Abstract
Heroin addiction, a chronic relapsing disorder characterized by excessive drug taking and seeking, requires constant psychotherapeutic and pharmacotherapeutic interventions to minimize the potential for further abuse. Vaccine strategies against many drugs of abuse are being developed that generate antibodies that bind drug in the bloodstream, preventing entry into the brain and nullifying psychoactivity. However, this strategy is complicated by heroin’s rapid metabolism to 6-acetylmorphine and morphine. We recently developed a “dynamic” vaccine that creates antibodies against heroin and its psychoactive metabolites by presenting multihaptenic structures to the immune system that match heroin’s metabolism. The current study presents evidence of effective and continuous sequestration of brain-permeable constituents of heroin in the bloodstream following vaccination. The result is efficient blockade of heroin activity in treated rats, preventing various features of drugs of abuse: heroin reward, drug-induced reinstatement of drug seeking, and reescalation of compulsive heroin self-administration following abstinence in dependent rats. The dynamic vaccine shows the capability to significantly devalue the reinforcing and motivating properties of heroin, even in subjects with a history of dependence. In addition, targeting a less brain-permeable downstream metabolite, morphine, is insufficient to prevent heroin-induced activity in these models, suggesting that heroin and 6-acetylmorphine are critical players in heroin’s psychoactivity. Because the heroin vaccine does not target opioid receptors or common opioid pharmacotherapeutics, it can be used in conjunction with available treatment options. Thus, our vaccine represents a promising adjunct therapy for heroin addiction, providing continuous heroin antagonism, requiring minimal medical monitoring and patient compliance.
Keywords: immunotherapy, antinociception, progressive ratio
Opiate abuse is a global problem, with nearly 20 million estimated users annually (1). Heroin/opium use is prevalent among roughly 0.5% of the world population, including within the United States, and nearly double this rate in Eastern European countries. Despite a rise in use, synthetic opioids still only significantly outnumbers heroin use within North America (2). Heroin dependence is associated with increased health risks for the user and substantial societal costs attributable to illicit use and loss of productivity. Heroin continues to be the illicit drug with the greatest associated risk of user mortality and criminal activity (3). In addition to traditional psychotherapy (4), numerous pharmaceutical agents are available to assist in the maintenance of opiate abstinence. Substitution therapy with long-acting opioid drugs, such as methadone (5) and buprenorphine (6) (or less commonly heroin itself; ref. 7), is effective at reducing craving for heroin and illicit opiate use. However, substitution therapy is fettered by an inherent risk for continued dependence symptomology (8) and relapse, in addition to potential adverse effects caused by continued opioid use (9–11). Heroin blockade with naltrexone, an opioid receptor antagonist, has also proven effective in the management of heroin addiction, with extended-release injection formulations and implants that improve compliance. However, there is some debate about the extent of side effects caused by long-term opioid receptor blockade (12, 13), including evidence of altered production of endogenous opioids and opioid-responsive hormones (14, 15). Additionally, resensitization of opioid receptors by naltrexone results in an increased risk of overdose of heroin or other opiates upon relapse (16).
Most evidence suggests that the continued and medically monitored use of available pharmacotherapies can provide a high likelihood of long-term abstinence (7). However, access to treatment programs can be difficult even within countries that permit such community- and physician-directed programs. Existing therapeutic options require uninterrupted access to medical providers for administration and monitoring, and low rates of compliance and retention in programs are highly predictive of relapse. Immunopharmacotherapy, designed to produce antibodies that bind drugs of abuse within the bloodstream and blunt passage into the brain, may provide an alternative to pharmacotherapy with comparatively less effort required to maintain patient compliance. Such an approach could provide a lasting defense against the drug’s psychoactivity, with a minimal risk of long-term side effects (17–19). Recent inquiries into immunopharmacotherapy against drugs of abuse have advanced our understanding of how to design vaccines to target addictive compounds, with heroin being an exceptional case.
Within seconds of injection, heroin undergoes rapid deacetylation to 6-acetylmorphine (6-AM) and subsequently morphine, both of which are active metabolites. Because of heroin’s metabolic lability and the diverse properties (e.g., opioid activity, brain permeation, and half-life) of its primary metabolites (20), designing an effective heroin vaccine requires targeting multiple chemical structures. Early opiate vaccine designs presented the alkaloid region of morphine, common to most drugs in the morphine class of molecules (21–23). As a consequence, these vaccines elicited nonselective opiate antibodies that were readily overcome by increasing the dose (24). We recently reported a vaccine designed for greater specificity for heroin, in which the bridgehead nitrogen was exploited for conjugation to protein, and the metabolically reactive region of the opioid scaffold was presented for immune recognition. In combination with alum adjuvant protection, this singular immunoconjugate was able to present itself to the immune system in a dynamic, multihaptenic fashion, allowing the elicitation of antibodies with high binding affinity for heroin and 6-AM and modest binding affinity for morphine. This vaccine generated high antibody titers that selectively prevented heroin-induced antinociception and the acquisition of heroin self-administration in prophylactically treated naïve rats (25). However, the extent to which immunotherapy against opiates, or any drug of abuse for that matter, can prevent compulsive drug seeking and taking in dependent animals remains to be established. The present study evaluated the “dynamic” heroin vaccine for selectivity and efficacy in prototypical models of heroin reward, relapse, and compulsive intake. The models used test the effects of heroin in both drug-naïve and drug-experienced rats (animals with a history of dependence), and required that the vaccine produce a blockade of heroin doses well above those previously examined in our own studies (25), as well as those of similar nonselective morphine-class vaccines (23, 26). We also demonstrated that this dynamic vaccine effectively acts as a long-term functional antagonist against heroin activity.
Heroin Vaccine Confers Selective Recognition
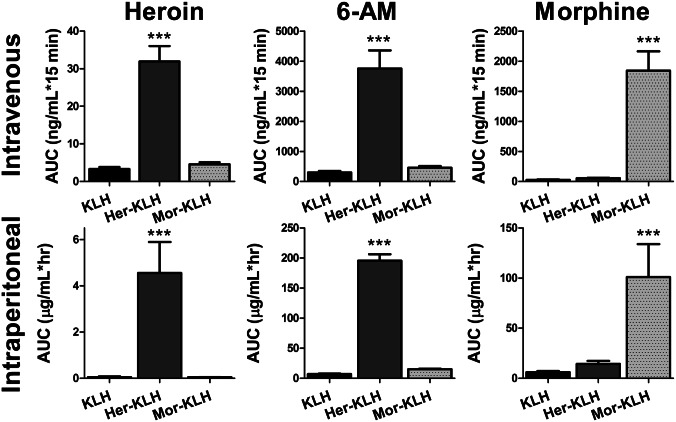
Rats were vaccinated with an alum adjuvant suspension containing 100 μg of the carrier protein keyhole limpet hemocyanin (KLH) alone or linked to with either heroin haptens (Her–KLH) or morphine haptens (Mor–KLH). A total of three doses of immunoconjugate were administered over a 28-d period, resulting in anti-heroin antibody titers of at least 1:30,000 (measured by serum ELISA, see Table S1) before behavioral testing. This procedure was previously established for this vaccine to produce maximal antibody production, and once completed has been shown to maintain sufficient titer levels (>50% maximal titer) for at least 52 d without further injections (25). Although titer measurements indicate antibody production in response to KLH presentation, due to the dynamic nature of the Her–KLH vaccine, the titer measured antibodies as judged by simple ELISA using Her-BSA will not accurately convey the true immune response metric being generated to the Her–KLH vaccine. Rather, a more accurate predictor of the efficacy of an active vaccination against heroin should be based upon retention of drug within the bloodstream, limiting distribution to the brain. Heroin metabolites were measured by liquid chromatography-tandem mass spectrometry (LC-MSMS) in serum after either i.v. (0.5 mg/kg) or i.p. (1.5 mg/kg) heroin administration. Her–KLH-treated rats showed significantly greater sequestration of heroin (F2,13 = 37, P < 0.001) and 6-AM (F2,13 = 26, P < 0.001) in blood compared with Mor–KLH- and KLH-treated controls (Fig. 1). Similarly, Mor–KLH-treated rats exhibited selective retention of morphine (F2,13 = 35, P < 0.001) in the bloodstream compared with the Her–KLH and KLH groups. The dynamics of i.v. heroin metabolite binding within the bloodstream were consistent with the known pharmacokinetics of heroin. Within minutes, the heroin concentration dropped precipitously, coinciding with increased levels of 6-AM. After ∼15 min, the serum morphine concentration began to rise (Fig. S1). Of particular significance in the i.p. time course, a lag in peak heroin concentration was apparently attributable to antibody sequestration after distribution through the visceral tissue into the bloodstream (F2,13 = 13, P = 0.001) and its primary metabolite 6-AM (F2,13 = 332, P < 0.001). Serum morphine levels again increased in Mor–KLH-treated rats (F2,13 = 8.6, P = 0.004) after 15 min. In Her–KLH-treated rats, serum 6-AM levels persisted longer than typical observations in heroin addicts (27, 28), suggesting that antibody–drug binding within the bloodstream may protect the labile 6-acetyl group against hydrolysis.
Fig. 1.
Heroin vaccination promotes binding of heroin and its metabolites in blood. The area-under-the-curve for blood serum concentrations of brain-penetrant metabolites (heroin, 6-AM, and morphine) of heroin are shown for rats injected with either 0.5 mg/kg into the tail vein (Upper) or 1.5 mg/kg intraperitoneally (Lower). Her–KLH vaccination promoted sequestration of heroin and 6-AM in blood, whereas Mor–KLH vaccination selectively bound morphine. n = 20–24 per curve. The data are expressed as mean ± SEM; ***P < 0.001, compared with all other vaccination groups (Tukey’s post hoc test).
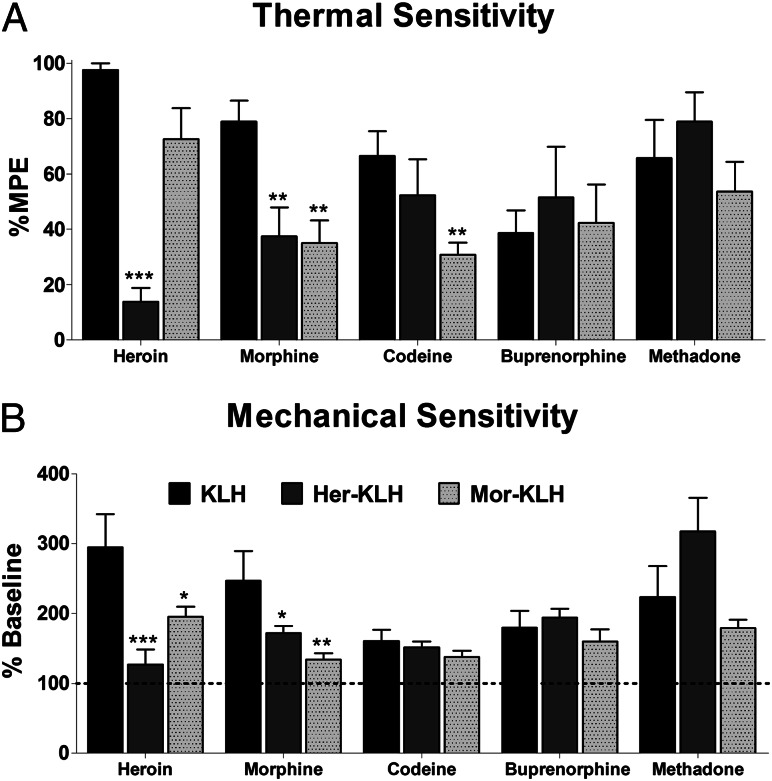
We previously reported that our Her–KLH vaccine prevented heroin-induced antinociception but did not mitigate antinociception using the structurally similar morphine-class analgesic oxycodone (25). To further evaluate the specificity of protection conferred by this vaccine design, we examined a fuller range of opiate analgesics, but also drugs typically used as part of opioid replacement therapy. Consistent with our previous findings, Her–KLH provided complete blockade of heroin’s antinociceptive properties in both the thermal hot plate (F2,14 = 21, P < 0.001) and mechanical von Frey filament (F2,15 = 7.4, P = 0.006) assays, whereas Mor–KLH failed to prevent heroin-induced antinociception (Fig. 2). Her–KLH-treated rats also showed sufficient protection against morphine-induced thermal (F2,33 = 5.3, P = 0.01) and mechanical (F2,33 = 5.1, P = 0.01) antinociception, similar to the effects of Mor–KLH. Mor–KLH prevented the decrease in codeine-induced thermal sensitivity (F2,15 = 3.8, P = 0.03), a result consistent with the observation that rats predominantly metabolize codeine to morphine (29). The responses of Her–KLH-vaccinated rats to therapeutic doses of codeine, buprenorphine, and methadone did not significantly differ from KLH controls, suggesting that the use of these drugs as therapeutics is still viable even after vaccination (Fig. 2 and Fig. S2).
Fig. 2.
Vaccination selectively prevents antinociception in designed opiate target, whereas therapeutic opioids maintain activity. Vaccinated rats were tested for thermal and mechanical sensitivity using the hot plate (A) and von Frey filament (B) tests, respectively. Her–KLH completely blocked heroin-induced antinociception, whereas codeine, buprenorphine, and methadone maintained effectiveness. Mor–KLH vaccination prevented morphine- and codeine-induced antinociception but not heroin-induced antinociception. n = 6–7 per group. The data are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, compared with KLH controls (Dunnett’s post hoc test).
In an attempt to quantify the vaccines’ functional antagonism of heroin psychoactivity, drug-naïve rats were administered cumulatively increasing heroin injections until full thermal antinociception was observed in the hot-plate assay. Rats in the KLH control group showed full heroin antinociception starting as low as 1 mg/kg, with the Mor–KLH vaccine rats showing a rightward shift in the dose–response about 2-fold, and Her–KLH rats requiring ∼6- to 7-fold more heroin to produce similar psychoactivity (Fig. S3). Regression analyses of the resulting dose–response curves demonstrated significant increases in the Her–KLH group’s 50% effective concentration (EC50; F2,23 = 7.7, P < 0.01) and maximally effective dose (F2,23 = 14.9, P < 0.001) compared with KLH controls. No correlation was observed between titer levels, as measured by ELISA, and shifts in heroin responding (R2 = 0.003).
Heroin Vaccine Prevents Heroin Seeking
Although the sequestration of drug in the bloodstream and prevention of its analgesic properties indicate vaccine efficacy, a more meaningful measure of efficacy when evaluating vaccines against drugs of abuse is whether the drug continues to exert rewarding effects. Vaccinated rats were given opiates repeatedly paired with a contextually distinct chamber over 4 d and then examined for drug-induced positive associations with those environmental cues [i.e., conditioned place preference (CPP)]. Saline controls spent slightly more time in the drug-paired side across all vaccine groups because the test drug was paired with the initially less-preferred chamber (“biased” procedure). However, to control for regression to the mean, preference in all of the drug-treated groups was compared with saline controls (as opposed to raw preference in “unbiased” procedure). In addition, it is clear that there was a significant increase in preference above the reversal of the nonpreferred side bias (KLH vs. saline, Fig. 3). Heroin (0.4 mg/kg, s.c.) generated robust place preference in the KLH and Mor–KLH groups (F3,31 = 3.1, P = 0.03), with average increases of 23% and 24%, respectively, in the time spent in the heroin-paired chamber compared with baseline. However, heroin failed to produce CPP in the Her–KLH group compared with saline (Fig. 3). Morphine (4 mg/kg, s.c.) also produced a preference for the drug-paired chamber in KLH controls (F3,31 = 2.9, P = 0.05) but not in Her–KLH- or Mor–KLH-vaccinated rats. Similar differences in heroin preference (KLH: 121.8 ± 52.9 s, Her–KLH: 28.9 ± 53.8 s; n = 5 per group) and similar differences in morphine preference (KLH: 209.0.8 ± 65.1 s, Her–KLH: 54.9 ± 37.1 s; n = 6 per group) scores were obtained from a small subset of rats that did not show an initial bias (<15%) for either chamber, indicating that the blockade of heroin place preference are independent of any chamber bias in the design. These results demonstrate that the Her–KLH vaccine capably prevented the expression of heroin and morphine reward in a common model of drug seeking.
Fig. 3.
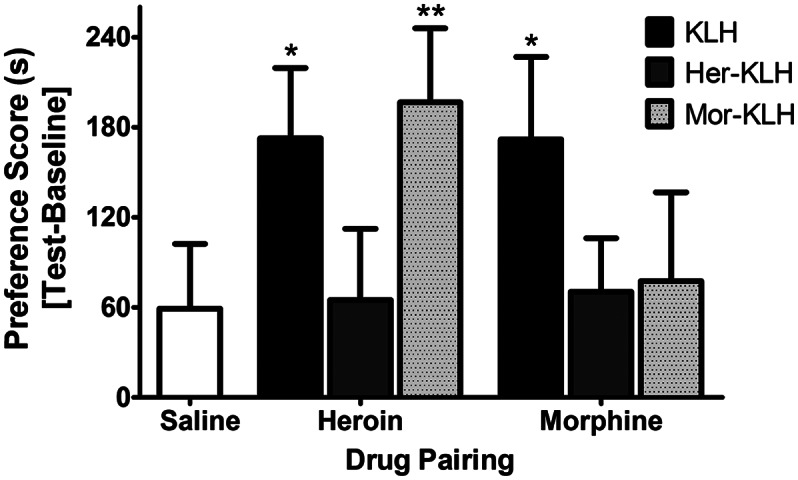
Opioid vaccination prevents acquisition of reward, reflected by conditioned place preference. Her–KLH rats failed to exhibit a preference for the heroin- or morphine-paired chamber, a preference demonstrated in KLH controls, following 4 d of twice-daily conditioning. Mor–KLH-treated rats exhibited no preference for morphine conditioning but still exhibited heroin-induced CPP. n = 9 per group. The data are expressed as mean ± SEM; *P < 0.05, **P < 0.01, greater than saline preference (Dunnett’s post hoc test).
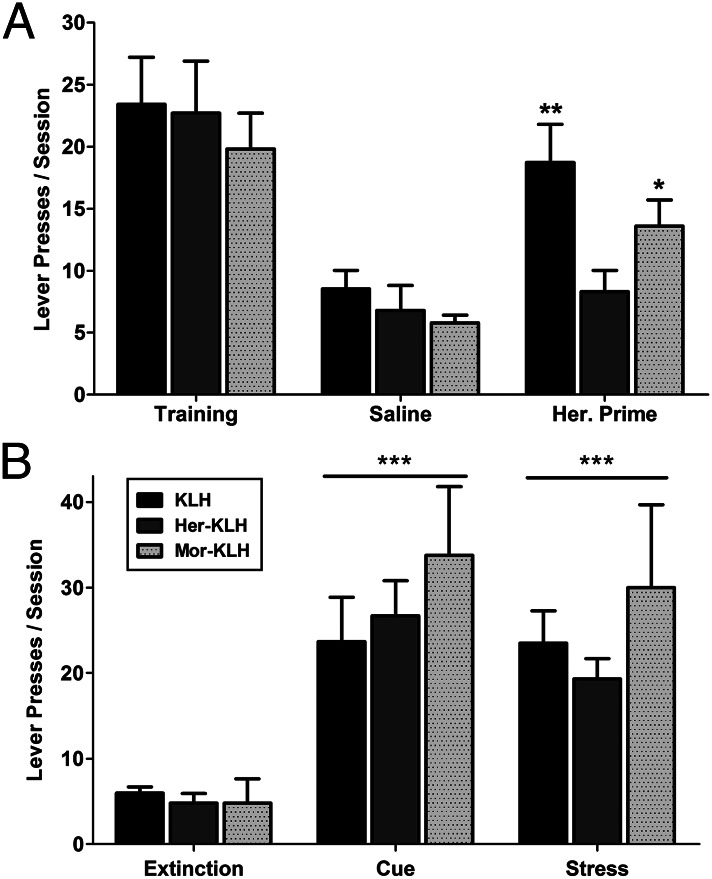
Having established the vaccine eliminated drug-context seeking, a common preclinical representation of relapse was examined, namely reinstatement of extinguished heroin self-administration. In addition to being a more active model of drug seeking, it allows the testing of drug-of-abuse vaccine efficacy in drug-experienced rats as opposed to prophylactic treatment in drug-naive rats. Studies using the reinstatement model show that learned extinction of reward seeking on a drug-associated lever can be abruptly reversed by noncontingent reexposure to drug, reintroduction of cues associated with drug reward, and introduction of a stressor (30–32). Rats were incrementally trained to press three times for a single heroin infusion [fixed-ratio 3 (FR3)] of 60 μg/kg, signaled by a 20-s cue light over the lever. During the period of vaccination (although not tested on the days of injection), the rats underwent extinction training during 2-h sessions in which pressing the drug-associated lever resulted in neither drug infusion nor activation of the cue light. Neither vaccine treatment altered the speed or extent of extinction learning (Fig. S4). Intravenous saline infusion just before an extinction session did not result in significant changes in drug seeking behavior over the 2-h session. However, an i.v. heroin infusion that was equivalent to three heroin doses (180 μg/kg) before the extinction session produced robust drug-associated lever pressing in KLH control rats (Fig. 4A). Heroin priming of Mor–KLH-treated rats produced a moderate increase in lever pressing. Notably, however, heroin priming was completely ineffective in altering drug responding in the Her–KLH-vaccinated group (Drug × Vaccine interaction: F2,14 = 4.7, P = 0.02). Whereas Her–KLH vaccination conferred protection against heroin-induced reinstatement, the drug vaccine had no impact on reinstatement elicited by reintroduction of the cue light (Cue: F2,14 = 51, P < 0.001; Fig. 4B). Reinstatement elicited by pretreatment with the α2-adrenergic antagonist yohimbine (1.25 mg/kg, i.p.), which produces stress responses via noradrenergic stimulation, was also insensitive to drug vaccine (Yohimbine: F2,14 = 26, P < 0.001). These data indicate that the Her–KLH vaccine was able to prevent heroin-induced, but not cue- or stress-induced relapse.
Fig. 4.
Her–KLH treatment prevents heroin-induced reinstatement of opiate seeking. (A) Rats previously extinguished from pressing for heroin (FR3) spontaneously reinstated heroin-seeking behavior upon i.v. heroin infusion (180 μg/kg), which was not apparent in the Her–KLH group. (B) All vaccination groups still exhibited reinstatement behavior in response to reintroduction of a heroin-associated cue light or yohimbine-induced (1.25 mg/kg, i.p.) stress. n = 9 per group. The data are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001, compared with respective group’s extinction response (Tukey’s post hoc test).
Halting the Progression of Compulsive Heroin Taking
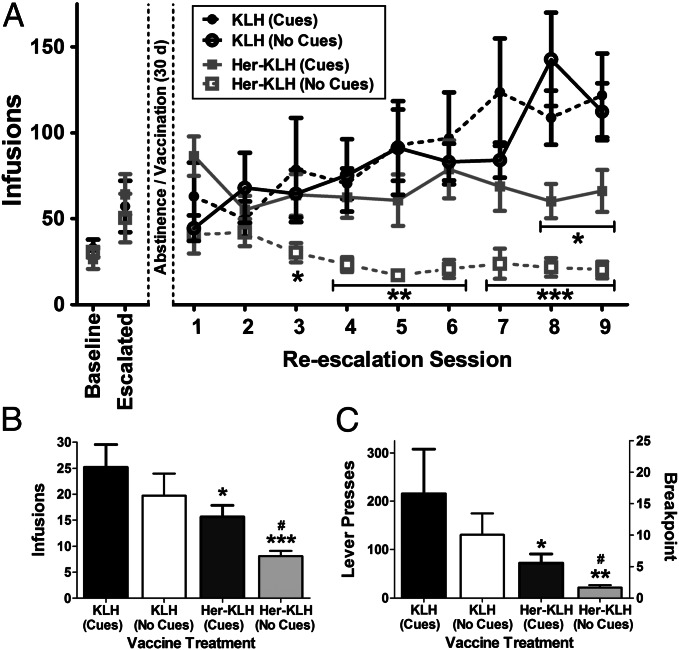
Finally, our most robust test of the therapeutic viability of the Her–KLH vaccine was to examine whether the vaccine alters the reestablishment of compulsive heroin self-administration in dependent rats with a history of compulsive heroin intake. The 12-h extended-access self-administration model results in significantly increased heroin intake over a period of several weeks, with rats becoming sufficiently tolerant until taking doses typically lethal to heroin-naïve animals. Rats also show signs of physical dependence, reduced food intake, self-mutilation, and increased motivation to work for heroin infusions (33, 34). A group of extended-access rats that exhibited increases in intake that nearly tripled across 17 sessions were subjected to forced heroin abstinence for 30 d. During this period, the rats were treated with the KLH or Her–KLH vaccine, and then resumed 12 h of free access to heroin. Both KLH and Her–KLH rats resumed lever pressing at levels that were comparable to preabstinence heroin intake, but the KLH group rapidly reescalated heroin intake until it again doubled in a 9-session period (Fig. 5A; i.e., half the time of the initial escalation). The Her–KLH group maintained steady intake at the level attained before vaccination, without further increasing heroin intake (Session × Vaccine interaction: F9,90 = 4.5, P < 0.001).
Fig. 5.
Her–KLH halts further escalation of intake in heroin-dependent rats, leading to reduced motivation to seek heroin. (A) Rats exposed to extended access heroin self-administration (12 h) escalated their intake to more than triple their initial infusions. Following vaccination, Her–KLH rats self-administer at prevaccination levels in 12 h sessions, whereas KLH controls further double their heroin intake. Replication without the presence of heroin-associated light cues, relying only on interoceptive stimuli, significantly reduced heroin taking behavior in the Her–KLH rats. After reescalation, Her–KLH rats worked less during a 6 h progressive-ratio test for heroin, resulting in fewer infusions (B) as a result of fewer lever presses (C). n = 6–8 per group. The data are expressed as represent mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.01 difference between KLH and Her–KLH groups (Dunnett’s post hoc test for escalation, Tukey’s post hoc test for progressive ratio). #P < 0.05 between Her–KLH cue groups (Tukey’s post hoc test).
To examine the reinforcing value of heroin infusions, a 6-h progressive-ratio test, in which the per-infusion workload (“price”) progressively increases until the rat reaches a breakpoint beyond which it no longer responds for heroin was performed a day after the final 12-h self-administration session. Rats in the KLH group pressed to receive significantly more heroin infusions (t10 = 2.3, P = 0.04; Fig. 5B), pressing nearly three times more during the session compared with their Her–KLH counterparts (t10 = 2.1, P = 0.05; Fig. 5C). Thus, Her–KLH-vaccinated rats were less “willing” to work for heroin, even after a prior history of compulsive heroin intake.
Despite evidence that vaccine treatment resulted in devaluation of heroin infusions, continued lever pressing suggests a potential role for the associated light cue accounting for some reinforcing properties. The results closely parallel those of the reinstatement studies, where vaccination prevented reemergence of lever responding behavior due to heroin exposure, but was ineffective against preventing similar behavior in response to cues. Continued minimal activity directed at the inactive lever, which does not significantly change across session or vaccine group (Fig. S5; F3,152 = 0.21, P = 0.89), suggesting a specific behavior directed at the cue-associated lever.
To test this hypothesis, the experiment was repeated with rats trained and tested without a light cue associated with an active lever infusion. The overall rate and amount of drug intake was not significantly different compared with the previous group with light cues. However, the animals without light cues showed an increased incidence of timeout lever pressing. Relying only on the interoceptive indicators of heroin infusion, the Her–KLH vaccination resulted in extinction of lever pressing behavior upon reescalation (Session × Vaccine interaction: F8,120 = 4.9, P < 0.001; Fig. 5A). Repetition of the progressive ratio test shows that Her–KLH-treated rats without visual cues are significantly less willing to work for heroin infusions than KLH controls, and less willing to work than Her–KLH-treated rats with visual cues (F2,17 = 16.1, P < 0.001; Fig. 5B). When looking at overall lever pressing during the 6-h period, Her–KLH rats without cues press only a tenth that of KLH-treated rats (t12 = 3.0, P < 0.01; Fig. 5C).
Discussion
The performance of our Her–KLH vaccine in heroin-induced CPP, reinstatement, and reescalation of intake suggests that a dynamic vaccine strategy that theoretically presents multiple haptenic epitopes to the immune system may have promising efficacy as an adjunct therapeutic. Indeed, using a condensed vaccination timetable of 30 d, sufficient antibody titers were observed to effectively confine heroin and its targeted metabolites to the bloodstream, because antibodies are incapable of crossing the blood–brain barrier. The distinct lack of correlation between measured titer response and functional blockade is readily reconciled due to the inability of currently available ELISA tools to measure for precise antibody recognition of not only heroin but also its metabolites 6-AM and morphine. Furthermore, the inability of the “static” Mor–KLH vaccine to impact heroin-induced behavioral responses is consistent with the current view of heroin as a brain-permeable prodrug, activated following quick passage across the blood–brain barrier as either heroin or 6-AM, beyond the reach of morphine-specific antibodies. Even when administered outside the bloodstream, the dynamic vaccine antibodies bind heroin and its metabolites as they diffuse through the viscera into the circulation, acting as a sink by sequestering heroin and 6-AM in the bloodstream. The capacity of these antidrug antibodies to prevent the passage of heroin or 6-AM into the brain translated to neutralization of the psychoactive effects across numerous measures, including analgesia, reward, and reinforcement (25).
One potential limitation of the present study is that heroin reward was measured by a biased place preference procedure where a less than robust place preference could simply reflect reversal of aversion rather than a true reward effect. However, the robust place preference reported here mitigates against such an interpretation because in fact the place preference is significantly higher than simply a reversal of the bias in the initial preference. Nevertheless, one cannot rule out that the reversal of bias may be contributing to the heroin reward effect in the present study.
Additionally, we demonstrated that Her–KLH vaccination may have promising therapeutic benefit subsequent to heroin use, preventing further instances of heroin exposure from promoting relapse behavior and blocking the reescalation of compulsive heroin intake with reexposure to extended access. This latter observation is a robust test of the validity of drug-of-abuse vaccines in general in that it represents the clinical situation most likely to be encountered by individuals enrolled in abstinence-based programs. Some persistent heroin self-administration without escalation, even after vaccination, in rats with heroin cues available cannot be merely explained by heroin intake that exceeds the capacity of the vaccine. Rats trained to self-administer heroin will increase their drug-taking several-fold in the presence of pharmacological opioid antagonism in attempts to overcome opioid receptor blockade (35). Furthermore, in testing the surmountability of the Her–KLH vaccine against cumulative doses of heroin, vaccinated rats required over 10 mg/kg to produce antinociceptive activity; this is roughly five times the lethal dose in naïve rats, and well above that self-administered even by the escalated intake rats over an entire 12-h session (∼6 mg/kg). Thus, previous contextual associations and drug-associated cues may maintain sufficient reinforcing value to sustain some lever pressing. Indeed, upon additional experimentation eliminating drug-predictive cues, we demonstrated that the Her–KLH vaccine can effectively eliminate the motivational valence of heroin psychoactivity, even in the original drug context. Numerous studies have demonstrated that heroin cues and contexts can produce reward activation in the brains of opiate addicts (36, 37) and stimulate dopamine release, which plays a role in the cognitive processing of drug associations (31, 38). Clearly, behavioral therapy, in conjunction with any vaccine treatment, will be needed to overcome such associative factors and further abate drug-seeking behavior. However, the compelling lack of escalation in intake, or even evidence of extinction, along with blunted responding on a progressive-ratio schedule in the Her–KLH groups demonstrated the dramatic ability of Her–KLH vaccination to prevent the reestablishment of compulsive heroin intake. Although the dynamic heroin vaccine is not targeted to treat the “addicted” brain, it represents a robust tool in the continuous blockade of all heroin activity.
The efficiency of our hapten design is due in part to its unfettered presentation of the metabolically vulnerable portion of the heroin molecule for anti-heroin and anti–6-AM antibodies to evolve during the immune response (25). By eliciting an array of antibodies that recognize heroin and its metabolites, this dynamic vaccine design produces a coordinated defense against heroin. The ineffectiveness of the Mor–KLH vaccine against heroin across numerous tests, despite effectively binding morphine in the bloodstream, suggests that heroin psychoactivity is mediated by its molecular antecedents entering the brain, and underscores the potential importance of 6-AM to the immediate actions of heroin (20). The hapten presentation of the current vaccine was developed to mimic heroin’s metabolic fate through multihaptenic display via a singular progenitor antigen facilitated by the chosen attachment site on the heroin structure. Other opioids, such as oxycodone, could also be targeted in a similar selective fashion. This approach is becoming increasingly pertinent, with the increase in prescription opiate abuse raising concerns about vaccinated heroin addicts transitioning to alternative opiates. The design of vaccines against oxycodone and hydrocodone were also recently reported (39, 40), along with examples of effective mixtures of multiple opiate vaccines (21, 41), pointing to progress that will widen the spectrum of potential opiate blockade via vaccination.
The prospect of heroin vaccine use in the treatment of addiction presents a high-payoff, low-risk opportunity. Drug vaccination requires minimal medical monitoring and compliance to maintain opiate resistance, allowing for greater potential worldwide accessibility. Furthermore, with no impact on endogenous opioid receptor or neurotransmitter function, drug vaccines represent a low risk for long-term side effects. We previously established that opioid analgesics can still be used in the concurrent treatment of pain, and opioid receptor therapy is still available for concomitant use. The current vaccine treatment strategy has been shown to maintain sufficient titers for effective blockade for at least 2–3 mo (25), but because the KLH protein conjugated to the heroin moiety here is not approved for clinical use, it is difficult to predict the level and sustainability of antibody production. Using this modular linker design (25), synthesis using a clinically approved alternative (i.e., tetanus or diphtheria toxoid) is practicable for development; such carrier protein–heroin hapten conjugates should produce robust and long-term immune response in humans with few immunizations. Although it may not be a “magic bullet” against all aspects of drug addiction, the dynamic nature of our heroin vaccine represents a promising and innovative adjunct therapy in the treatment of heroin addiction.
Materials and Methods
Male Wistar rats (n = 145; Charles River) arrived weighing 250–275 g. The rats were housed three per cage in a temperature-controlled (22 °C) vivarium on a 12-h light cycle with ad libitum access to food and water. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. The production of vaccine haptens, immunization schedules, evaluation of serum titers via ELISA, and behavioral assessment of antinociception using a 54 °C hot plate were identical to the procedures of Stowe et al. (25). Von Frey filaments were tested using the “up–down” method, sufficient for analyses using the nonparametric method of Dixon (42). Heroin (1 mg/kg) and codeine (50 mg/kg) were evaluated 30 min after s.c. injection, whereas morphine (10 mg/kg), buprenorphine (3 mg/kg), and methadone (5 mg/kg) were evaluated at 60 min. For cumulative dose–response analysis, heroin was given in 1 mg/kg intervals up to 3 mg/kg total, then given in 2 mg/kg intervals, then evaluated 10 min after each injection. Dosing was completed once response latencies of greater than 30 s were observed.
The rats were killed at set times following either a 0.5 mg/kg tail vein or 1.5 mg/kg i.p. heroin injection (2, 5, 10, and 15 min, i.v.; 5, 10, 30, and 60 min, i.p.) using isoflurane followed by rapid decapitation. The LC-MSMS procedures followed those described by Karinen et al. (43), with methods and parameters (Table S2) fully detailed in SI Materials and Methods.
Conditioned place preference paradigm consisted of a two-chambered apparatus, differentiated by wall (dots or stripes) and textured plastic flooring (prismatic or cracked ice), with a small central alley (44). The rats were video-recorded in 15-min preconditioning sessions and then were scored by a blind observer for the time spent on each side of the apparatus. Because of the limited supply of vaccinated rats, a biased procedure was used, with drug always paired to the least-preferred side. Conditioning consisted of 4 d of counterbalanced morning/afternoon 30-min pairings of saline or test drug (saline, 0.4 mg/kg heroin, 4 mg/kg morphine; all s.c.) while restricted to one chamber. The overall test was balanced by the side initially preferred and test drug. On day 5, the rats were again videotaped while being allowed to freely explore both chambers, and the preference score was calculated as the additional time spent on the drug-paired side following conditioning compared with the initial time.
The rats that required heroin self-administration underwent i.v. catheterization surgery to implant catheters in the right jugular vein, with experiments performed in modular chambers as described (34, 45). All of the self-administration procedures used 60 μg/kg/infusion unit doses, a 4-s infusion of 100 μL, and a 20-s cue light above the lever denoting a timeout period. Reinstatement rats were trained in 2-h sessions and transitioned to a FR3 schedule over at least 17 sessions, followed by extinction for at least 12 sessions, in which neither lever had any programmed consequences. The rats were vaccinated entirely during the extinction phase. Drug-induced reinstatement was produced by heroin drug priming (180 μg/kg, i.v.) just before the session, but without any programmed consequences for lever pressing. Cue-induced reinstatement was precipitated by reintroduction of the cue light on an FR3 schedule but without infusion or other consequences. Stress-induced reinstatement was performed similarly to Banna et al. (30), using a yohimbine injection (1.25 mg/kg, i.p.) 30 min before session to induce a stress response.
Reescalation followed published 12-h FR1 extended-access escalation procedures (34), followed by vaccination during a 30-d abstinence period and then reintroduction of 12-h heroin for nine sessions. The rats were given a day off following their final reescalation session and then tested in a 6-h progressive-ratio session. The experiment was exactly replicated, with the exception that the 20-s timeout light cue associated with an active lever infusion was turned off throughout training, escalation, and reescalation.
The hot plate data were transformed to maximum possible effect (%MPE) using a standard formula: (Test – Baseline)/(Cutoff – Baseline) × 100, with a cutoff of 30 s. The statistical analyses of transformed antinociceptive data and CPP scores were performed using between-subjects analysis of variance (ANOVA). Raw antinociception data, reinstatement lever pressing, and reescalation were analyzed by repeated-measures ANOVA, with vaccination as a between-subjects factor. Significant results in the ANOVA were followed by Dunnett’s or Tukey’s post hoc test where appropriate. Statistical analyses, including calculations of the areas-under-the-curve (AUCs), were performed using either MedCalc 12.3 or SAS Statview 5.0 software. Values of P < 0.05 were considered statistically significant. All of the data are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of Andrew Pham and Joe Torres as a part of their independent research internships at the University of California, San Diego. We also thank Dr. Gery Schulteis and Dr. Marc Azar for use of the equipment for antinociceptive measurements and Michael Arends for assistance in manuscript preparation and editing. This work was supported by The Scripps Research Institute, Pearson Center for Alcoholism and Addiction Research, and National Institutes of Health Grants DA026625, DA004043, and AA007456. This is Manuscript 21985 from The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219159110/-/DCSupplemental.
References
- 1.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 2.United Nations Office for Drug Control and Crime Prevention . World Drug Report 2012. Vienna, Austria: United Nations; 2012. 112. [Google Scholar]
- 3.Nutt DJ, King LA, Phillips LD. Independent Scientific Committee on Drugs Drug harms in the UK: A multicriteria decision analysis. Lancet. 2010;376(9752):1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- 4.Mayet S, Farrell M, Ferri M, Amato L, Davoli M. Psychosocial treatment for opiate abuse and dependence. Cochrane Database Syst Rev. 2005;(1):CD004330. doi: 10.1002/14651858.CD004330.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Farrell M, et al. Methadone maintenance treatment in opiate dependence: A review. BMJ. 1994;309(6960):997–1001. doi: 10.1136/bmj.309.6960.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: A randomised, placebo-controlled trial. Lancet. 2003;361(9358):662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 7.Blanken P, Hendriks VM, van Ree JM, van den Brink W. Outcome of long-term heroin-assisted treatment offered to chronic, treatment-resistant heroin addicts in the Netherlands. Addiction. 2010;105(2):300–308. doi: 10.1111/j.1360-0443.2009.02754.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller NS, Gold MS. Opiate prescription medication dependence and pain perceptions. J Addict Dis. 2007;26(Suppl 1):65–71. doi: 10.1300/J069v26S01_07. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Vital signs: Risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493–497. [PubMed] [Google Scholar]
- 10.Bell JR, Butler B, Lawrance A, Batey R, Salmelainen P. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009;104(1-2):73–77. doi: 10.1016/j.drugalcdep.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Mégarbane B, et al. Prospective comparative assessment of buprenorphine overdose with heroin and methadone: Clinical characteristics and response to antidotal treatment. J Subst Abuse Treat. 2010;38(4):403–407. doi: 10.1016/j.jsat.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Malcolm R, O’Neil PM, Von JM, Dickerson PC. Naltrexone and dysphoria: A double-blind placebo controlled trial. Biol Psychiatry. 1987;22(6):710–716. doi: 10.1016/0006-3223(87)90202-2. [DOI] [PubMed] [Google Scholar]
- 13.Crowley TJ, Wagner JE, Zerbe G, Macdonald M. Naltrexone-induced dysphoria in former opioid addicts. Am J Psychiatry. 1985;142(9):1081–1084. doi: 10.1176/ajp.142.9.1081. [DOI] [PubMed] [Google Scholar]
- 14.Bronstein DM, Day NC, Gutstein HB, Trujillo KA, Akil H. Pre- and posttranslational regulation of beta-endorphin biosynthesis in the CNS: Effects of chronic naltrexone treatment. J Neurochem. 1993;60(1):40–49. doi: 10.1111/j.1471-4159.1993.tb05820.x. [DOI] [PubMed] [Google Scholar]
- 15.Kosten TR, Kreek MJ, Ragunath J, Kleber HD. A preliminary study of beta endorphin during chronic naltrexone maintenance treatment in ex-opiate addicts. Life Sci. 1986;39(1):55–59. doi: 10.1016/0024-3205(86)90437-6. [DOI] [PubMed] [Google Scholar]
- 16.Ritter AJ. Naltrexone in the treatment of heroin dependence: Relationship with depression and risk of overdose. Aust N Z J Psychiatry. 2002;36(2):224–228. doi: 10.1046/j.1440-1614.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- 17.Hatsukami DK, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2005;78(5):456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Kosten TR, et al. Human therapeutic cocaine vaccine: Safety and immunogenicity. Vaccine. 2002;20(7-8):1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 19.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58(2):158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Selley DE, et al. mu Opioid receptor-mediated G-protein activation by heroin metabolites: Evidence for greater efficacy of 6-monoacetylmorphine compared with morphine. Biochem Pharmacol. 2001;62(4):447–455. doi: 10.1016/s0006-2952(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 21.Stowe GN, et al. Developing a vaccine against multiple psychoactive targets: A case study of heroin. CNS Neurol Disord Drug Targets. 2011;10(8):865–875. doi: 10.2174/187152711799219316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24(16):3232–3240. doi: 10.1016/j.vaccine.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 23.Li QQ, et al. A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J Neurochem. 2011;119(6):1271–1281. doi: 10.1111/j.1471-4159.2011.07502.x. [DOI] [PubMed] [Google Scholar]
- 24.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;252(5485):708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 25.Stowe GN, et al. A vaccine strategy that induces protective immunity against heroin. J Med Chem. 2011;54(14):5195–5204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK, Pentel PR. Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J Pharmacol Exp Ther. 2013;344(2):397–406. doi: 10.1124/jpet.112.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rook EJ, Huitema AD, van den Brink W, van Ree JM, Beijnen JH. Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: Review of the literature. Curr Clin Pharmacol. 2006;1(1):109–118. doi: 10.2174/157488406775268219. [DOI] [PubMed] [Google Scholar]
- 28.Rook EJ, et al. Pharmacokinetics and pharmacodynamics of high doses of pharmaceutically prepared heroin, by intravenous or by inhalation route in opioid-dependent patients. Basic Clin Pharmacol Toxicol. 2006;98(1):86–96. doi: 10.1111/j.1742-7843.2006.pto_233.x. [DOI] [PubMed] [Google Scholar]
- 29.Oguri K, Hanioka N, Yoshimura H. Species differences in metabolism of codeine: Urinary excretion of codeine glucuronide, morphine-3-glucuronide and morphine-6-glucuronide in mice, rats, guinea pigs and rabbits. Xenobiotica. 1990;20(7):683–688. doi: 10.3109/00498259009046884. [DOI] [PubMed] [Google Scholar]
- 30.Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208(1):144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27(46):12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32(3):616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- 33.Chen SA, et al. Unlimited access to heroin self-administration: Independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31(12):2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- 34.Vendruscolo LF, et al. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98(4):570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koob GF, Pettit HO, Ettenberg A, Bloom FE. Effects of opiate antagonists and their quaternary derivatives on heroin self-administration in the rat. J Pharmacol Exp Ther. 1984;229(2):481–486. [PubMed] [Google Scholar]
- 36.Li Q, et al. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: An event-related fMRI study. Brain Res. 2012;1469:63–72. doi: 10.1016/j.brainres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Zijlstra F, Booij J, van den Brink W, Franken IH. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 2008;18(4):262–270. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Franken IH, Hendriks VM, Stam CJ, Van den Brink W. A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol. 2004;14(6):503–508. doi: 10.1016/j.euroneuro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Pravetoni M, et al. An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther. 2012;341(1):225–232. doi: 10.1124/jpet.111.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pravetoni M, et al. Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J Med Chem. 2013;56(3):915–923. doi: 10.1021/jm3013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pravetoni M, et al. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine. 2012;30(31):4617–4624. doi: 10.1016/j.vaccine.2012.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 43.Karinen R, et al. Determination of heroin and its main metabolites in small sample volumes of whole blood and brain tissue by reversed-phase liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2009;33(7):345–350. doi: 10.1093/jat/33.7.345. [DOI] [PubMed] [Google Scholar]
- 44.Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30(1):90–98. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- 45.Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260(5115):1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.