the EM results of the LEAV RNP suggest a filamentous mode of packing of the viral genome. Sequence alignment reveals that structures of N of viruses belonging to this genus are conserved. Thus, the structure of LEAV N could be used to design broad-spectrum inhibitors that could be effective in combating diseases caused by viruses belonging to the Orthobunyvirus genus.
Materials and Methods
Detailed discussion of materials and methods is given in SI Materials and Methods.
Protein Production.
The N proteins from four orthobunyaviruses (LEAV, BUNV1, BUNV2, and LACV) were overexpressed using E. coli. Cells were lysed by sonication. Cell debris was removed by centrifugation and the clarified supernatant was loaded onto a 5-mL nickel-nitrilotriacetic acid resin gravity column (Qiagen). After thorough washing, the bound LEAV N protein was eluted using imidazole. The protein was further purified using SEC on a Superdex G200 SEC column (Amersham). The RNA-free N protein of LEAV (A260/280 ∼0.58) was produced by the addition of 1 M NaCl and 3 M urea to the lysis buffer during ultrasonication followed by on-column refolding using a Ni-affinity chromatography column. A gradient (3 M to 0 M) of urea was used to wash the bound protein for refolding. The reconstituted LEAV N–ssRNA/ssDNA complex was produced by incubating RNA-free N protein with 24-nt ssRNA or 24-nt ssDNA at a molar ratio of 1:2 for 1 h in an ice bath. Excess nucleic acids were removed by gel-filtration chromatography.
Crystallization and Data Collection.
All of the N proteins with or without ssRNA/ssDNA were initially screened for crystallization by the hanging drop vapor-diffusion method using commercially available sparse matrix screens. The screening was done at 16 °C using a Mosquito (TTP LabTech) robot. Crystals were optimized manually by mixing 1 μL of the protein solution with an equal volume of a reservoir solution and equilibrating the mixed drop against 500 μL of reservoir solution. Diffraction data for native LEAV N–ssRNA/ssDNA complexes were collected at 100 K using an ADSC Q315 CCD detector on beamline BL17U1 of the Shanghai Synchrotron Radiation Facility and at beamline BL5.0.1 of the Advanced Light Source, Lawrence Berkeley National Laboratory. Anomalous diffraction data for the selenomethionine LEAV N–ssRNA complex was collected to 3.08 Å at 100 K using an ADSC Q315 CCD detector on beamline BL17A at the Photon Factory, High Energy Accelerator Research Organization.
Data Processing and Structure Determination.
All datasets were indexed, integrated, and scaled using the HKL2000 software package (40). The LEAV N–ssRNA complex crystallized in space group P1 with unit cell parameters of a = 49.84, b = 73.83, and c = 79.88 Å. The initial phases were determined using X2DF structure determination pipeline (28, 29) by the Se–SAD method (27) and the initial model was built by PHENIX AutoBuild (41). The LEAV N–ssDNA complex structure was solved by Molecular Replacement (301) using the solved LEAV N–ssRNA structure as a search model. The LEAV N–ssRNA/ssDNA complex models were manually improved in Coot (42). Refinement was carried out using Refmac (43) and PHENIX Refine (41), alternately.
Negative Staining and Electron Microscopy.
The LEAV N–ssRNA complex eluted as two peaks during Superdex G200 SEC, which were analyzed separately by electron microscopy. The sample was diluted to 0.1 mg/mL in a buffer containing 20 mM Tris⋅HCl (pH 7.5) and 150 mM NaCl and negative-stained with uranyl acetate as described previously (44). The images were recorded using a Tecnai F20 electron microscope (FEI) equipped with a field emission gun and operated at an acceleration voltage of 200 kV.
Supplementary Material
Acknowledgments
The authors thank the staff at synchrotron beamlines (17U1 of the Shanghai Synchrotron Radiation Facility, 17A of the High Energy Accelerator Research Organization, and 5.0.1 of Advanced Light Source) for their kind help with data collection; Y. Han, Y. Y. Chen, and Y. Wang at the Protein Science Core Facility of the Institute of Biophysics for technical help with X-ray diffraction studies, isothermal titration calorimetry experiments, and automated crystallizations; and Julie Liu for preparing the illustration chart of LEAV N with RNA filamentous formation. This work is supported by Ministry of Health of China Grant 2013ZX10004-602, Ministry of Science and Technology of China Grants 2013CB911103, 2009CB918803, and 2011CB911103, and National Natural Science Foundation of China Grants 31270795, 31200559, 31070660, and 31021062.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4J1G and 4J1J).
See Commentary on page 8769.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300035110/-/DCSupplemental.
References
- 1.Hastie KM, King LB, Zandonatti MA, Saphire EO. Structural basis for the dsRNA specificity of the Lassa virus NP exonuclease. PLoS ONE. 2012;7(8):e44211. doi: 10.1371/journal.pone.0044211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi X, et al. Cap binding and immune evasion revealed by Lassa nucleoprotein structure. Nature. 2010;468(7325):779–783. doi: 10.1038/nature09605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, et al. Crimean-Congo hemorrhagic fever virus nucleoprotein reveals endonuclease activity in bunyaviruses. Proc Natl Acad Sci USA. 2012;109(13):5046–5051. doi: 10.1073/pnas.1200808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albertini AA, et al. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science. 2006;313(5785):360–363. doi: 10.1126/science.1125280. [DOI] [PubMed] [Google Scholar]
- 5.Green TJ, Zhang X, Wertz GW, Luo M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science. 2006;313(5785):357–360. doi: 10.1126/science.1126953. [DOI] [PubMed] [Google Scholar]
- 6.Tawar RG, et al. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science. 2009;326(5957):1279–1283. doi: 10.1126/science.1177634. [DOI] [PubMed] [Google Scholar]
- 7.Hastie KM, et al. Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding. Proc Natl Acad Sci USA. 2011;108(48):19365–19370. doi: 10.1073/pnas.1108515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond DD, Piper ME, Gerrard SR, Skiniotis G, Smith JL. Phleboviruses encapsidate their genomes by sequestering RNA bases. Proc Natl Acad Sci USA. 2012;109(47):19208–19213. doi: 10.1073/pnas.1213553109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savji N, et al. Genomic and phylogenetic characterization of Leanyer virus, a novel orthobunyavirus isolated in northern Australia. J Gen Virol. 2011;92(Pt 7):1676–1687. doi: 10.1099/vir.0.028308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter CT, Barr JN. Recent advances in the molecular and cellular biology of bunyaviruses. J Gen Virol. 2011;92(Pt 11):2467–2484. doi: 10.1099/vir.0.035105-0. [DOI] [PubMed] [Google Scholar]
- 11.Alfadhli A, et al. Hantavirus nucleocapsid protein oligomerization. J Virol. 2001;75(4):2019–2023. doi: 10.1128/JVI.75.4.2019-2023.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaukinen P, Koistinen V, Vapalahti O, Vaheri A, Plyusnin A. Interaction between molecules of hantavirus nucleocapsid protein. J Gen Virol. 2001;82(Pt 8):1845–1853. doi: 10.1099/0022-1317-82-8-1845. [DOI] [PubMed] [Google Scholar]
- 13.Kaukinen P, et al. Oligomerization of Hantavirus N protein: C-terminal alpha-helices interact to form a shared hydrophobic space. J Virol. 2004;78(24):13669–13677. doi: 10.1128/JVI.78.24.13669-13677.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaukinen P, Vaheri A, Plyusnin A. Mapping of the regions involved in homotypic interactions of Tula hantavirus N protein. J Virol. 2003;77(20):10910–10916. doi: 10.1128/JVI.77.20.10910-10916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferron F, et al. The hexamer structure of Rift Valley fever virus nucleoprotein suggests a mechanism for its assembly into ribonucleoprotein complexes. PLoS Pathog. 2011;7(5):e1002030. doi: 10.1371/journal.ppat.1002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter SD, et al. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J Virol. 2012;86(20):10914–10923. doi: 10.1128/JVI.01555-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, et al. 2012. Structure of Crimean-Congo haemorraghic fever virus nucleoprotein: Superhelical homo-oligomers and the role of caspase-3 cleavage. J Virol 86(22):12294–12303.
- 18.Leonard VH, Kohl A, Osborne JC, McLees A, Elliott RM. Homotypic interaction of Bunyamwera virus nucleocapsid protein. J Virol. 2005;79(20):13166–13172. doi: 10.1128/JVI.79.20.13166-13172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tauro LB, Venezuela RF, Spinsanti LI, Konigheim BS, Contigiani MS. First case of human infection with a Bunyamwera serogroup virus in Argentina. J Clin Virol. 2012;54(1):98–99. doi: 10.1016/j.jcv.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Beer M, Conraths FJ, van der Poel WH. ‘Schmallenberg virus’—A novel orthobunyavirus emerging in Europe. Epidemiol Infect. 2013;141(1):1–8. doi: 10.1017/S0950268812002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann B, et al. Novel orthobunyavirus in cattle, Europe, 2011. Emerg Infect Dis. 2012;18(3):469–472. doi: 10.3201/eid1803.111905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuckly KG, Wright PJ. Characterization of Leanyer virus: Resemblance to Bunyavirus. Aust J Exp Biol Med Sci. 1983;61(Pt 2):193–200. doi: 10.1038/icb.1983.18. [DOI] [PubMed] [Google Scholar]
- 23.Mohl BP, Barr JN. Investigating the specificity and stoichiometry of RNA binding by the nucleocapsid protein of Bunyamwera virus. RNA. 2009;15(3):391–399. doi: 10.1261/rna.1367209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter CT, Bento DF, Alonso AG, Barr JN. Amino acid changes within the Bunyamwera virus nucleocapsid protein differentially affect the mRNA transcription and RNA replication activities of assembled ribonucleoprotein templates. J Gen Virol. 2011;92(Pt 1):80–84. doi: 10.1099/vir.0.024240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green TJ, et al. Access to RNA encapsidated in the nucleocapsid of vesicular stomatitis virus. J Virol. 2011;85(6):2714–2722. doi: 10.1128/JVI.01927-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang S, et al. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36(6):1073–1086. doi: 10.1016/j.immuni.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendrickson WA. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science. 1991;254(5028):51–58. doi: 10.1126/science.1925561. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z-J, et al. Parameter-space screening: A powerful tool for high-throughput crystal structure determination. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 5):520–527. doi: 10.1107/S0907444905003239. [DOI] [PubMed] [Google Scholar]
- 29.Ru H, et al. S-SAD phasing study of death receptor 6 and its solution conformation revealed by SAXS. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 5):521–530. doi: 10.1107/S0907444912004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Holm L, Rosenström P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010;38(Web Server issue) suppl 2:W545-9. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouet P, Courcelle E, Stuart DI, Métoz F. ESPript: Analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15(4):305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- 35.Barr JN, Rodgers JW, Wertz GW. Identification of the Bunyamwera bunyavirus transcription termination signal. J Gen Virol. 2006;87(Pt 1):189–198. doi: 10.1099/vir.0.81355-0. [DOI] [PubMed] [Google Scholar]
- 36.Osborne JC, Elliott RM. RNA binding properties of bunyamwera virus nucleocapsid protein and selective binding to an element in the 5′ terminus of the negative-sense S segment. J Virol. 2000;74(21):9946–9952. doi: 10.1128/jvi.74.21.9946-9952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr JN, Elliott RM, Dunn EF, Wertz GW. Segment-specific terminal sequences of Bunyamwera bunyavirus regulate genome replication. Virology. 2003;311(2):326–338. doi: 10.1016/s0042-6822(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 38.Emekli U, Schneidman-Duhovny D, Wolfson HJ, Nussinov R, Haliloglu T. HingeProt: Automated prediction of hinges in protein structures. Proteins. 2008;70(4):1219–1227. doi: 10.1002/prot.21613. [DOI] [PubMed] [Google Scholar]
- 39. Li B, et al. (2013) Bunyamwera virus possesses a distinct nucleocapsid protein to facilitate genome encapsidation. Proc Natl Acad Sci USA 110:9054–9059. [DOI] [PMC free article] [PubMed]
- 40.Otwinowski Z, Minor W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Part A, Methods in Enzymology, eds Carter, Jr. CW, Sweet RM (Academic, New York), Vol 276, pp 307–326.
- 41.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 44.Ohi M, Li Y, Cheng Y, Walz T. Negative staining and image classification — Powerful tools in modern electron microscopy. Biol Proced Online. 2004;6(1):23–34. doi: 10.1251/bpo70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.