Abstract
Posttranslational knockdown of a specific protein is an attractive approach for examining its function within a system. Here we introduce phospho-dependent proteolysis targeting chimeras (phosphoPROTACs), a method to couple the conditional degradation of targeted proteins to the activation state of particular kinase-signaling pathways. We generated two phosphoPROTACs that couple the tyrosine phosphorylation sequences of either the nerve growth factor receptor, TrkA (tropomyosin receptor kinase A), or the neuregulin receptor, ErbB3 (erythroblastosis oncogene B3), with a peptide ligand for the E3 ubiquitin ligase von Hippel Lindau protein. These phosphoPROTACs recruit either the neurotrophic signaling effector fibroblast growth factor receptor substrate 2α or the survival-promoting phosphatidylinositol-3-kinase, respectively, to be ubiquitinated and degraded upon activation of specific receptor tyrosine kinases and phosphorylation of the phosphoPROTACs. We demonstrate the ability of these phosphoPROTACs to suppress the short- and long-term effects of their respective activating receptor tyrosine kinase pathways both in vitro and in vivo. In addition, we show that activation of phosphoPROTACs is entirely dependent on their kinase-mediated phosphorylation, as phenylalanine-containing null variants are inactive. Furthermore, stimulation of unrelated growth factor receptors does not induce target protein knockdown. Although comparable in efficiency to RNAi, this approach has the added advantage of providing a degree of temporal and dosing control as well as cell-type selectivity unavailable using nucleic acid-based strategies. By varying the autophosphorylation sequence of a phosphoPROTAC, it is conceivable that other receptor tyrosine kinase/effector pairings could be similarly exploited to achieve other biological effects.
Keywords: protein degradation, SH2 domain, signal transduction
Antagonizing the function of a protein is an effective strategy for determining its role within a cellular context. For some enzymes and/or receptor proteins, this can be accomplished by incubation with a small molecule inhibitor or antagonist. However, specific small molecule inhibitors have not been discovered or developed for many proteins. As alternative strategies, gene deletion and RNAi approaches function at the DNA and mRNA levels to reduce protein expression, thus offering the ability to control function even for those proteins lacking a specific inhibitor (1, 2). However, such nucleic acid-based approaches do not afford the same rapid, direct assessment of protein function as posttranslational intervention; furthermore, in vivo application of these strategies is even today still complicated and relatively cumbersome. Combining the attractive qualities of both RNAi and small molecule inhibitor approaches, we developed a strategy, proteolysis targeting chimeras (PROTACs) for targeted posttranslational knockdown of proteins. Each PROTAC is heterodimeric, consisting of an E3 ubiquitin ligase-binding moiety linked to a ligand that binds to the target protein (3). As such, each PROTAC recruits its target protein to the E3 ubiquitin ligase, resulting in target protein ubiquitination by the E3 ligase and, ultimately, proteasome-mediated degradation of the target (4–6). Targeting proteins for degradation using PROTACs offers direct intervention at the protein level without the restriction to targets with established small molecule inhibitors because ligands that bind to any site on the target protein can be incorporated into a PROTAC. Moreover, given the irreversibility of protein degradation, PROTAC-mediated control of protein function yields physiological consequences akin to those of RNAi and/or irreversible small molecule inhibitors.
As originally conceived, PROTACs work constitutively to induce targeted protein ubiquitination/degradation irrespective of any intracellular signaling context (4, 6, 7). Here, we advance this paradigm by coupling PROTAC-mediated protein degradation to the activation state of a particular signaling pathway. Specifically, we tested whether a PROTAC can selectively distinguish between an activated versus a quiescent receptor tyrosine kinase-signaling pathway. Because phosphorylated tyrosine residues can serve as ligands for the binding of phosphotyrosine-binding (PTB) and Src homology 2 (SH2) domain-containing proteins (8), a cell-permeable PROTAC molecule that incorporates a specific tyrosine kinase substrate sequence may do the same (9). Thus, phosphorylation of such a PROTAC by a receptor tyrosine kinase (RTK) could generate a binding site for a downstream PTB or SH2 domain-containing protein and its subsequent recruitment to the ubiquitin/proteasome degradation pathway for destruction, thereby disrupting tyrosine kinase signaling (Fig. 1 A and B). In this manner, the phosphoPROTAC would serve as a PROTAC whose activity was inducible, or “conditional” upon activation of a given receptor tyrosine kinase, thus creating an artificial negative feedback mechanism. Our previous study regarding the regulation of nerve growth factor (NGF) activity by small molecules (10) suggested that TrkA (the NGF receptor) signaling would be an interesting and suitable pathway in which to investigate the possibility and/or utility of our signaling context-dependent protein knockdown strategy. The effector protein fibroblast growth factor receptor substrate 2α (FRS2α) (also known as SNT-1) (11, 12) emerged as an attractive target protein candidate, given its importance in TrkA signaling and its lack of any enzymatic activity that could be blocked with traditional small molecule inhibitors. Our success in this system subsequently led us to expand the study to the erythroblastosis oncogene (Erb)B2/ErbB3 RTK pathway to show that the phosphoPROTAC strategy can be adapted for other receptor tyrosine kinase pathways and may even hold therapeutic potential.
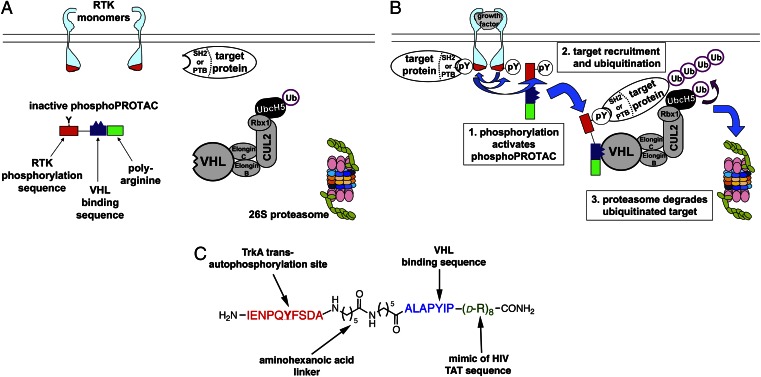
Fig. 1.
(A and B) Schematic diagram of conditional posttranslational target protein knockdown by a phosphoPROTAC. (A) In the absence of an activated RTK, the phosphoPROTAC is not phosphorylated and fails to bind to the target protein, sparing it from degradation. (B) Upon activation of the RTK, the phosphoPROTAC is phosphorylated, creating a binding site for the SH2- or PTB-domain–containing effector protein and its subsequent recruitment for ubiquitination by VHL and proteasomal degradation. (C) Schematic of phosphoPROTAC TrkAPPFRS2α: the red peptide sequence corresponds to the TrkA autophosphorylation site; the blue peptide sequence enables binding to the E3 ubiquitin ligase VHL; and the green poly-d-arginine motif permits cell permeability of the peptide.
Results
Designing a PhosphoPROTAC Activated by NGF-TrkA Signaling.
Following sustained NGF treatment, rat pheochromocytoma PC12 cells differentiate into a postmitotic neuronal phenotype (13). Thus, PC12 cells have been used extensively to study NGF-dependent neuronal differentiation and the signaling induced by NGF binding to its receptor tyrosine kinase, TrkA. NGF-induced dimerization results in TrkA transautophosphorylation on specific tyrosine residues in its intracellular domain (14), thereby fully activating the kinase. In addition, transautophosphorylation on other tyrosine residues creates binding sites for SH2 and PTB domain-containing substrate proteins, which, upon recruitment, are themselves phosphorylated by TrkA. One such substrate is FRS2α, a myristoylated, membrane-tethered protein and a major downstream effector for TrkA-driven neuronal differentiation. The PTB domain-containing FRS2α binds to phosphorylated TrkA and, following its own phosphorylation, serves as a scaffolding protein to coordinate downstream signaling pathways (15). FRS2α-mediated signaling includes the B-rapidly accelerated fibrosarcoma (B-Raf)/mitogen-activated erk kinase (MEK)/extracellular signal-regulated kinase 1 and 2 (Erk1/2) pathway, sustained activation of which is essential to drive the changes in protein expression and cell morphology characteristic of neuronal differentiation (16).
We designed a phosphorylation-dependent PROTAC, or phosphoPROTAC (PP) that would knockdown FRS2α levels upon NGF activation of TrkA. We designated this molecule, “TrkAPPFRS2α.” This phosphoPROTAC contains a TrkA-derived decapeptide sequence (Fig. 1C) whose central tyrosine is phosphorylated by TrkA following NGF binding and receptor dimerization (12). We hypothesized that this sequence in TrkAPPFRS2α would also be phosphorylated by NGF-bound TrkA and thus enable TrkAPPFRS2α to similarly bind FRS2α. In turn, to target the FRS2α:TrkAPPFRS2α complex for ubiquitination of FRS2α, the phosphoPROTAC also contains a seven-amino-acid sequence derived from the transcription factor hypoxia-inducible factor-1α(HIF-1α), which serves as a recognition sequence for binding to the von Hippel Lindau (VHL) tumor suppressor (17). VHL is the substrate recognition component of a larger E3 ubiquitin ligase complex that ubiquitinates lysine residues of bound proteins, targeting them to the proteasome for proteolytic degradation. In addition, a poly-d-arginine tag was incorporated (Fig. 1C) to enhance cell permeability via the same mechanism used by the HIV transactivator of transcription (Tat) protein (6, 18–20). PhosphoPROTAC peptides were synthesized using standard solid-phase Fmoc coupling, and the cleaved peptide product was typically between 82% and 88% pure (Figs. S1 and S2).
Incubation of this peptide with freshly prepared membrane fraction from NGF-treated PC12 cells supplemented with ATPγ[32P] revealed the incorporation of [32P]phosphate into TrkAPPFRS2α (Fig. S3) compared with incubation of the peptide with membrane fraction from untreated PC12 cells. This increase in 32P content was not observed when we incubated membrane fraction from NGF-treated cells with a phosphoPROTAC variant possessing a phenylalanine instead of the tyrosine residue within the TrkA transautophosphorylation motif. The variant peptide cannot be phosphorylated, or is a “null” phosphoPROTAC (NP), which we designated as TrkANPFRS2α. Furthermore, incubation of TrkAPPFRS2α with membrane fraction from cells treated with epidermal growth factor (EGF) or insulin-like growth factor-1 (IGF1), neither of which activate TrkA, did not increase 32P incorporation into TrkAPPFRS2α. This indicated that activation of the phosphoPROTAC should be specific to NGF treatment.
Efficacy and Selectivity of a FRS2α-Targeting PhosphoPROTAC.
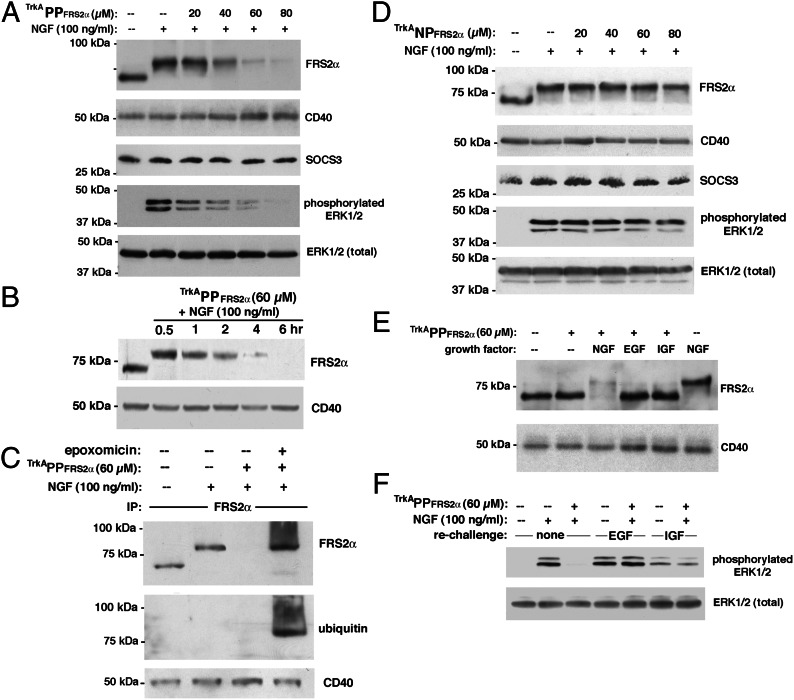
To test the activity of TrkAPPFRS2α, we incubated PC12 cells with increasing concentrations of the phosphoPROTAC in the presence of NGF (100 ng/mL) for a total of 7 h (Fig. 2A). Whereas treatment with NGF alone resulted in the phosphorylation of FRS2α (as evidenced by the upward band shift), addition of TrkAPPFRS2α at 40 µM reduced FRS2α levels by ∼50%. Furthermore, concentrations ≥60 µM of TrkAPPFRS2α were maximally effective and knocked down FRS2α to <10% of control. We saw no decrease in the levels of two other unrelated proteins—the integral membrane protein CD40 (21) as well as the SH2 domain-containing protein suppressor of cytokine signaling 3 (SOCS3) (22), which suggested that protein knockdown by TrkAPPFRS2α was selective for the target. To confirm the functional significance of FRS2α protein knockdown, we next investigated the ability of NGF to activate Erk1/2 in the presence of TrkAPPFRS2α. Concordant with FRS2α knockdown, Erk1/2 activation by NGF was inhibited by TrkAPPFRS2α. However, this reduction of Erk1/2 activation by NGF was not due to overall reduction of Erk1/2 levels, confirming decreased NGF-mediated signaling in TrkAPPFRS2-treated cells.
Fig. 2.
Function of TrkAPPFRS2α, a phosphoPROTAC peptide that conditionally targets FRS2α for degradation upon TrkA activation. (A) Selective knockdown of FRS2α by TrkAPPFRS2α and inhibition of NGF-dependent Erk1/2 activation in PC12 cells. Cells were incubated with the phosphoPROTAC for total of 7 h. (B) Knockdown of FRS2α by TrkAPPFRS2α is time dependent. Cells were incubated with 60 µM TrkAPPFRS2α for 30 min to 6 h. (C) Activated TrkAPPFRS2α recruits FRS2α to be ubiquitinated. PC12 cells were pretreated with 100 nM epoxomicin for 1 h before incubation with 60 µM TrkAPPFRS2α and with NGF for 7 h before lysis in boiling RIPA buffer. (D) Phosphorylation dependence of FRS2α knockdown by phosphoPROTAC. Phospho-null variant, TrkANPFRS2α, is inactive in the presence of NGF. (E) Knockdown of FRS2α by TrkAPPFRS2α is dependent on activation of TrkA but not on other receptor tyrosine kinases. NGF, EGF, and IGF1 were all used at 100 ng/mL. In lanes where NGF was not added, tonic activity of TrkA was blocked by supplementation with TrkA inhibitor (1 µM). (F) Knockdown of FRS2α by TrkAPPFRS2α does not interfere with signaling of other RTKs through Erk1/2. NGF, EGF, and IGF1 were all used at 100 ng/mL.
In addition to being concentration dependent, knockdown of FRS2α was time dependent as well (Fig. 2B). To confirm that TrkAPPFRS2α, like our previous PROTACs, causes knockdown of target by recruitment to the ubiquitin-proteasome pathway, we pretreated the PC12 cells with the proteasome inhibitor epoxomicin: whereas immunoblotting for FRS2α in the presence of NGF and TrkAPPFRS2α alone showed little remaining immunoreactivity, the additional presence of epoxomicin not only stabilized FRS2α but also revealed the presence of larger molecular weight forms of the target (Fig. 2C). These larger forms were verified to be ubiquitin conjugates that had accumulated due to proteasome inactivation. To investigate whether TrkAPPFRS2α-induced FRS2α degradation was dependent on phosphorylation by TrkA, we performed immunoblot analysis of PC12 cells that had been incubated with the phenylalanine-containing TrkANPFRS2α. The null phosphoPROTAC was not able to reduce FRS2α levels and did not block NGF-triggered Erk1/2 activation (Fig. 2D). This demonstrates the phosphorylation-dependent activity of the phosphoPROTAC, i.e., the “conditional” degradation of FRS2α.
Similar to NGF, EGF and IGF1 stimulate Erk1/2 activation in PC12 cells, albeit via their own cognate receptor tyrosine kinases ErbB1 and IGF-1R, respectively. However, their cognate receptor transautophosphorylation sequences differ from that of TrkA such that these other growth factors are unable to cause phosphorylation of the phosphoPROTAC, as underscored by the results in Fig. S3. Therefore, EGF and IGF1 should spare FRS2α from degradation by TrkAPPFRS2α. To test the specificity of activation of TrkAPPFRS2α in intact cells, we treated PC12 cells with TrkAPPFRS2α in the presence of NGF, EGF, or IGF1 and measured their levels of FRS2α. Due to the presence of a small, ligand-independent (“tonic”) level of TrkA activity in the PC12 cells (23), which could over time partially activate the phosphoPROTAC even in the absence of NGF, it was necessary to reduce this tonic activity of TrkA in non-NGF–treated cells with an oxindole-based, small molecule, selective TrkA inhibitor (24). Using this strategy, we were able to activate selectively either the EGF receptor or the IGF-1R in the absence of tonic TrkA activity and monitor the corresponding effect of TrkAPPFRS2α on FRS2α levels (Fig. 2E). We found that TrkAPPFRS2α-induced FRS2α degradation was induced by NGF, but not by other growth factors. Moreover, addition and activation of TrkAPPFRS2α in PC12 cells did not significantly interfere with subsequent EGF- or IGF-triggered downstream signaling to Erk1/2 (Fig. 2F), underscoring the lack of off-target effects of the phosphoPROTAC.
PhosphoPROTAC-Dependent Blockade of Neuronal Differentiation.
We next determined whether incubation of PC12 cells with TrkAPPFRS2α could inhibit the cellular consequences of NGF treatment: biologically meaningful protein knockdown of FRS2α should reduce the induction of neuronal differentiation by NGF. As shown in Fig. S4A, increasing concentrations of TrkAPPFRS2α dose-dependently inhibited NGF-driven neurite extension in PC12 cells. In addition to this phenotype, PC12 cell differentiation can be detected by measuring the amount of the neuron-associated cytoskeletal protein neurofilament, which is up-regulated by NGF (25). Indeed, treatment of PC12 cells with NGF for 48 h resulted in a large increase in neurofilament expression, which was blocked by cotreatment with TrkAPPFRS2α at concentrations that also blocked neurite extension (Fig. S4B). Taken together, the results demonstrate the feasibility of a conditional phosphoPROTAC that is critically dependent on phosphorylation by a distinct RTK to induce posttranslational degradation of a specific target protein.
PhosphoPROTAC Targeting of Phosphatidylinositol-3-Kinase in Response to Neuregulin-ErbB2/ErbB3 Signaling.
To test the applicability of our conditional knockdown strategy beyond the NGF-TrkA-FRS2α pathway, we turned to a different receptor tyrosine kinase pathway, the ErbB2/ErbB3-phosphatidylinositol-3-kinase (PI3K) pathway. Studies have firmly established the importance of this signal transduction pathway in mitogenesis as well as in suppression of apoptosis. Heterodimerization of the EGF receptor family RTKs ErbB2 and ErbB3 occurs upon binding of the latter to its agonist, neuregulin (NRG). Formation of the heterodimer and transphosphorylation to activate signaling can also occur from overexpression of ErbB2, as frequently occurs in breast cancer and ovarian cells (26, 27). ErbB3 phosphorylation results in the recruitment of the lipid kinase PI3K via a SH2 domain of the PI3K p85 regulatory subunit (28). In turn, this results in PI3K activation and culminates in stimulation of the survival-promoting kinase Akt (protein kinase B), which is critically important in tumor cells for inactivation of proapoptotic mechanisms. Much attention has recently focused on inhibiting the ErbB3/PI3K-signaling pathway as an antitumor strategy.
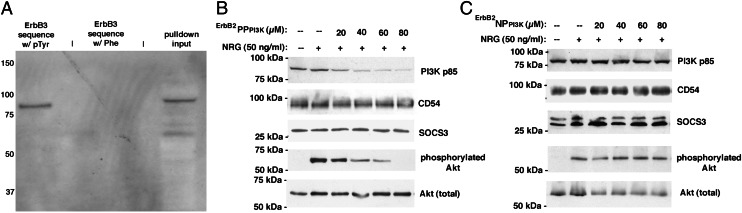
To develop a phosphoPROTAC targeting PI3K in response to activated ErbB2/EbB3, we synthesized another phosphoPROTAC composed of the same VHL-binding sequence and octa-d-Arg motif at its C terminus but having a different, 24-amino acid stretch at the N terminus (Fig. S5A) taken from a PI3K-binding domain on the intracellular region of ErbB3. Upon phosphorylation of the dual tyrosine residues in this motif by ErbB2, this 24-amino-acid sequence is predicted to bind PI3K and recruit it for ubiquitination and degradation. Accordingly, we designated this phosphoPROTAC as ErbB2PPPI3K. We also synthesized a phospho-null variant of this phosphoPROTAC, ErbB2NPPI3K (Fig. S5B). As ErbB3 has more than one predicted PI3K-binding site, we first verified by pulldown assay that our selected 24-amino-acid targeting sequence was sufficient to recruit PI3K. We synthesized biotinylated versions (Fig. S5 A and B) of the 24-amino-acid targeting sequences of ErbB2PPPI3K and ErbB2NPPI3K: these pulldown peptides each possess a C-terminal biotin group instead of the VHL-binding/octa-d-Arg moiety and tyrosines that are already phosphorylated. Upon incubating each peptide in whole-cell lysates from human breast cancer cell line MCF-7 followed by streptavidin-agarose pulldown, we detected the p85 subunit of PI3K in the pulldown from the phosphotyrosine-containing peptide, but not in the phenylalanine-substituted peptide (Fig. 3A). This demonstrated that our selected 24-amino-acid sequence from ErbB3 is sufficient to bind PI3K.
Fig. 3.
ErbB2PPPI3K, a phosphoPROTAC that conditionally targets PI3K for degradation upon ErbB2 activation. (A) Streptavidin pulldowns from MCF-7 cells using biotinylated peptides demonstrate efficacy and phospho-dependence of a selected dual phosphotyrosine-containing ErbB3 sequence to bind PI3K p85. Pulldowns electrophoresed and immunoblotted with anti-p85 antibody as described in Materials and Methods. (B) ErbB2PPPI3K-mediated degradation of PI3K is target specific and blocks NRG-dependent Akt activation in MCF-7 cells. (C) Null-phosphoPROTAC ErbB2NPPI3K cannot cause degradation of PI3K p85 even in the presence of neuregulin-activated ErbB2/ErbB3 signaling.
To test whether this PI3K-targeting phosphoPROTAC would work as effectively in living cells as the FRS2α-targeting phosphoPROTAC, we treated MCF-7 cells with increasing concentrations of ErbB2PPPI3K in the presence of neuregulin-1-α (Fig. 3B). Encouragingly, a dose-dependent decrease in p85 PI3K levels was detected by immunoblotting. Similarly to TrkAPPFRS2α in PC12 cells, concentrations of ErbB2PPPI3K ≤20 µM were not effective at knocking down PI3K, and maximum target knockdown was achieved at concentrations above 40 µM. Furthermore, the loss of PI3K resulted in decreased Akt activation, indicating an important biological consequence of the PI3K knockdown by ErbB2PPPI3K. Immunoblotting for CD54 (an unrelated membrane-associated protein) and SOCS3 showed no decreases in their abundance upon TrkAPPFRS2α treatment, reflecting a degree of target selectivity by the phosphoPROTAC. Moreover, treatment of MCF-7 cells with the cognate phospho-null variant ErbB2NPPI3K (Fig. 3C) reaffirmed the pulldown result: that tyrosine phosphorylation of the peptide was necessary for it to be active, indicating the conditional nature of the knockdown.
Knockdown of PI3K in Cancer Cells Results in Loss of Viability.
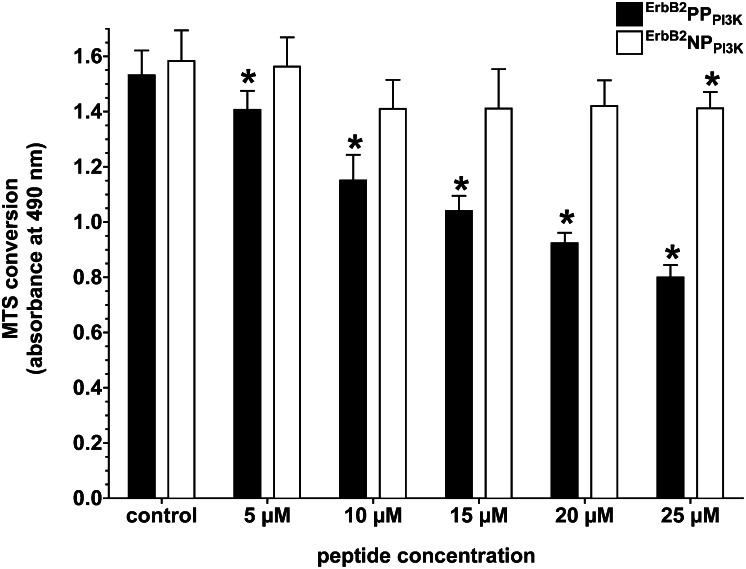
Because PI3K knockdown occurred in a phospho-dependent manner, we next tested whether ErbB2PPPI3K was able to produce the typical cytotoxic effect in cancer cells following inhibition of PI3K by small molecule inhibitors. For example, treatment of MCF-7 cells with the selective PI3K inhibitor LY294002 will decrease cell viability due to the resultant loss of Akt activation. Similarly, incubation of MCF-7 cells with increasing concentrations of ErbB2PPPI3K caused a loss of viability (Fig. 4). Note that, due to the longer time intervals required to monitor a decrease in cell viability (days vs. hours), concentrations of ErbB2PPPI3K lower than those needed to see a biochemical knockdown in a shorter time frame were able to mediate the toxic response. However, treatment of MCF-7 cells with equivalent concentrations of ErbB2NPPI3K caused no statistically significant changes in 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) conversion relative to control cells (except at 25 µM), indicating a strongly attenuated toxic response to this null variant. The toxic effect seen with either peptide at 25 µM must not be due to a phosphorylation event, as ErbB2NPPI3K cannot be phosphorylated.
Fig. 4.
Inhibition of PI3K signaling due to targeted knockdown by ErbB2PPPI3K is phospho-dependent and reduces cell viability. MCF-7 cells were incubated with varying concentrations of ErbB2PPPI3K or ErbB2NPPI3K for 48 h, following which viability was assessed using MTS conversion. Bars represent the mean ± SD of three replicate experiments. Asterisk denotes significant difference in MTS conversion compared with control (untreated) cells determined by Student t test (P < 0.05).
Consistent with the prediction that ErbB2PPPI3K reduces cell viability through inhibition of PI3K signaling, combined treatment of MCF-7 cells with both ErbB2PPPI3K and LY294002 led to a more pronounced reduction in MTS conversion than the sum of either treatment done separately (Fig. S6A). For example, 2.5 µM LY294002 by itself causes a small 5% decrease in MTS conversion after 2 d, and 5 µM ErbB2PPPI3K alone also causes a small decrease of 5%; however, when added as a combined treatment to MCF-7 cells, the decrease in viability is close to 36%, rather than the sum of the individual treatments (10%). This synergy of inhibition supports the notion that both molecules are negatively affecting the same target, albeit in different modes: ErbB2PPPI3K causes stoichiometric knockdown of the PI3K regulatory subunit (p85), whereas LY294002 binds to and inhibits the catalytic subunit p110.
To confirm the phospho-dependent cytotoxicity in another cell line, we similarly tested both ErbB2PPPI3K and ErbB2NPPI3K in MDA-MB-175 cells. Like MCF-7 cells, MDA-MB-175 cells are breast cancer derived; unlike MCF-7 cells, MDA-MB-175 cells secrete their own neuregulin as part of an autocrine loop. MDA-MB-175 cells were even more sensitive (Fig. S6B) to the cytotoxic effect of ErbB2PPPI3K than were the MCF-7 cells. In agreement with this, ErbB2PPPI3K more potently knocked down PI3K levels in MDA-MB-175 cells than in MCF-7 cells (Fig. S7A). In addition to MDA-MB-175 and MDA-MB-231 (Fig. S7B) breast cancer cells, ErbB2PPPI3K was also able to knock down PI3K in two independent ovarian cancer cell lines, OVCAR8 and SK-OV-3 (Fig. S7B). The small differences in ErbB2PPPI3K-induced PI3K knockdown among various cell lines may reflect variations in signaling component levels or perhaps the relative degree to which the cells tested are dependent upon PI3K signaling for survival.
PhosphoPROTAC-Mediated Inhibition of Tumor Growth in Vivo.
Given the activity of phosphoPROTACs to elicit biological responses in vitro, we tested whether ErbB2PPPI3K could inhibit tumor growth in vivo. We elected to use the OVCAR8 cells for our model tumor line due to their demonstrated in vitro sensitivity to ErbB2PPPI3K, the lack of need for estrogen administration to the mice (a necessary adjuvant for MCF-7 cell tumorigenesis), and OVCAR8 dependence on activated ErbB2/ErbB3-to-Akt signaling for tumorigenicity. Athymic mice were s.c. implanted dorsally with OVCAR8 cells following which ErbB2PPPI3K was administered daily by i.p. injection at 10 mg/kg, which we determined to be the maximum tolerated dose as evidenced by lack of toxicity symptoms and steady weight gain by the mice over the course of the experiment (Table S1). For comparison, other mice were injected daily with either an equal dose of ErbB2NPPI3K or an equal volume of PBS vehicle (“control”). After a total of 47 d of daily peptide injection, mice were killed, and the s.c. tumors (for control, n = 16; for ErbB2PPPI3K, n = 16; and for ErbB2NPPI3K, n = 18) were surgically removed and weighed (Fig. S8). Mice that were treated daily with ErbB2PPPI3K showed an average tumor weight that was ∼40% less than that in control mice. Conversely, mice that had received daily i.p. ErbB2NPPI3K injections developed tumors that were on average 10% smaller than in control mice. There was a statistically significant difference between the groups as determined by one-way ANOVA [F(2,46) = 3.802, P = 0.030]. Newman–Keuls post hoc analysis further specified where these differences exist: tumor growth in ErbB2PPPI3K-receiving mice was significantly different from that in control mice (P < 0.05) and in ErbB2NPPI3K-receiving mice as well (P < 0.05). However, there was no significant difference between the control and the ErbB2NPPI3K-receiving groups (P > 0.05). These data show that ErbB2PPPI3K retains its anticancer activity in live animals and further strongly suggest that in vivo activity of phosphoPROTACs is still dependent on phosphorylation of the peptide.
Discussion
Delineating the importance of various tyrosine kinase pathways in cell biology is an enormous challenge given overlapping downstream effectors and the limited number of kinase-specific small molecule inhibitors. In this report, we describe an approach to inhibit tyrosine kinase pathways that may take advantage of the intrinsic selectivity inherent in each signaling pathway. The first level of specificity exploited by this phosphoPROTAC approach arises from the specificity that individual tyrosine kinases possess for their respective substrates. By incorporating peptide sequences known to be phosphorylated by particular kinases, we take advantage of the natural specificity of individual signaling pathways. This was demonstrated by the lack of FRS2α degradation by IGF-1R and ErbB1 (Fig. 2E), despite the ability of both kinases (shared with TrkA) to activate downstream MAPK. This methodology also inhibits particular signaling cascades not by targeting the kinases themselves but by inducing the degradation of particular downstream-signaling components such as FRS2α and PI3K. Again, by using the natural affinity inherent in phosphotyrosine-containing peptides that serve as ligands for these SH2/PTB domain-containing proteins, we can effectively couple their degradation to the activation state of their cognate signaling cascade within the cell. Thus, these two levels of selectivity, i.e., at the kinase/substrate level and the effector protein/phosphoPROTAC binding level, co-opt nature’s ability to delineate particular signaling pathways from one another, alleviating the need to artificially engineer specificity into these cellular probes.
In addition to its ability to distinguish the activation state of various signaling pathways within a cell, this methodology may offer the ability to distinguish normal tissue from diseased tissue. Because dysregulation of various tyrosine kinase pathways leads to uncontrolled cell proliferation, the ability of a phosphoPROTAC to couple protein degradation to a particular growth factor-signaling pathway offers a possible cell-type selective anticancer strategy. Use of nucleic acid-based approaches for target protein knockdown in a mature, whole organism are being developed; conversely, use of peptide therapeutics, although not without caveats regarding their delivery and biological half-life, is well established. Futhermore, the conditional nature of the activation makes phosphoPROTACs suitable for the selective treatment of malignant cells. For example, one could envision that ubiquitous PROTAC-mediated knockdown of a protein involved in cell growth or survival could have systemic deleterious effects (e.g., inhibition of PI3K by the small molecule inhibitor LY294002 has been found to be toxic in animal studies). In this study, we were able to administer to mice a dose of phosphoPROTAC that was able to blunt tumor growth with no apparent ill effects on animal health (as evidenced by the maintenance of body mass and lack of toxicity symptoms over the course of the experiment). The signaling context-dependent phosphoPROTAC knockdown of PI3K in only those cells with high levels of ErbB2/ErbB3 signaling could preferentially inhibit breast cancer or ovarian or pancreatic cancer cell proliferation (29).
Another strength of this approach is that it is unlikely to elicit drug-resistant mutations. Current antitumor tyrosine kinase inhibitors such as Tarceva and Iressa halt cell proliferation via inhibition of receptor tyrosine kinase activity, providing a strong selective pressure for target mutations that mitigate inhibitor binding and preserve kinase activity. In contrast, conditional phosphoPROTACs depend on the misregulated kinase activity found in many tumor types to stop cellular signaling and growth. Thus, mutation of kinase structure to prevent phosphoPROTAC activation would likely result in a loss of kinase signaling as well.
Finally, a key advantage of this methodology is its accessibility to the cell biology community. The generation of kinase-specific small molecule inhibitors currently requires the concerted efforts of chemists, pharmacologists, and biochemists. However, as shown here, these signaling pathway-specific cellular probes can be readily synthesized using an automated peptide synthesizer. Moreover, the “portability” of this approach is clear: because many of the important tyrosine kinase phosphotyrosine residue:SH2- or PTB-domain–containing protein pairings are already known, it is conceivable that additional phosphoPROTACs may prove useful in the exploration of the cell biology of different signaling pathways, especially in instances where no specific small molecule inhibitor currently exists.
Materials and Methods
Reagents.
Neuregulin (catalog no. 296-HR) and IGF1 (catalog no. 291-G1-200) were purchased from R&D Systems; NGF, EGF, and other tissue culture reagents were obtained from Invitrogen; and collagen-coated tissue culture dishes were obtained from BD Biosciences. TrkA inhibitor (catalog no. 648450) was from EMD Biosciences, and remaining reagents were from Sigma unless otherwise indicated.
Cell Culture and Neurite Outgrowth.
PC12 cells were a generous gift from Randy Pittman (University of Pennsylvania, Philadelphia). PC12 cells were routinely cultured in RPMI medium 1640 supplemented with 10% heat-inactivated horse serum, 5% (vol/vol) heat-inactivated FBS, 100 units/mL penicillin G, and 100 µg/mL streptomycin sulfate. Unless otherwise noted, PC12 cells were grown on collagen-coated dishes. For neurite outgrowth, proliferating cells were switched to reduced serum conditions [2% (vol/vol) horse serum and 1% (vol/vol) FBS] and treated for 48 h with NGF ± PROTAC, with fresh reagents added after 24 h. Cells were then photographed to document the extent of neurite outgrowth. MCF-7 cells were provided by Anton Bennett (Yale University, New Haven, CT); MDA-MB-175, MDA-MB-231, and SK-OV-3 cells were purchased from ATCC; and OVCAR8 cells were provided by Joyce Liu (Dana Farber Cancer Institute, Boston); all were cultured in RPMI medium 1640 supplemented with 10% heat-inactivated FBS, 100 units/mL penicillin G, and 100 µg/mL streptomycin sulfate.
Streptavidin Pulldown.
MCF7 cells were grown to near confluence, harvested by trypsinization, washed in PBS, and frozen at –80 °C until used. Thawed cells were lysed in lysis buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 20 µM leupeptin, 1 mM PMSF, 1 mM NaF, 1 mM β-glycerophosphate, 2 mM sodium orthovanadate, 2 mM EDTA, 10% glycerol, 1% Nonidet P-40) by pipetting and then clarified at 14,000 × g for 10 min. Biotinylated peptides dissolved in PBS were added at a final concentration of 100 µM to neutravidin beads (Pierce Chemicals) and then washed three times with lysis buffer. Beads were boiled in 2× Laemmli sample buffer and then analyzed by immunoblotting as described in SI Materials and Methods. Please consult SI Materials and Methods for other technical information concerning the experiments described here.
Supplementary Material
Acknowledgments
The authors thank Ashley Schneekloth (Yale University) for her assistance in the preliminary experimentation and Randy Pittman (University of Pennsylvania) for his generous contribution of both PC12 cells and expertise in their culturing. We appreciate the valuable comments on this manuscript provided by the members of the C.M.C. laboratory. This work was supported by National Institutes of Health Grant R33CA118631 and by the Yale Cancer Center. T.W.C. was the Canadian Institutes of Health Research Jean-François St-Denis Fellow in Cancer Research and a Bisby Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217206110/-/DCSupplemental.
References
- 1.Ng DP, Krolewski AS. Molecular genetic approaches for studying the etiology of diabetic nephropathy. Curr Mol Med. 2005;5(5):509–525. doi: 10.2174/1566524054553504. [DOI] [PubMed] [Google Scholar]
- 2.Micklem DR, Lorens JB. RNAi screening for therapeutic targets in human malignancies. Curr Pharm Biotechnol. 2007;8(6):337–343. doi: 10.2174/138920107783018426. [DOI] [PubMed] [Google Scholar]
- 3.Corson TW, Aberle N, Crews CM. Design and applications of bifunctional small molecules: Why two heads are better than one. ACS Chem Biol. 2008;3(11):677–692. doi: 10.1021/cb8001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto KM, et al. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA. 2001;98(15):8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneekloth JS, Jr, Crews CM. Chemical approaches to controlling intracellular protein degradation. ChemBioChem. 2005;6(1):40–46. doi: 10.1002/cbic.200400274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneekloth JS, Jr, et al. Chemical genetic control of protein levels: Selective in vivo targeted degradation. J Am Chem Soc. 2004;126(12):3748–3754. doi: 10.1021/ja039025z. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto KM, et al. Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol Cell Proteomics. 2003;2(12):1350–1358. doi: 10.1074/mcp.T300009-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Schlessinger J, Lemmon MA. SH2 and PTB domains in tyrosine kinase signaling. Sci STKE. 2003;2003(191):RE12. doi: 10.1126/stke.2003.191.re12. [DOI] [PubMed] [Google Scholar]
- 9.Liu BA, et al. SH2 domains recognize contextual peptide sequence information to determine selectivity. Mol Cell Proteomics. 2010;9(11):2391–2404. doi: 10.1074/mcp.M110.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hines J, Groll M, Fahnestock M, Crews CM. Proteasome inhibition by fellutamide B induces nerve growth factor synthesis. Chem Biol. 2008;15(5):501–512. doi: 10.1016/j.chembiol.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouhara H, et al. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89(5):693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 12.Meakin SO, MacDonald JIS, Gryz EA, Kubu CJ, Verdi JM. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem. 1999;274(14):9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- 13.Obin M, et al. Neurite outgrowth in PC12 cells. Distinguishing the roles of ubiquitylation and ubiquitin-dependent proteolysis. J Biol Chem. 1999;274(17):11789–11795. doi: 10.1074/jbc.274.17.11789. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham ME, Stephens RM, Kaplan DR, Greene LA. Autophosphorylation of activation loop tyrosines regulates signaling by the TRK nerve growth factor receptor. J Biol Chem. 1997;272(16):10957–10967. doi: 10.1074/jbc.272.16.10957. [DOI] [PubMed] [Google Scholar]
- 15.Ong SH, et al. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol Cell Biol. 2000;20(3):979–989. doi: 10.1128/mcb.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao S, Jaiswal RK, Kolch W, Landreth GE. Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem. 2001;276(21):18169–18177. doi: 10.1074/jbc.M008870200. [DOI] [PubMed] [Google Scholar]
- 17.Hon WC, et al. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417(6892):975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 18.Fawell S, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91(2):664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothbard JB, et al. Arginine-rich molecular transporters for drug delivery: Role of backbone spacing in cellular uptake. J Med Chem. 2002;45(17):3612–3618. doi: 10.1021/jm0105676. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Gonzalez A, et al. Targeting steroid hormone receptors for ubiquitination and degradation in breast and prostate cancer. Oncogene. 2008;27(57):7201–7211. doi: 10.1038/onc.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan J, et al. CD40 is expressed and functional on neuronal cells. EMBO J. 2002;21(4):643–652. doi: 10.1093/emboj/21.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett. 2000;486(1):33–37. doi: 10.1016/s0014-5793(00)02205-5. [DOI] [PubMed] [Google Scholar]
- 23.Zaccaro MC, Ivanisevic L, Perez P, Meakin SO, Saragovi HU. p75 co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J Biol Chem. 2001;276(33):31023–31029. doi: 10.1074/jbc.M104630200. [DOI] [PubMed] [Google Scholar]
- 24.Wood ER, et al. Discovery and in vitro evaluation of potent TrkA kinase inhibitors: Oxindole and aza-oxindoles. Bioorg Med Chem Lett. 2004;14(4):953–957. doi: 10.1016/j.bmcl.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Lindenbaum MH, Carbonetto S, Mushynski WE. Nerve growth factor enhances the synthesis, phosphorylation, and metabolic stability of neurofilament proteins in PC12 cells. J Biol Chem. 1987;262(2):605–610. [PubMed] [Google Scholar]
- 26.Holbro T, et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100(15):8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern DF. ERBB3/HER3 and ERBB2/HER2 duet in mammary development and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(2):215–223. doi: 10.1007/s10911-008-9083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276(45):42153–42161. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- 29.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008;15(7):413–448. doi: 10.1038/cgt.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.