Significance
DNA transposons move by excision and reintegration mediated by the element-encoded transposase. Unlike most transposases, the transposon Tn7 transposase is heteromeric, containing TnsB protein, which recognizes the transposon ends and mediates 3′ end breakage and joining, and TnsA protein, which mediates 5′ end breakage. We show that TnsA and TnsB interact and that TnsA can stimulate two key TnsB activities, end binding and end pairing. We also show that an end-pairing deficiency underlies the inability of a Tn7 containing two left ends to transpose. These findings reveal new aspects of protein–protein and protein–DNA interactions that underlie Tn7 transposition.
Keywords: protein–protein interaction, transposition, protein-DNA interaction, asymmetric transposon ends
Abstract
The transposon Tn7 transposase that recognizes the transposon ends and mediates breakage and joining is heteromeric. It contains the Tn7-encoded proteins TnsB, which binds specifically to the transposon ends and carries out breakage and joining at the 3′ ends, and TnsA, which carries out breakage at the 5′ ends of Tn7. TnsA apparently does not bind specifically to DNA, and we have hypothesized that it is recruited to the ends by interaction with TnsB. In this work, we show that TnsA and TnsB interact directly and identify several TnsA and TnsB amino acids involved in this interaction. We also show that TnsA can stimulate two key activities of TnsB, specific binding to the ends and pairing of the Tn7 ends. The ends of Tn7 are structurally asymmetric (i.e., contain different numbers of TnsB-binding sites), and Tn7 also is functionally asymmetric, inserting into its specific target site, attachment site attTn7 (attTn7) in a single orientation. Moreover, Tn7 elements containing two Tn7 right ends can transpose, but elements with two Tn7 left ends cannot. We show here that TnsA + TnsB are unable to pair the ends of a Tn7 element containing two Tn7 left ends. This pairing defect likely contributes to the inability of Tn7 elements with two Tn7 left ends to transpose.
Transposable elements are discrete DNA segments that can move from one genomic location to another. Transposition requires the positioning of the element-encoded transposase at the transposon ends by the recognition of specific DNA end sequences. The transposase also mediates pairing of the ends and subsequently the DNA breakage and joining reactions that underlie the process (1). We are interested in the protein–protein interactions that dictate the mechanism and regulation of transposition and particularly in the highly evolved bacterial transposon Tn7 which moves by a cut-and-paste mechanism (2, 3).
The Tn7 transposition machinery is elaborate, requiring four Tn7-encoded proteins, TnsA, TnsB, TnsC, and TnsD, to insert into its specific target site attTn7 (2, 3). Many transposases contain a single type of polypeptide (1), but the Tn7 transposase is heteromeric, containing two polypeptides, TnsA and TnsB (4, 5). Hundreds of elements containing TnsA-like and TnsB-like genes are present in bacterial genomes (6, 7).
TnsB binds specifically to multiple sites within the ends of Tn7 (8, 9) and mediates 3′ end breakage and joining (5), whereas TnsA mediates 5′ end breakage (4). Because TnsA does not bind specifically to DNA (10, 11), and no TnsB end breakage is seen in the absence of TnsA (12), we have proposed that interaction of TnsA with TnsB recruits TnsA to the Tn7 ends and activates TnsB (10, 12). However, direct evidence for this interaction is lacking.
Tn7 transposition involves additional proteins that control the activity of the TnsAB transposase and direct transposon insertion to particular target sites (3, 13). TnsD and TnsE are alternative target-site selectors. TnsD directs Tn7 to insert site and orientation specifically into its chromosomal target site, attachment site attTn7 (attTn7), by binding to attTn7 (14, 15), and TnsE directs insertion to replicating DNA (13, 16). TnsC is the central regulator of Tn7 transposition (3, 17) and stimulates TnsAB to carry out breakage and joining upon TnsC interaction with a target complex containing the target-selector protein, TnsD or TnsE, and target DNA. TnsC, an ATPase- and ATP-dependent DNA-binding protein (18), is itself activated by interaction with the target DNA (10, 14) and activates transposition by direct interaction with both TnsA (19–22) and TnsB (23). Transposition occurs upon the formation of a nucleoprotein complex containing the transposon, the target DNA, and the appropriate subset of Tns proteins (e.g., TnsABC+D) (10, 24, 25).
No transposition is observed in vivo in the presence of only wild-type TnsAB. However, we have isolated gain-of-function mutants, including TnsAE185K and TnsBA325T, that, when combined in vivo, promote TnsAB transposition (22). They also promote transposition when combined in vitro, albeit in the presence of 20% (vol/vol) glycerol rather than the standard 5% (vol/vol) contained in our in vitro reactions. The A325T amino acid change in TnsB is located in the catalytic domain, but, because the structure of TnsB remains to be determined, it is not yet possible to rationalize the effect of this amino acid change on TnsB at the molecular level. The structure of TnsA has been determined (26), and the position of TnsAE185K is intriguing. TnsA has two domains, an N-terminal catalytic domain containing the TnsA active site, and a C-terminal domain, which resembles histone H5. TnsAE185K is located on a surface-exposed helix in the C-terminal domain that could provide an interaction region for TnsB.
DNA breakage and joining by wild-type TnsAB in vitro also occurs in the presence of Mn2+ and high (20%, vol/vol) glycerol, when double-strand breaks (DSBs) are catalyzed at the ends of Tn7 and intramolecular end-to-end joining reactions are observed (12). Notably, no breakage by TnsB alone is observed under these conditions, and TnsA still is required, even if it is catalytically inactive (4).
In this work we isolate and characterize additional TnsA gain-of-function mutants in the C-terminal domain of TnsA that are active in vivo and in vitro in the presence of wild-type TnsB. Notably the mutations are located from TnsAY180 to TnsAE185, supporting the view that this helix provides a region for interaction with TnsB. We also show that TnsA can stimulate the binding of TnsB to the ends of Tn7 and TnsB-dependent pairing of the Tn7 ends.
Although TnsB binds to both ends in vitro under similar conditions, different numbers of TnsB-binding sites are present in each end of Tn7, making the ends structurally asymmetric (8, 9). The ends also are functionally asymmetric: Tn7 inserts into attTn7 in only a single orientation, with the Tn7 right end (Tn7R) adjacent to the 3′ end of the glucosamine synthetase gene (glmS), a highly conserved and essential bacterial gene (2, 3, 15). A possible biological rational for this bias is that transcription from glmS could enter the tns genes, which are oriented with their 5′ ends toward glmS, a possible route for connection between cellular metabolism and the control of transposition. We have shown previously that transcription from glmS into attTn7 reduces attTn7 target activity (27). How then is orientation-specific insertion imposed? It seems likely that orientation-specific insertion is related to another functional asymmetry: Although a Tn7 element containing one Tn7 left end and one right end and an element containing two Tn7 right ends can transpose, an element containing two Tn7 left ends cannot transpose (28). We also show here that the ends of an element containing two Tn7 left ends do not pair in the presence of TnsAB, suggesting that this end-pairing defect underlies the transposition defect found with elements with two left ends.
Results
Characterization of TnsA Gain-of-Function and Loss-of-Function Mutants.
To probe the unresolved physical and functional interactions between TnsA and TnsB, we isolated additional TnsA gain-of-function mutants using a screen done in the presence of the previously isolated gain-of-function mutant TnsBA325T. As described in Materials and Methods, we isolated several TnsA mutations located in the C-terminal domain including Y180H, S181P, V182A, and E185G. Notably, these mutations, like the previously isolated gain-of-function mutant E185K, lie on the solvent-exposed helix that we previously suggested could be a region of TnsA–TnsB interaction (22).
To examine the TnsA mutants in vitro, we expressed these proteins in Escherichia coli, affinity purified them, and assayed them in modified transposition reactions in the presence of 20% (vol/vol) glycerol as described previously (22) rather than the 5% (vol/vol) glycerol in our standard TnsABC+D reactions (Fig. 1A). In the presence of TnsBA325T, we find that the C-terminal domain Y180H, S181P, V182A, E185G, and E185K TnsA gain-of-function mutants generate a markedly increased level of DSBs at each end of Tn7, DSB.Left (DSB.L) and DSB.Right (DSB.R), and an excised linear transposon (ELT) resulting from DSBs at both ends, with TnsAY180H and TnsAS181P being the most active in promoting DSBs. We also observed increased levels of end cleavage with the TnsA mutants in the presence of wild-type TnsB.
Fig. 1.
In vivo and in vitro comparisons of TnsA gain-of-function mutants. (A) In vitro Tn7 transposition assays with TnsA gain-of-function mutants. Reactions were carried out in high glycerol (20%, vol/vol) and 15 mM Mg2+, digested with a restriction enzyme that cleaved once in the donor backbone. DNA DSBs at the right or left end of Tn7 result in DSB.R and DSB.L, respectively. DSBs at both ends result in an ELT. M, size markers. (B) Comparison of TnsA gain-of-function mutants by papillation in cells containing TnsA mutants and wild-type TnsB. Cell suspensions as indicated were spotted on MacConkey lactose plates. (C) In vivo lambda hop assay with Tns mutants. The frequency of the transposition of a miniTn7Kan element from an integration-defective lambda phage into the E. coli chromosome was measured in strains containing the various tnsA, tnsB, and tnsC genes. The results are the average of three independent experiments and are shown as the number of transposition events.
Using two different assays, we also evaluated the in vivo activity of the C-terminal domain Y180H, S181P, V182A, and E185G TnsA gain-of-function mutants with wild-type TnsB. In one assay we used a promoter capture papillation assay (20, 22), which allows us to visualize transposition in individual colonies. The host strain contains a tnsA plasmid, a tnsB plasmid and a miniTn7lac donor plasmid, which lacks a promoter for the β-galactosidase (lac) gene and results in a strain that is white on MacConkey lactose plates. When transposition occurs, so that the miniTn7lac element is downstream of a promoter, red papillae are seen on the plates. Although the Y180H, S181P, V182A, and E185G mutants were isolated in the presence of the gain-of-function mutant TnsBA325V, we find that they also can promote transposition in vivo in the presence of wild-type TnsB (Fig. 1B).
In another system, we used the lambda hop assay, in which we quantitatively can measure the ability of a miniTn7 element encoding a kanamycin-resistance gene (miniTn7 KanR) to translocate from an infecting phage to the genome to generate a drug-resistant strain capable of forming a colony on selective media (Fig. 1C). As a baseline, we used a TnsABCA225V system in which the gain-of-function TnsCA225V allele promotes TnsABC transposition in the absence of TnsD or TnsE (29). A 2.5-fold increase in transposition was seen with the gain-of-function TnsAS181P allele. In contrast, changing TnsA to TnsAY180A/S181A reduced transposition to undetectable levels, suggesting that TnsAY180A/S181A is a loss-of-function TnsA allele.
TnsA Interacts Directly with TnsB.
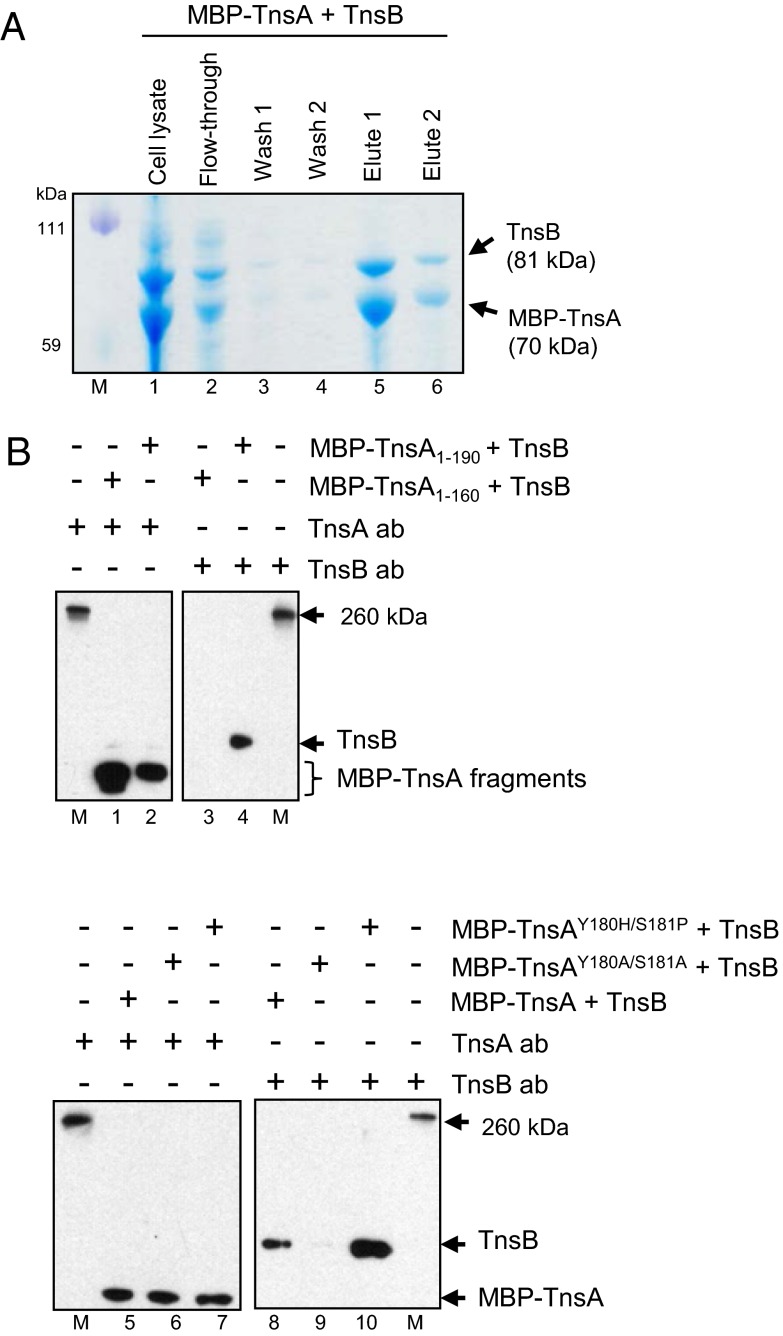
To look for direct interactions between TnsA and TnsB, we asked if TnsB would copurify with TnsA when TnsA was purified via an affinity tag. We cloned tnsAB into a maltose-binding protein (MBP) expression vector so that TnsA was fused to the C terminus of MBP to form MBP–TnsA. Lysates of E. coli containing MBP–TnsA and TnsB were passed over an amylose column, the column was washed, and MBP–TnsA was eluted from the column with maltose. We then displayed the crude extract and eluted fractions on SDS/PAGE gels and examined their content by Coomassie blue staining and Western blotting with anti-TnsA and anti-TnsB antibodies. As shown in Fig. 2A, proteins of the size expected for MBP–TnsA and TnsB were both present in the crude extract. Notably, both of these proteins also were present in the fraction eluted with maltose. These proteins react appropriately with anti-TnsA and anti-TnsB (Fig. S1A). No binding of TnsB to the amylose column was observed in the absence of MBP–TnsA (Fig. S1B). These copurification assays provide evidence that TnsA and TnsB can interact directly by protein–protein interactions.
Fig. 2.
TnsA interacts directly with TnsB. (A) The elution profiles of MBP–TnsA and TnsB from an amylose column. Fractions were displayed on an SDS/PAGE gel and stained with Coomassie blue. A single gel is shown. M, size markers; lane 1, crude extract; lane 2, flow-through fraction; lanes 3 and 4, wash fractions; lanes 5 and 6, fractions eluted with maltose buffer. (B) Western blot of maltose-eluted fractions using extracts containing MBP–TnsA mutated proteins with TnsA or TnsB antibody as indicated. M, size markers; lanes 1 and 3, maltose-eluted fraction using MBP–TnsA1–160 blotted with TnsA and TnsB antibodies; lanes 2 and 4, maltose-eluted fraction using MBP–TnsA1–190 blotted with TnsA and TnsB; lanes 5 and 8, maltose-eluted fraction using TnsA blotted with TnsA and TnsB antibodies; lanes 6 and 9, maltose-eluted fraction using TnsAY180A/S181A blotted with TnsA and TnsB antibodies; lanes 7 and 10, maltose-eluted fraction using TnsAY180H/S181P blotted with TnsA and TnsB antibodies. Equivalent reactions were run on the same gel, and different lanes were blotted with either anti-TnsA or anti-TnsB antibody.
What regions of TnsA and TnsB mediate this interaction? To define the region of TnsA that interacts with TnsB, we used the copurification assay described above to examine the binding of TnsB with C-terminal–truncated TnsA fragments (Fig. 2B). We found that although MBP–TnsA1–190 was able to interact with TnsB, a MBP–TnsA1–160 truncation mutant that lacks the TnsA180–185 region which contains the gain-of-function mutations did not interact with TnsB. We also found that the introduction of alanine substitutions into the TnsA180–185 region was the reason the loss-of-function allele MBP–TnsAY180A/S181A mutant decreased TnsB retention. In contrast when MBP–TnsA contained the gain-of-function mutations MBP–TnsAY180H/S181P, retention of TnsB was increased over wild-type TnsB. These results support the view that TnsA and TnsB interact directly and that the solvent-exposed region of TnsAY180 to TnsAS181 likely plays a key role in this interaction.
TnsA Stimulates TnsB Binding to the Tn7 Ends.
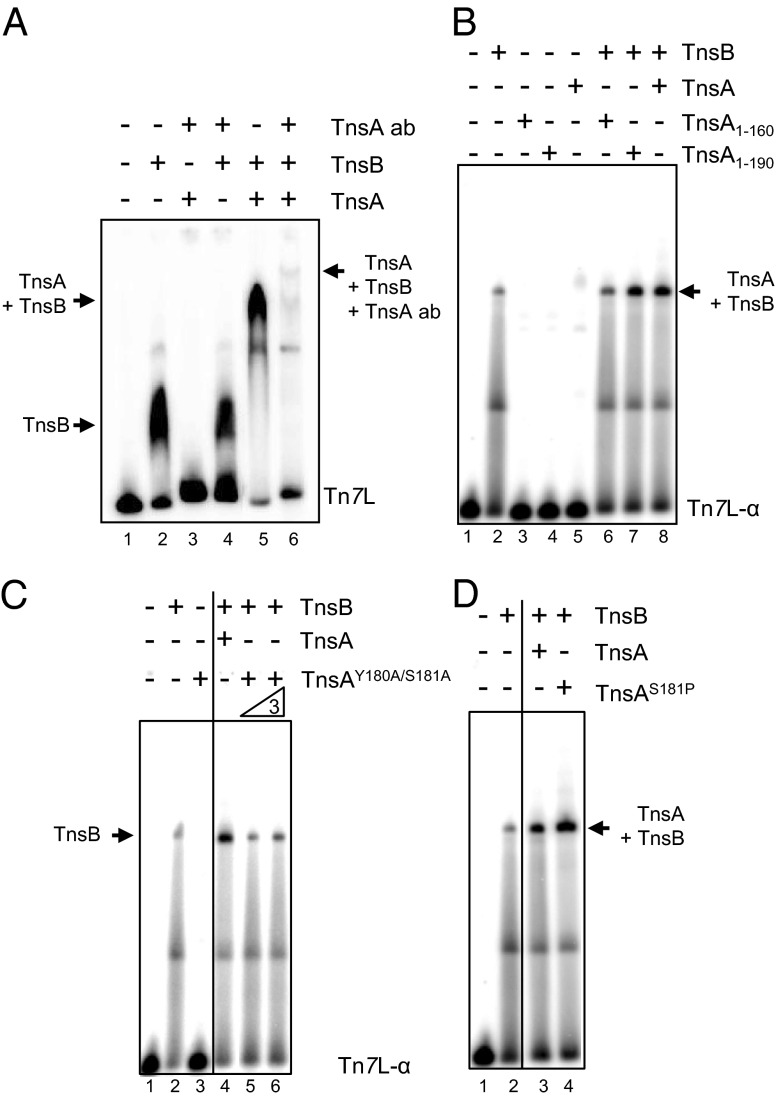
During transposition, the specific binding of TnsB to the Tn7 ends (8, 9) and TnsB-mediated end pairing (23, 24) are key steps that occur before DNA breakage and joining. To determine whether TnsA can influence TnsB DNA binding, we used a gel retardation assay whose products are displayed on an 8% acrylamide gel analyzed the binding of TnsB to a DNA fragment containing the Tn7 left-end sequences (Tn7L, 161 bp) which contain the α, β, and γ TnsB-binding sites. As shown in Fig. 3A, TnsA stimulates TnsB end binding and results in a new complex with slower mobility, suggesting that both TnsB and TnsA are in the complex. Furthermore, mobility of this TnsA–TnsB complex is slowed further to form another new complex in the presence of TnsA antibody, as is consistent with the view that both TnsB and TnsA are in the slower mobility complex.
Fig. 3.
TnsA stimulates TnsB binding at the end of Tn7. The binding of TnsB to Tn7L1–166 and Tn7L1–30 is evaluated by gel retardation assays. (A) TnsB–Tn7L can be supershifted with TnsA and an antibody against TnsA. TnsA makes a new complex from the TnsB–Tn7L end complex. Lane 1, no protein; lane 2, TnsB; lane 3, TnsA + anti-TnsA antibody; lane 4, TnsB + anti-TnsA antibody; lane 5, TnsB + TnsA; lane 6, TnsB+TnsA and anti-TnsA antibody. (B) TnsA stimulation of TnsB binding requires TnsA161–190. Lane 1, no protein; lane 2, TnsB; lane 3, TnsA1–160; lane 4, TnsA1–190; lane 5, TnsA; lane 6, TnsB + TnsA1–160; lane 7, TnsB + TnsA1–190; lane 8, TnsB + TnsA. (C) TnsAY180A/S181A does not stimulate TnsB binding. Lane 1, no protein; lane 2. TnsB; lane 3, TnsAY180A/S181A; lane 4, TnsB + TnsA; lane 5, TnsB+TnsAY180A/S181A; lane 6, TnsB+ 3× TnsAY180A/S181A. As indicated by the vertical line, different sections from the same gel have been juxtaposed next to each other. (D) The gain-of-function mutant TnsAS181P stimulates TnsB binding more than TnsA. Lane 1, no protein; lane 2, TnsB; lane 3, TnsB + TnsA; lane 4, TnsB + TnsAS181P. As indicated by the vertical line in C and D, different sections from the same gel have been juxtaposed next to each other.
We also examined the effect of TnsA on TnsB binding using a single TnsB-binding site, the Tn7L-α 30-bp fragment which contains the Tn7 terminal α TnsB site. In these experiments, we displayed the reaction products on a 6% acrylamide gel (Fig. 3B). Although we still observed stimulation of TnsB end binding by TnsA, there was no change in the mobility of the complex as is observed with a 8% acrylamide gel as described above. The TnsA1–190 fragment stimulated TnsB binding, whereas the TnsA1–160 fragment, which lacks the TnsA180–185 region, does not. Furthermore, the TnsA loss-of-function mutant TnsAY180A/S181A, which we show in Fig. 1C to be defective in transposition activity, does not stimulate formation of the TnsB–Tn7L-α complex (Fig. 3C), whereas the gain-of-function mutant TnsAS181P, which results in increased transposition activity, stimulates formation of the TnsB–Tn7L-α complex to a greater degree than does wild-type TnsA (Fig. 3D). These findings are consistent with the view that the TnsA180–185 region is key to TnsA stimulation of the formation of TnsB–Tn7 end complex.
TnsA–TnsB Interaction Domain Is Located Within TnsB440–480.
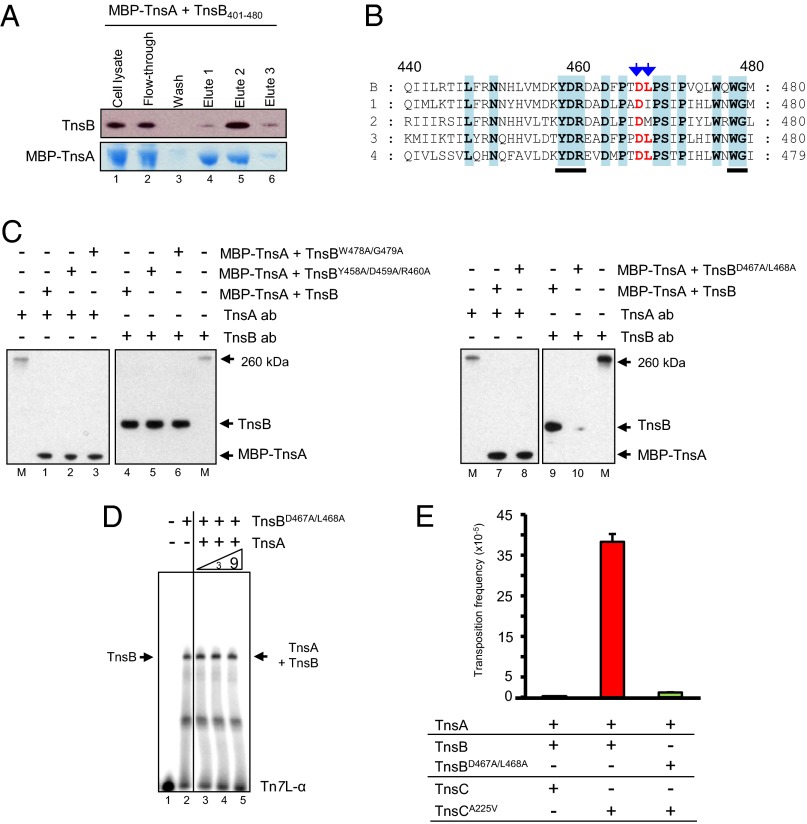
The experiments above suggested that TnsA180–185 interacts directly with TnsB. To identify the region(s) of TnsB that are important for TnsA interaction, we coexpressed several N-terminally and C-terminally truncated TnsB derivatives with MBP–TnsA and applied the extracts to an amylose resin, eluted MBP–TnsA with maltose, and analyzed the eluted fractions for the presence of the TnsB variants. We found that TnsB1–500 was sufficient to interact with MBP–TnsA (Fig. S2A) and that TnsB401–702 also can interact with TnsA (Fig. S2B). As shown Fig. 4A, a TnsB401–480 fragment interacts with MBP–TnsA. Furthermore, MBP–TnsA was able to bind TnsB1–480 but was unable to bind TnsB1–420 and TnsB1–440, suggesting that the region of TnsB that interacts with TnsA is within TnsB440–480 (Fig. S2C).
Fig. 4.
Interaction of TnsB mutants with TnsA. (A) Copurification of TnsB401–480 with MBP–TnsA. The copurification of TnsB401–480 with MBP–TnsA after elution from an amylose column with maltose was evaluated by SDS/PAGE, followed by Western blotting with TnsB antibody and staining with Coomassie blue. Lane 1, cell lysate; lane 2, flow-through fraction; lane 3, wash fraction; lanes 4, 5, and 6, fractions eluted with maltose. Equivalent reactions were run on the same gel, and different lanes were blotted with either anti-TnsA or anti-TnsB antibody. (B) Multiple sequence alignment of TnsBs between amino acids 440 and 480 produced using the software T-Coffee (www.ebi.ac.uk/Tools/msa/tcoffee/) (38). B, E. coli (NP_065319) (TnsB in this work); 1, Shewanella putrefaciens CN-32 (YP_001185440); 2, Tolumonas auensis DSM 9187 (YP_002891520); 3, Acinetobacter baumannii ATCC17978 (ABO12968); 4, Idiomarina loihiensis L2TR (YP_156993). Conserved positions are highlighted in blue. Changes at positions indicated by blue arrows lead to loss of TnsB interaction with TnsA. Changes at underscored positions did not affect interaction with TnsA. (C) TnsBD467A/L468A does not interact with MBP–TnsA. Analysis of the copurification of TnsB truncations with MBP–TnsA after elution from an amylose column with maltose was evaluated by SDS/PAGE, followed by Western blotting with anti-TnsA and anti-TnsB antibodies as indicated. M, size markers; lanes 1, 4, 7, and 9, MBP–TnsA + TnsB; lanes 2 and 5, MBP–TnsA + TnsBY458A/D459A/R460A; lanes 3 and 6, MBP–TnsA + TnsBW478A/G479A; lanes 8 and 10, MBP–TnsA + TnsBD467A/L468A. Equivalent reactions were run on the same gel, and different lanes were blotted with either anti-TnsA or anti-TnsB antibody. (D) TnsBD467A/L468A binding to a Tn7 end is not stimulated by TnsA. TnsB binding to Tn7L-α was evaluated by EMSA with different concentrations of TnsA on the same gel. Lane 1, no TnsB; lane 2, TnsBD467A/L468A; lane 3, TnsBD467A/L468A + 1× TnsA; lane 4, TnsBD467A/L468A + 3× TnsA; lane 5, TnsBD467A/L468A + 9× TnsA. As indicated by the vertical line, different sections from the same gel have been juxtaposed next to each other. (E) TnsBD467A/L468A is inactive in transposition in vivo. Transposition in the presence of TnsA, TnsB, and TnsC was measured in a lambda hop assay. The results are the average of three independent experiments and are shown as the number of transposition events.
To identify individual TnsB residues within TnsB440–480 that are important to this interaction, we tested the effects of changing amino acids within TnsB440–480 on the interaction of MBP–TnsA and –TnsB. Alignment of the amino acid sequences in this region of several TnsBs from different organisms revealed multiple conserved amino acids in the TnsB440–480 region (Fig. 4B). We changed several amino acids residues within this TnsB440–480 region to alanine and examined the effect on TnsA interaction, finding that TnsBD467A/L468A interacts poorly with MBP–TnsA but that the other mutants interact with MBP–TnsA in the same way as wild-type TnsB (Fig. 4C). This finding supports the view that the TnsB region within TnsB440–480 mediates the TnsA–TnsB interaction.
We also found that TnsA does not stimulate Tn7 end binding by TnsBD467A/L468A (Fig. 4D). Moreover, TnsBD467A/L468A is not active in transposition in vivo as evaluated by a lambda hop assay (Fig. 4E), a failure we attribute to its inability to interact with TnsA.
TnsA Can Stimulate TnsB-Mediated Pairing of the Tn7 Ends.
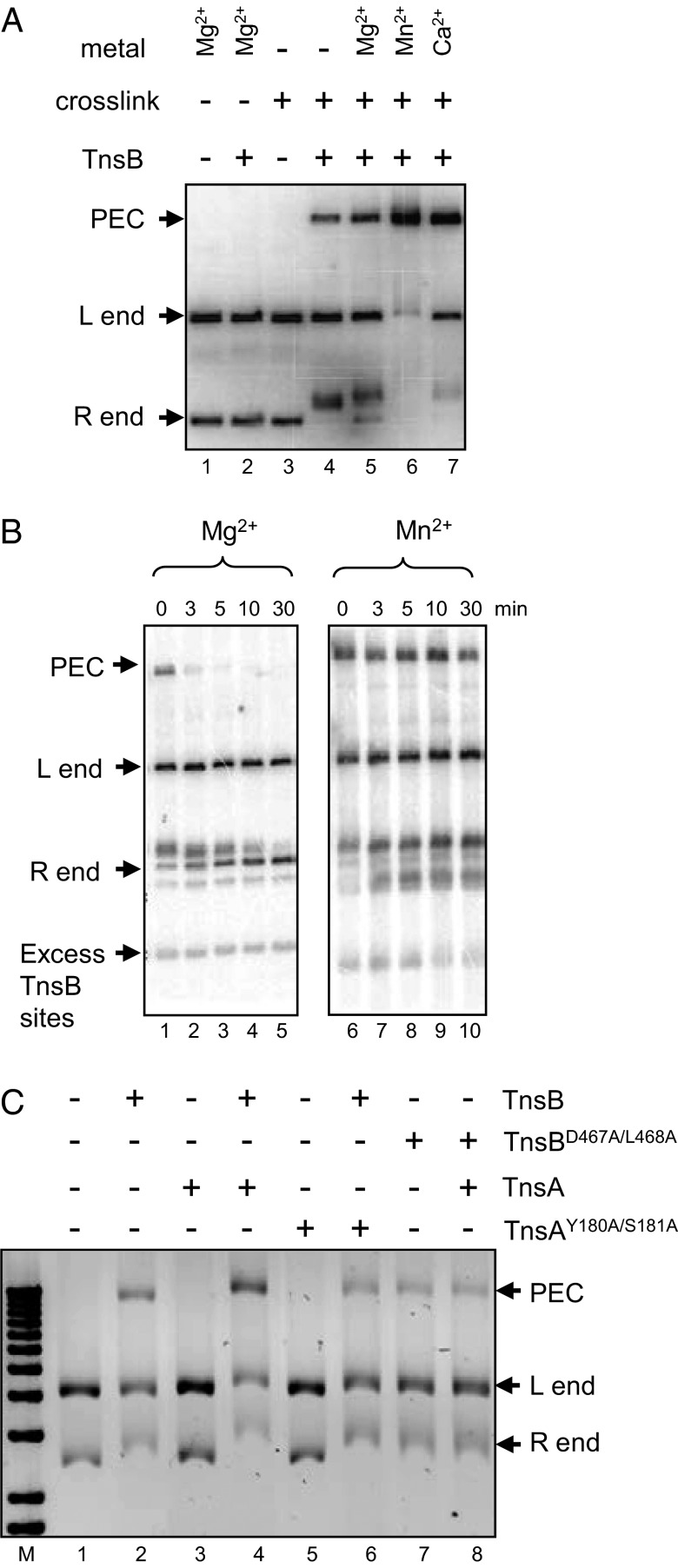
One of the key steps in recombination is the pairing of the transposon ends. We have shown previously that TnsB alone can pair the ends of Tn7 (23, 24) to form the paired-end complex (PEC). As shown in Fig. 5A, detection of the PEC is dependent on crosslinking (glutaraldehyde) and the presence of divalent metal. The stimulation of PEC formation by Mn2+ (Fig. 5B) also likely allows intramolecular Tn7 transposition to occur in the presence of Mn2+ and high glycerol (12) but not with Mg2+ and low glycerol. We show in Fig. S3 that the PEC is an intermediate in transposition.
Fig. 5.
In vitro formation of the PEC by TnsB and TnsA. (A) The formation of a PEC is examined under various conditions as indicated. (B) The stability of the PEC is different in Mg2+ and 5% (vol/vol) glycerol vs. Mn2+ and 20% (vol/vol) glycerol. The stability over time of a PEC formed in the presence of either Mg2+ and 5% (vol/vol) glycerol (lanes 1–5) or Mn2+ and 20% (vol/vol) glycerol (lanes 6–10) is examined after the addition of excess TnsB-binding sites. (C) TnsA stimulates TnsB to form the PEC. M, size markers; lane 1, no protein addition; lane 2, TnsB, lane 3, TnsA; lane 4, TnsB + TnsA; lane 5. loss-of-function TnsAY180A/S181A; lane 6, TnsB + loss-of-function TnsAY180A/S181A; lane 7, loss-of-function TnsBD467A/L468A; lane 8, loss-of-function TnsBD467A/L468A + TnsA.
PEC formation also was stimulated by the addition of TnsA to the pairing reactions (Fig. 5C). However, this stimulation was not evident with the loss-of-function mutants TnsAY180A/S181A or with TnsBD467A/L468A, which are defective in TnsA–TnsB interaction. Thus, although the ability to pair the ends is an intrinsic activity of TnsB, it can be stimulated by TnsA.
TnsAB Cannot Pair the Ends of a Tn7 Element with Two Tn7 Left Ends.
Although both ends of Tn7 contain TnsB-binding sites, the left and right ends are structurally asymmetric: Tn7L contains three widely spaced TnsB-binding sites whereas Tn7R contains four adjoining sites (8, 9). The ends of Tn7 also are functionally asymmetric as revealed by the fact that Tn7 inserts in a single preferred orientation in attTn7. Another indication of the functional asymmetry of the Tn7 ends is that, although elements with two right ends can be transposed, elements with two left ends cannot (28). At what step in transposition does the restriction against Tn7L–Tn7L transposition occur? As shown in Fig. 6, even TnsAB cannot pair the ends of a Tn7L–Tn7L element under conditions in which pairing of the ends of Tn7L–Tn7R and Tn7R–Tn7R elements does occur. We suggest that this end-pairing defect contributes to the inability of Tn7L–Tn7L elements to transpose.
Fig. 6.
Formation of PEC with various Tn7 elements. End pairing of Tn7L–Tn7R, Tn7L–Tn7L, and Tn7R–Tn7R donor plasmids was analyzed in the presence by TnsB and TnsA as indicated. Lanes 1–4, analysis of Tn7L–Tn7R; lanes 5–8, analysis of Tn7L–Tn7L; lanes 9–1, analysis of Tn7R–Tn7R.
Discussion
The Tn7 transposition machinery is distinguished by its complexity: Insertion into its specific target site, attTn7, requires four Tn7-encoded proteins, TnsABC+D, and two substrate DNAs, Tn7 in its donor site and the attTn7 target site. Assembly of these components into a complex prompts the initiation of DNA breakage and joining (2, 3, 25). The Tn7 transposase contains two proteins, TnsA and TnsB. TnsA mediates cleavage from the donor site at the 5′ ends of the transposon, and TnsB binds specifically to the transposon ends and mediates cleavage and joining to the target DNA at the 3′ ends of the transposon.
We have shown previously in vitro that TnsA plus TnsB in the absence of other Tns proteins, but in the permissive conditions of Mn2+ and high concentrations of glycerol, can promote end cleavage and intramolecular joining, showing that they are sufficient for DNA breakage and joining (12). Notably, TnsA is required for TnsB breakage and joining, even if TnsA is catalytically inactive (4). Thus, we have speculated previously that TnsA is recruited to the transposon ends by interaction with TnsB and that DNA breakage and joining also involve interactions between TnsA and TnsB (4, 5, 25). Previous fluorescence anisotropy studies first suggested a direct interaction between TnsA and TnsB (30).
Here we provide evidence for direct interaction by showing that TnsA and TnsB copurify when coexpressed. We also show that this interaction plays a role in transposase activity, showing that TnsA stimulates several fundamental activities of TnsB. To probe the interaction of TnsA and TnsB at higher resolution, we isolated and characterized TnsA gain-of-function and loss-of-function mutants that affect the ability of TnsA to interact with TnsB. These mutations are clustered on a region of TnsA, Y180 to E185, that is solvent-exposed in the TnsA crystal and thus lie on a potential surface for TnsA–TnsB interaction.
We also isolated and characterized a TnsB loss-of-function mutation, TnsBD467A/L468A, that likely identifies a surface on TnsB involved in TnsA–TnsB interaction. To demonstrate a direct interaction between TnsA and TnsB, we developed a copurification assay in which we show that TnsB copurifies with an MBP–TnsA fusion protein isolated by affinity purification on an amylose resin. We find that the region of TnsA containing the gain-of-function mutations is required for TnsA–TnsB interaction and that presence of the TnsA gain-of-function mutations increases TnsA–TnsB interaction, whereas the TnsA loss-of-function mutations decrease TnsA–TnsB interaction. The presence of the TnsB loss-of-function mutation also decreases TnsA–TnsB interaction.
We also examined the effect of TnsA on two central activities of TnsB, its ability to bind specifically to the Tn7 ends and its ability to pair the Tn7 ends (8, 24). We find that TnsA stimulated the end-binding activity. As with TnsA–TnsB interaction, we found that TnsA stimulates TnsB end binding and that end binding is increased in the presence of the TnsA gain-of-function mutations that increase TnsA–TnsB binding. TnsB end binding is not stimulated in the presence of TnsA mutants containing mutations that decrease interactions between TnsA and TnsB.
A key step in transposition is pairing between the ends, a control step that assures that DNA cleavage does not occur on a single, isolated end. Previously we have shown that TnsB can pair the ends of Tn7 to form a PEC. We now have found that TnsA can stimulate end pairing, showing that this stimulation does not occur when either TnsA or TnsB contains mutations that block TnsA–TnsB interaction.
What is the molecular mechanism by which TnsA stimulates TnsB end binding and end pairing? Although we have not detected DNA binding by TnsA alone, the fact that it contains the active site for 5′ end cleavage indicates that it must interact with DNA. Increased end binding by TnsB complexed with TnsA thus may reflect the combined action of their two DNA-binding interfaces. Alternatively, interaction of TnsA with TnsB may provoke a conformational change in TnsB that increases end binding, providing more TnsB and end complex for pairing. We also propose that interaction of TnsA with TnsB promotes a conformational change in TnsB that prompts end cleavage and joining in the nonstandard conditions presence of Mn2+ and 20% (vol/vol) glycerol (12). Defining the nature of these changes will be the next key steps in analysis of TnsAB.
Although we have shown that interactions between TnsA and TnsB are central to multiple steps in transposition, the presence of only TnsA and TnsB is not sufficient for transposition in the presence of Mg2+. Key to the activation of the transposase is the regulator of transposition, TnsC, an ATPase- and ATP-dependent DNA-binding protein that activates transposition when it interacts with the target DNA, and Tn7’s alternative targeting proteins, TnsD, which directs transposition to attTn7, and TnsE, which directs transposition to replicating DNA (3). TnsC also interacts directly with both TnsA (21, 22, 29) and TnsB (23). Thus, our view is that transposition is modulated by a network of protein–protein interactions between TnsA and TnsB, between TnsA and TnsC, and between TnsB and TnsC. We have argued previously that TnsA, TnsC, and TnsD interact with attTn7 to form the TnsACD–attTn7 target complex and that TnsB is involved in a transposon end complex so that the active TnsAB transposase forms only when these target and end nucleoprotein complexes are juxtaposed (23, 25). Thus, direct interaction between TnsA and TnsB provides a mechanism for assembly and activation of the transposase. The molecular role(s) of TnsC interaction with TnsA and TnsB also will be interesting to determine.
The ends of Tn7 are both structurally and functionally asymmetric: They contain different numbers of TnsB binding sites, and Tn7 insertion into attTn7 is orientation-dependent; that is, the Tn7R end also inserts adjacent to glmS (2, 3). Another indication of this asymmetry is that, although miniTn7 elements containing two Tn7R ends are functional, those containing two Tn7L ends are not active in vivo (28). We have now found that the ends of a Tn7L–Tn7L element cannot pair in the presence of TnsB or TnsA + TnsB, suggesting that a deficiency in end pairing contributes to the inability of a Tn7L–Tn7L element to transpose. We are attracted to the view that for Tn7, as for bacteriophage Mu (31–33), having two closely abutted TnsB-binding sites on the terminus of the right end is key to transpososome activity because they can support interend pairing. Further studies will be required to determine how the asymmetry of the Tn7 ends is communicated to attTn7.
Materials and Methods
Screening for TnsA Hyperactive Mutants Using a Papillation Assay.
We screened for TnsA gain-of-function mutants that promoted transposition in the presence of only TnsBA325T, a gain-of-function TnsB mutant (22). To mutagenize tnsA, we used the pUC19-based plasmid pCW43 (34), a pUC19 plasmid containing Tn7R42–1235. tnsA is located at Tn7R135–956, and a partial tnsB gene is located at Tn7R943–1235. Tn7R42 is flanked by the pUC19 polylinker, and Tn7R1235 is flanked by a miniMu sequence. A fragment extending from the pUC19 polylinker adjacent to Tn7R42 to the miniMu segment beyond Tn7R1235 was PCR amplified in the presence of Mn2+ using primers GCTATGACCATGATTACGCCAAGCT in the pUC19 polylinker and GCCCTTCCCAACAGTTGCGCAGCC in the miniMu segment. The PCR fragment was digested with NcoI and BglII and ligated to pCW43 digested with NcoI and BglII. The ligation mixture was transformed into Escherichia coli CW51 containing a pACYC plasmid encoding tnsBA325T (22) and pOXminiTn7lac, which has no promoter in the transposon that directs lac expression and which is in a donor site in which lac is not expressed. Transformants were selected on MacConkey lactose plates, and the colonies with greater numbers of red papillae were screened for among about 6,500 transformants. Plasmids from the colonies with increased numbers of papillae were retested and sequenced. Seven TnsA mutants were identified: Q82R, V103A, D139N, Y180H, S181P (four colonies), V182A (three colonies), and E185G.
Purification of Tns Proteins.
The tnsA, tnsB, and tnsAB derivatives were PCR amplified with PicoMaxx High Fidelity Master Mix (Stratagene) and MJ Research PTC-200 Thermo Cycler (Bio-Rad). The PCR-generated fragments were gel-purified with the Qiaquick Gel Extraction kit (Qiagen) and then were inserted into the intein pCYB1 vector (New England BioLabs) between NdeI and EcoRI (New England BioLabs) sites and transformed into E. coli ER2566 (New England BioLabs). For purification, cells were grown to OD600nm 0.5–0.7 at 37 °C in LB broth before induction by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.4 mM. The cultures were grown for 16 h at 17 °C. All subsequent steps were performed at 4 °C. Cells were harvested by centrifugation and resuspended in 10 mL column buffer [20 mM Tris (pH 8.0), 0.5 M NaCl, and 1 mM EDTA]. Cells were lysed by sonication, and cell debris was removed by centrifugation. The soluble material was filtered through a 0.45-μm filter (Millipore) before loading onto a column of chitin beads (New England BioLabs) that had been equilibrated with column buffer and then was shaken gently for 20 min. The column was incubated for 2 d at 4 °C in column buffer containing 50 mM DTT, and the bound protein was eluted by washing of the column. Proteins were dialyzed into TnsA storage buffer [25 mM Hepes (pH 8.0), 1 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.25 mM PMSF, and 5% (vol/vol) glycerol] or TnsB storage buffer [25 mM Hepes (pH 8.0), 0.5 M NaCl, 2 mM DTT, and 25% (vol/vol) glycerol]. Proteins were analyzed by display on a 4–12% NuPAGE SDS/PAGE gel (Invitrogen).
In Vitro Transposition Assay.
In vitro transposition reactions (10) in which end cleavage occurred contained 0.25 nM pEMΔ, a 5.9-kb plasmid which contains a 1.6-kb miniTn7 KanR element (i.e., a kanamycin gene segment between 166-bp Tn7L and 99-bp Tn7R ends), a 2.1-nM attTn7 target plasmid, Tns proteins, and buffer including 20% (vol/vol) glycerol. The Tns proteins were 22 ng TnsA, 15 ng TnsB, 30 ng TnsC, and 30 ng TnsD. The buffers contain 2.5 mM Tris (pH 7.6), 25 mM Hepes (pH 7.6), 2 mM DTT, 50 μg/mL BSA, 100 μg/mL tRNA, 25 μM ATP, 20% (vol/vol) glycerol, and 15 mM MgAc (12). The reactions were performed at 30 °C for 30 min in a final volume of 100 μL. DNA was extracted with phenol:chloroform:isoamylalcohol (25:24:1), was precipitated with ethanol and digested with NdeI with added RNase A, and then was analyzed on a 1.0% (wt/vol) agarose gel in 1× Tris-borate-EDTA (TBE) buffer. The DNAs were transferred to GeneScreen Plus membrane (NEN Research Products) and then were Southern blotted with a miniTn7-specific probe. The blots were analyzed using a Molecular Dynamics PhosphorImager.
Papillation Assay.
Papillation was evaluated in CW51 containing wild-type or mutant tnsA in pCW43, wild-type tnsB in a pACYC plasmid (22), and pOXminiTn7lac.
In Vivo Lambda Hop Transposition Assays.
In vivo transposition was evaluated using the lambda hop assay in which a replication- and integration-defective lambda phage containing miniTn7KanR is infected into a cell containing tns genes as indicated and for KanR cells in which transposition into the chromosome has occurred are selected (35). Two plasmids, pTA106-tnsAB (13) containing wild-type or several mutated tnsA or tnsB genes and pACYC184-tnsC containing tnsC or tnsCA225V (20), were cotransformed into E. coli NLC51-competent cells (36). Transposition frequency was determined by scoring the number of kanamycin-resistant colonies per infectious phage particle as measured by plaque-forming units.
Construction of Tns Plasmids.
The tnsA and tnsB genes were isolated from pCW4 (34). Oligonucleotides pairs for gene amplification are shown in Table S1. tnsAB segments were PCR amplified using pCW4, which contains tnsABCDE, as a template with oligonucleotides. The PCR products were digested and isolated by gel purification. This fragment was ligated and cloned between the NdeI and EcoRI sites of pCYB1 vector (New England Bio-Labs), making intein–TnsA, and TnsB. The tnsAB genes were cloned between the KpnI and EcoRI sites of plasmid pMAL vector (New England Bio-Labs) so that a MBP–TnsA fusion resulted, generating the plasmid pMAL–TnsA + TnsB. Various tnsAB site-specific mutated segments were amplified by overlap PCR. Every reaction terminal primer set is the same as in the tnsAB cloning and overlap primer sets and was used as described (Table S1). To assay the copurification of MBP-fused C-terminal–truncated TnsA + TnsB or MBP–TnsA + N- or C-terminal–deleted TnsB, overlap PCR was performed as described above.
TnsA + TnsB Copurification Assays.
Cultures of ER2566 pMAL-TnsA + TnsB (100 mL) were grown at 37 °C to OD 600nm 0.5–0.7 and were induced with 0.3 mM IPTG for 2 h at 37 °C. All subsequent steps were performed at 4 °C. Cells were harvested by centrifugation, were resuspended in 10 mL column buffer [20 mM Tris (pH 8.0), 0.5 M NaCl, 1 mM EDTA, 1 mM DTT, and 0.1 mM PMSF], and were lysed by sonication. The soluble material was filtered through a 0.45-μm filter (Millipore) before loading onto an amylose resin column (New England Bio-Labs) that was equilibrated with column buffer and then was shaken gently for 30 min. The column then was washed extensively with column buffer by gravity flow, and bound protein was eluted with column buffer containing 20 mM maltose.
Western Blotting Analysis.
Proteins displayed on an SDS/PAGE gel were electrotransferred onto PVDF membrane (Millipore) by TurboBlotter (Bio-Rad) at 18 V for 50 min. Western blot analysis used TnsA or TnsB antibodies purified from rabbit polyclonal antisera to TnsA or TnsB (37) and ECL anti-rabbit IgG HRP-linked whole antibody (GE Healthcare). The membrane was soaked with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) for 1–2 min and exposed to Kodak film.
Gel Mobility Shift Assay and Supershift Assay with Anti-TnsA Antibody.
One of the DNA substrates, Tn7L, a 161-bp fragment containing the three TnsB-binding sites of the Tn7 left end, and the other DNA substrate, Tn7L-α, containing the terminal Tn7L TnsB-binding α site, ccctgcacttatctctgttaTGTGGGCGGACAAAATAGTTGGGAACTGGGtcggacagctttaataaa (uppercase indicates Tn7), were PCR amplified and purified following display on a 1.5% agarose gel in 1× Tris-acetate-EDTA (TAE) buffer. The probe was labeled at its 5′ ends with 32P-γ-ATP (Perkin-Elmer) and T4 polynucleotide kinase (New England Bio-Labs) and purified with a Quick Spin Column G-50 (Roche). The 20-μL binding reactions contained binding buffer [20 mM Hepes (pH 8.0), 2.5 mM Tris (pH 8.0), 10 mM NaCl, 0.0625 mM EDTA, 5 mM DTT, 0.005% BSA, 1 μg poly(dI-dC), and 5% (vol/vol) glycerol] and 25 ng of either TnsA or TnsB. Reaction mixtures were assembled and incubated for 30 min at 30 °C. In the TnsA Ab supershift assay, reactions also contained anti-TnsA polyclonal antibody (37), which was added after preincubation of the protein–DNA mixture. The reactions were electrophoresed through 6% (gm/100 mL) or 8% (gm/100 mL) polyacrylamide gels (37.5:1 acrylamide:N,N′-methylene-biacrylamide) in 1× TBE buffer at 4 V/cm for 15 h at 4 °C or 10 V/cm for 3 h at 25 °C. Gels were vacuum-dried and viewed with a Molecular Dynamics PhophorImager and Typhoon (GE Healthcare Life Science).
PEC Assays.
The 20-μL PEC formation reactions were carried out as in vitro transposition assays with pEMΔ, Tns proteins, and metal ions as described above. The donor plasmids and Tns proteins as indicated were incubated with 15 mM CaAc at 30 °C for 10 min, followed by incubation with glutaraldehyde (Sigma Aldrich) to a final concentration of 0.01% (vol/vol). The cross-linker was quenched by the addition of Tris (pH 8.0) to 25 mM and lysine to 5 mM for 10 min at room temperature. Eight units of PflMI (New England Bio-Labs) and 3 μL New England Bio-Labs #3 buffer were added and digested for 1 h at 30 °C. The reactions were analyzed by 0.9% agarose gel electrophoresis in 1× TAE buffer at 50 V for 100 min at 25 °C.
Supplementary Material
Acknowledgments
We thank members of the N.L.C. laboratory, particularly Jason Holder, for helpful discussions related to this work and Patti Kodeck for her assistance with the manuscript. N.L.C. is an Investigator of the Howard Hughes Medical Institute. This work was supported by National Institutes of Health Grant R01GM076425.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305716110/-/DCSupplemental.
References
- 1.Craig NL, Craigie R, Gellert M, Lambowitz A. Mobile DNA II. Washington, DC: ASM; 2002. [Google Scholar]
- 2.Peters JE, Craig NL. Tn7: Smarter than we thought. Nat Rev Mol Cell Biol. 2001;2(11):806–814. doi: 10.1038/35099006. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Craig NL, Peters JE. 2013. in Bacterial Integrative Mobile Genetic Elements, eds Roberts A, Mullany P (Landes Bioscience, London), pp. 1–32.
- 4.May EW, Craig NL. Switching from cut-and-paste to replicative Tn7 transposition. Science. 1996;272(5260):401–404. doi: 10.1126/science.272.5260.401. [DOI] [PubMed] [Google Scholar]
- 5.Sarnovsky RJ, May EW, Craig NL. The Tn7 transposase is a heteromeric complex in which DNA breakage and joining activities are distributed between different gene products. EMBO J. 1996;15(22):6348–6361. [PMC free article] [PubMed] [Google Scholar]
- 6.Parks AR, Peters JE. Transposon Tn7 is widespread in diverse bacteria and forms genomic islands. J Bacteriol. 2007;189(5):2170–2173. doi: 10.1128/JB.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rådström P, et al. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J Bacteriol. 1994;176(11):3257–3268. doi: 10.1128/jb.176.11.3257-3268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arciszewska LK, Craig NL. Interaction of the Tn7-encoded transposition protein TnsB with the ends of the transposon. Nucleic Acids Res. 1991;19(18):5021–5029. doi: 10.1093/nar/19.18.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arciszewska LK, McKown RL, Craig NL. Purification of TnsB, a transposition protein that binds to the ends of Tn7. J Biol Chem. 1991;266(32):21736–21744. [PubMed] [Google Scholar]
- 10.Bainton RJ, Kubo KM, Feng J-N, Craig NL. Tn7 transposition: Target DNA recognition is mediated by multiple Tn7-encoded proteins in a purified in vitro system. Cell. 1993;72(6):931–943. doi: 10.1016/0092-8674(93)90581-a. [DOI] [PubMed] [Google Scholar]
- 11.Bainton RJ, Gamas P, Craig NL. Tn7 transposition in vitro proceeds through an excised transposon intermediate generated by staggered breaks in DNA. Cell. 1991;65(5):805–816. doi: 10.1016/0092-8674(91)90388-f. [DOI] [PubMed] [Google Scholar]
- 12.Biery MC, Lopata M, Craig NL. A minimal system for Tn7 transposition: The transposon-encoded proteins TnsA and TnsB can execute DNA breakage and joining reactions that generate circularized Tn7 species. J Mol Biol. 2000;297(1):25–37. doi: 10.1006/jmbi.2000.3558. [DOI] [PubMed] [Google Scholar]
- 13.Peters JE, Craig NL. Tn7 recognizes transposition target structures associated with DNA replication using the DNA-binding protein TnsE. Genes Dev. 2001;15(6):737–747. doi: 10.1101/gad.870201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuduvalli PN, Rao JE, Craig NL. Target DNA structure plays a critical role in Tn7 transposition. EMBO J. 2001;20(4):924–932. doi: 10.1093/emboj/20.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra R, McKenzie GJ, Yi L, Lee CA, Craig NL. Characterization of the TnsD-attTn7 complex that promotes site-specific insertion of Tn7. Mob DNA. 2010;1(1):18. doi: 10.1186/1759-8753-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters JE, Craig NL. Tn7 transposes proximal to DNA double-strand breaks and into regions where chromosomal DNA replication terminates. Mol Cell. 2000;6(3):573–582. doi: 10.1016/s1097-2765(00)00056-3. [DOI] [PubMed] [Google Scholar]
- 17.Stellwagen AE, Craig NL. Mobile DNA elements: Controlling transposition with ATP-dependent molecular switches. Trends Biochem Sci. 1998;23(12):486–490. doi: 10.1016/s0968-0004(98)01325-5. [DOI] [PubMed] [Google Scholar]
- 18.Gamas P, Craig NL. Purification and characterization of TnsC, a Tn7 transposition protein that binds ATP and DNA. Nucleic Acids Res. 1992;20(10):2525–2532. doi: 10.1093/nar/20.10.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stellwagen AE, Craig NL. Avoiding self: Two Tn7-encoded proteins mediate target immunity in Tn7 transposition. EMBO J. 1997;16(22):6823–6834. doi: 10.1093/emboj/16.22.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stellwagen AE, Craig NL. Gain-of-function mutations in TnsC, an ATP-dependent transposition protein that activates the bacterial transposon Tn7. Genetics. 1997;145(3):573–585. doi: 10.1093/genetics/145.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronning DR, et al. The carboxy-terminal portion of TnsC activates the Tn7 transposase through a specific interaction with TnsA. EMBO J. 2004;23(15):2972–2981. doi: 10.1038/sj.emboj.7600311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu F, Craig NL. Isolation and characterization of Tn7 transposase gain-of-function mutants: A model for transposase activation. EMBO J. 2000;19(13):3446–3457. doi: 10.1093/emboj/19.13.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skelding Z, Queen-Baker J, Craig NL. Alternative interactions between the Tn7 transposase and the Tn7 target DNA binding protein regulate target immunity and transposition. EMBO J. 2003;22(21):5904–5917. doi: 10.1093/emboj/cdg551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skelding Z, Sarnovsky R, Craig NL. Formation of a nucleoprotein complex containing Tn7 and its target DNA regulates transposition initiation. EMBO J. 2002;21(13):3494–3504. doi: 10.1093/emboj/cdf347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holder JW, Craig NL. Architecture of the Tn7 posttransposition complex: An elaborate nucleoprotein structure. J Mol Biol. 2010;401(2):167–181. doi: 10.1016/j.jmb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickman AB, et al. Unexpected structural diversity in DNA recombination: The restriction endonuclease connection. Mol Cell. 2000;5(6):1025–1034. doi: 10.1016/s1097-2765(00)80267-1. [DOI] [PubMed] [Google Scholar]
- 27.DeBoy RT, Craig NL. Target site selection by Tn7: attTn7 transcription and target activity. J Bacteriol. 2000;182(11):3310–3313. doi: 10.1128/jb.182.11.3310-3313.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arciszewska LK, Drake D, Craig NL. Transposon Tn7. cis-Acting sequences in transposition and transposition immunity. J Mol Biol. 1989;207(1):35–52. doi: 10.1016/0022-2836(89)90439-7. [DOI] [PubMed] [Google Scholar]
- 29.Stellwagen AE, Craig NL. Analysis of gain-of-function mutants of an ATP-dependent regulator of Tn7 transposition. J Mol Biol. 2001;305(3):633–642. doi: 10.1006/jmbi.2000.4317. [DOI] [PubMed] [Google Scholar]
- 30.Holder JW. Assembly and Architecture of Tn7 Transpososomes. 2006. PhD dissertation. (The Johns Hopkins University, Baltimore), p. 216.
- 31.Savilahti H, Rice PA, Mizuuchi K. The phage Mu transpososome core: DNA requirements for assembly and function. EMBO J. 1995;14(19):4893–4903. doi: 10.1002/j.1460-2075.1995.tb00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker TA, Mizuuchi K. the DNA-promoted assembly of the active tetramer of the Mu transposase. Genes Dev. 1992;6(11):2221–2232. doi: 10.1101/gad.6.11.2221. [DOI] [PubMed] [Google Scholar]
- 33.Montaño SP, Pigli YZ, Rice PA. The Mu transpososome structure sheds light on DDE recombinase evolution. Nature. 2012;491(7424):413–417. doi: 10.1038/nature11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waddell CS, Craig NL. Tn7 transposition: Two transposition pathways directed by five Tn7-encoded genes. Genes Dev. 1988;2(2):137–149. doi: 10.1101/gad.2.2.137. [DOI] [PubMed] [Google Scholar]
- 35.McKown RL, Orle KA, Chen T, Craig NL. Sequence requirements of Escherichia coli attTn7, a specific site of transposon Tn7 insertion. J Bacteriol. 1988;170(1):352–358. doi: 10.1128/jb.170.1.352-358.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKown RL, Waddell CS, Arciszewska LK, Craig NL. Identification of a transposon Tn7-dependent DNA-binding activity that recognizes the ends of Tn7. Proc Natl Acad Sci USA. 1987;84(22):7807–7811. doi: 10.1073/pnas.84.22.7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orle KA, Craig NL. Identification of transposition proteins encoded by the bacterial transposon Tn7. Gene. 1991;104(1):125–131. doi: 10.1016/0378-1119(91)90478-t. [DOI] [PubMed] [Google Scholar]
- 38.Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302(1):205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.