Significance
The integrity of DNA must be preserved to pass genetic information onto the next generation and to prevent genomic instability. One of the key enzymes involved in DNA metabolism is the nuclease-helicase Dna2, which is required for both DNA replication and the repair of broken DNA. Our work revealed that Dna2 from Saccharomyces cerevisiae possesses a potent but cryptic helicase capacity, which is controlled by the nuclease activity of the same polypeptide. Regulating the interplay between both enzymatic activities might explain how Dna2 can take on its distinct cellular roles.
Keywords: DNA nuclease, replication protein-A, Sgs1
Abstract
Dna2 is a nuclease-helicase involved in several key pathways of eukaryotic DNA metabolism. The potent nuclease activity of Saccharomyces cerevisiae Dna2 was reported to be required for all its in vivo functions tested to date. In contrast, its helicase activity was shown to be weak, and its inactivation affected only a subset of Dna2 functions. We describe here a complex interplay of the two enzymatic activities. We show that the nuclease of Dna2 inhibits its helicase by cleaving 5′ flaps that are required by the helicase domain for loading onto its substrate. Mutational inactivation of Dna2 nuclease unleashes unexpectedly vigorous DNA unwinding activity, comparable with that of the most potent eukaryotic helicases. Thus, the ssDNA-specific nuclease activity of Dna2 limits and controls the enzyme's capacity to unwind dsDNA. We postulate that regulation of this interplay could modulate the biochemical properties of Dna2 and thus license it to carry out its distinct cellular functions.
The Dna2 enzyme functions at the crossroads of key DNA metabolic processes. It was initially identified in screens for Saccharomyces cerevisiae mutants deficient in DNA replication (1, 2), and its importance was underscored by the finding that dna2Δ mutants are not viable (3). When the DNA2 gene was cloned, it was shown to be conserved in evolution from yeast to humans and found to contain conserved nuclease and helicase motifs (4). Further work identified a number of genetic and physical interactions of Dna2 with factors required for the synthesis and maturation of the lagging strand during DNA replication, including Rad27 [homolog of human Flap endonuclease 1, FEN1 (5)]. Overexpression of Rad27 rescued growth defects of some dna2 point mutants, and, conversely, overexpression of Dna2 suppressed rad27Δ defects (5). Subsequent work established that Dna2 functions together with Rad27 in the removal of single-stranded (ss) flaps generated at the 5′ termini of Okazaki fragments by polymerase δ-catalyzed strand displacement. Although Rad27 seems to have a more general role in flap processing, Dna2 is required to cleave only a subset of longer flaps bound by Replication Protein A (RPA), which stimulates its nuclease activity while inhibiting cleavage by Rad27. In this process, Dna2 and Rad27 were proposed to function in a sequential manner, with Dna2 loading first onto the flap termini and shortening them with its 5′–3′ nuclease. Rad27 was then proposed to further cleave the shortened flaps at the ss/dsDNA junctions, creating thus ligatable substrates for Cdc9 (DNA ligase 1), which completes Okazaki fragment maturation (6–8). The role of Dna2 in Okazaki fragment processing is now generally accepted although it still remains somewhat puzzling why dna2Δ cells are inviable whereas rad27Δ mutants are not.
More recently, it has been shown that Dna2 has an independent and conserved function in dsDNA break repair (9). Specifically, Dna2 belongs to one of the pathways that resect 5′ ends of dsDNA breaks to initiate homologous recombination. This process leads to the formation of long 3′ overhangs, which become coated by the strand exchange protein Rad51, and which also prime DNA synthesis during the downstream steps in homologous recombination. Genetic and later biochemical work established that Dna2 functions together with the vigorous Sgs1 helicase (Bloom in human cells) downstream of the Mre11-Rad50-Xrs2 (MRX) complex (9–14). MRX first recognizes dsDNA breaks and is likely involved in their initial processing, and subsequently helps recruit Sgs1 and Dna2. Sgs1 helicase then unwinds the DNA from the break and the ssDNA is coated by RPA, which stimulates the 5′–3′ nuclease activity of Dna2. The specific degradation of the 5′ end of the unwound DNA ensures the correct polarity of resection, which is required for homologous recombination (12, 13). However, the roles of Dna2 are not limited to Okazaki fragment and dsDNA break processing. Dna2 was also shown to function in telomere maintenance (15), aging (16), long-patch base excision repair (17), and prevention of reversal of stalled replication forks (18). The expression of human DNA2 was increased in human cancers and negatively correlated with disease outcome, indicating that DNA2 function is relevant for human health (19). However, the role of Dna2/DNA2 in these latter processes remains poorly defined.
The potent nuclease activity of Dna2 seems to be critical for all of its functions, including replication and recombination. Point mutants lacking the nuclease activity are inviable as a dna2Δ strain (20). The nuclease activity of Dna2 is ssDNA-specific, and shows both 5′–3′ and 3′–5′ polarities. Because RPA stimulates the 5′–3′ nuclease and inhibits the 3′–5′ activity, it is likely that only the 5′–3′ directionality is important in vivo (12, 13). It has been shown that the Dna2 nuclease can load only on a free ssDNA tail, and that it subsequently cleaves DNA endonucleolytically into short oligonucleotides (21, 22).
Much less is known about the function of the helicase activity of Dna2. To date, very weak 5′–3′ unwinding capacity has been demonstrated for the yeast enzyme (4, 7, 23). Interestingly, similarly to the Dna2 nuclease, also the helicase domain requires a free DNA end (22). In contrast, no unwinding activity could be detected in the Xenopus laevis Dna2. Whether human DNA2 possesses helicase activity remains controversial (24–27). Yeast point mutants lacking helicase activity show impaired growth but are viable under most conditions (23). Overexpression of Rad27 partially rescues dna2 helicase-deficient mutants, suggesting that the helicase activity might play a supportive but nonessential role in DNA replication (28). The helicase-deficient mutants show a dramatic sensitivity to the DNA alkylating drug methylmethanesulfonate (MMS), pointing to an as-yet uncharacterized role of Dna2 helicase in the repair of DNA damage (29). In contrast, Dna2 helicase activity had no detectable effect on DNA end resection (9). Due to the limited unwinding capacity of Dna2 observed in vitro, the motor was proposed to function as an ssDNA translocase to aid positioning of the nuclease domain on ssDNA and to remove secondary structures from ssDNA, rather than as a DNA helicase to unwind dsDNA (23).
In this work, we expressed S. cerevisiae Dna2 and its variants and optimized the purification of these polypeptides. We now show that Dna2 is a potent but cryptic DNA helicase. It functionally interacts with RPA, which enables it to unwind tens of kilobases of dsDNA. Surprisingly, the nuclease of wild-type Dna2 interferes with this remarkable helicase capacity by cleaving ssDNA tails that the helicase requires for loading onto DNA to initiate unwinding. The interplay between the two main biochemical activities of Dna2 might fine-tune its behavior to suit its distinct cellular roles.
Results
Expression and Purification of Wild-Type Dna2 and Nuclease- and Helicase-Dead Variants.
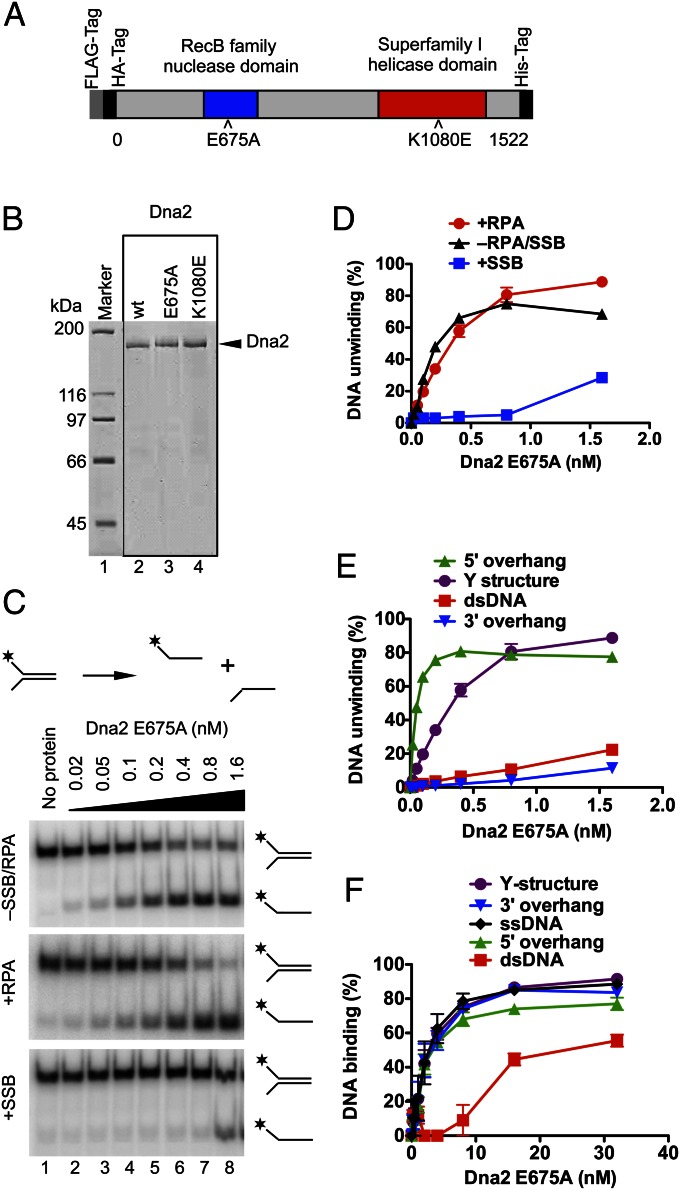
S. cerevisiae Dna2 is a large protein (172 kDa) consisting of 1,522 amino acids (Fig. 1A). We noticed previously that recombinant Dna2 was rather unstable, rapidly losing activity during extended dialysis procedures used in earlier preparation protocols (12). Furthermore, it was sensitive to the omission of reducing agents, possibly due to the presence of an oxidation-prone iron–sulfur cluster (30, 31). Modification of the purification procedure allowed us to obtain Dna2 with a high specific nuclease activity (12). In this work, we further optimized and shortened the purification process (Materials and Methods) and obtained nearly homogenous wild-type Dna2 and nuclease-dead Dna2 E675A, as well as helicase-dead Dna2 K1080E variants (Fig. 1 A and B and Fig. S1 A and B).
Fig. 1.
The nuclease-inactive Dna2 E675A variant possesses a vigorous DNA helicase activity. (A) A schematic representation of the recombinant Dna2 protein used in this study. The polypeptide contains an N-terminal FLAG and HA tags, and a C-terminal 6xHis tag. Positions of mutations inactivating the nuclease activity (E675A) or helicase activity (K1080E) are indicated. (B) Purified Dna2 wild-type (wt), E675A, and K1080E variant proteins (550 ng each) used in this study were stained with Coomassie blue. (C) Representative polyacrylamide gels (10%) showing the DNA helicase activity of Dna2 E675A on a 32P-labeled Y-structure DNA substrate (1 nM). The length of both ssDNA arms was 19 nt. As indicated, the reactions were supplemented with either S. cerevisiae RPA or E. coli SSB (both 22 nM). *, position of the 32P label. (D) Quantitation of the helicase assays such as shown in C. Error bars, SE, n = 3. (E) Quantitation of the helicase assays such as shown in Fig. S2. Error bars, SE, n = 3. (F) Quantitation of electrophoretic mobility shift assays showing the binding of Dna2 E675A to various 32P-labeled DNA substrates (1 nM). Error bars, SE, n = 3.
Dna2 Possesses a Vigorous DNA Helicase Activity.
The ssDNA-specific nuclease activity of Dna2 was proposed to obscure the detection of its limited unwinding property (22). Therefore, the nuclease-dead Dna2 variants were used to characterize its helicase activity. One of these mutants is Dna2 E675A, which had been designed based on the homology between Escherichia coli RecB and S. cerevisiae Dna2 nuclease sites (20). Indeed, mutation of the conserved glutamate at position 675 to alanine largely inactivated the nuclease activity of Dna2, and Dna2 E675A has been shown to exhibit a weak helicase activity (22, 23). Here, having used the optimized preparation procedure, we set out to examine the helicase activity of this variant. Dna2 E675A could readily unwind a synthetic Y-structure oligonucleotide-based substrate containing 31 base pairs of dsDNA (Fig. 1C). To our great surprise, the helicase was active at subnanomolar concentrations (Fig. 1C). Previously, ∼20 nM enzyme was needed to unwind a similar length of dsDNA (22, 23), indicating that our preparation possesses >20-fold higher specific DNA unwinding activity. In agreement with previous data (23), supplementing the reaction with S. cerevisiae replication protein A (RPA) did not further stimulate its unwinding capacity whereas E. coli Single Strand DNA Binding protein (SSB) strongly inhibited DNA unwinding (Fig. 1 C and D). We show that a 5′-tailed DNA is a preferred structure for Dna2 E675A unwinding, with ∼0.05 nM Dna2 E675A required to unwind 50% of the DNA substrate, which was used at 1 nM concentration (Fig. 1E and Fig. S2). The unwinding of the 5′-tailed DNA substrate was therefore clearly catalytic, with one enzyme molecule being capable of unwinding at least ∼10 DNA substrate molecules during the time course of the reaction. The vigorous activity stands in contrast with previous studies where ∼15- to 100-fold excess enzyme over DNA substrate was required to detect DNA unwinding (22, 23). We further show that ∼0.3 nM Dna2 E675A was required to unwind 50% of the Y-structure DNA substrate; thus, the presence of an additional 3′ ssDNA arm (Y-structure vs. 5′ overhang) inhibited DNA unwinding ∼sixfold (Fig. 1E and Fig. S2). As anticipated for a 5′–3′ DNA helicase, the unwinding of 3′-tailed or fully dsDNA substrates was inefficient (Fig. 1E and Fig. S2). The difference between the specific DNA unwinding activity presented here and in earlier reports (22, 23) is likely due to the optimized enzyme preparation procedure used in this work (Materials and Methods and Discussion).
Next, we used electrophoretic mobility shift assays to assess the binding of Dna2 E675A to DNA. We saw that Dna2 E675A binds rather indiscriminately (Kd ∼5 nM) to structures that contain ssDNA. A 5′ ssDNA overhang was bound equally well as a 3′ ssDNA overhang, despite the fact that the former structure is an excellent substrate for the Dna2 E675A helicase whereas the latter is not (Fig. 1F and Fig. S3A). Similar results were obtained in the presence of competitor DNA in the electrophoretic mobility shift assays (Fig. S3B). These results show that Dna2 E675A is a vigorous DNA helicase with high affinity for DNA.
RPA Stimulates Dna2 E675A to Unwind Long Stretches of DNA.
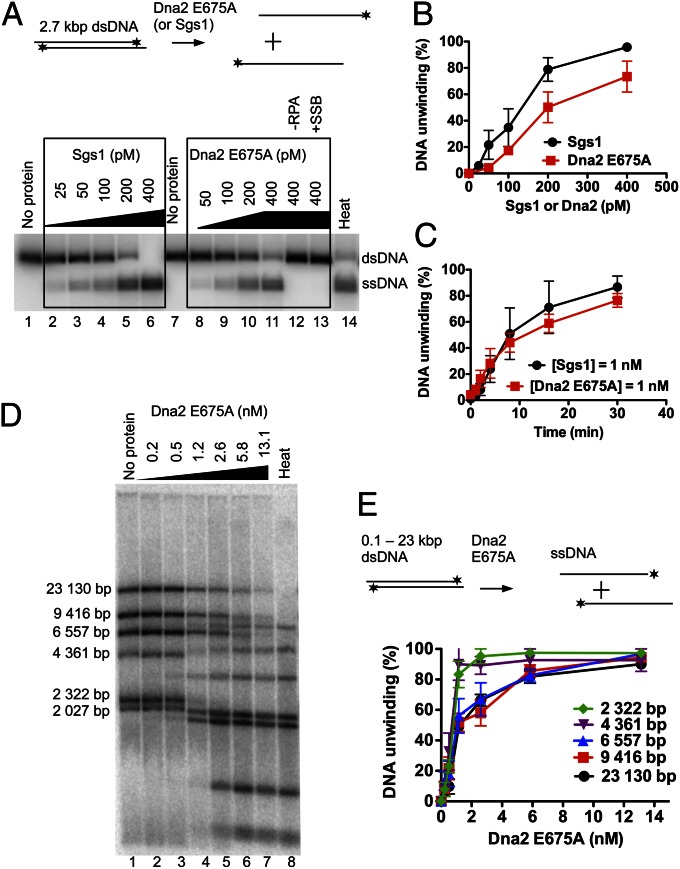
Dna2 nuclease-deficient variants were previously shown to be only capable of unwinding short, oligonucleotide-based DNA structures. The fraction of unwound substrate decreased dramatically with the length of the duplex DNA; the unwinding of a 91-bp duplex was ∼15-fold less efficient than the unwinding of a 30-bp duplex (23). Is has been proposed that Dna2 is a weak and nonprocessive DNA helicase (23). We have decided to analyze unwinding of long-length DNA with our preparation of Dna2 E675A. Surprisingly, the enzyme could readily unwind plasmid-based 2.7-kbp dsDNA containing a 3-nt-long 5′ ssDNA tail (Fig. 2A). Half of the DNA substrate was unwound by only 200 pM Dna2 E675A, a concentration similar to that required for the unwinding of oligonucleotide-based DNA substrates (Fig. 1 C and E). However, unlike in the case of the oligonucleotide-based substrates, the unwinding of the 2.7-kbp dsDNA was absolutely dependent on RPA, as no DNA unwinding was observed when RPA was omitted (Fig. 2A, lane 12). E. coli SSB could not replace RPA (Fig. 2A, lane 13), indicating that species-specific interaction between yeast Dna2 and yeast RPA plays an essential role in promoting long-length DNA unwinding by the Dna2 helicase.
Fig. 2.
Dna2 E675A can unwind long lengths of dsDNA in the presence of RPA. (A) Representative agarose gels (0.8%) showing the helicase activity of Dna2 E675A or Sgs1 on a 32P-labeled 2.7-kbp-long dsDNA substrate with a 3-nt 5′ ssDNA overhang (1 nM). The reactions were supplemented with S. cerevisiae RPA (0.4 μM) except lane 12 (RPA omitted) and lane 13 (RPA replaced with E. coli SSB). Heat, heat-denatured DNA substrate; *, position of the 32P label. (B) Quantitation of the helicase assays as in A. Error bars, SE, n = 3. (C) Kinetics of DNA unwinding of 5′-tailed 2.7-kbp-long dsDNA (1 nM) by either Dna2 E675A (1 nM) or Sgs1 (1 nM) in the presence of RPA (0.4 μM). Error bars, SE, n = 3. (D) λ phage DNA was digested with HindIII to produce dsDNA fragments ranging from 125 bp to 23 kbp in length, and 32P-labeled with Klenow fragment of DNA polymerase I. The resulting DNA fragments had 5′ ssDNA tails of 3 nt in length at their ends. Various concentrations of Dna2 E675A were incubated with the restricted DNA (2.4 nM of DNA ends) in the presence of RPA (1.08 μM). Heat, heat-denatured substrate. The panel shows a representative agarose gel (1%). (E) Quantitation of the helicase assays such as shown in D. Error bars, SE, n = 3.
Sgs1 is the most vigorous DNA helicase characterized to date in eukaryotes (32). Given that it functions in DNA end resection together with Dna2 (9, 12, 13), we set out to compare the unwinding capacities of Sgs1 and Dna2 E675A. Sgs1 helicase can initiate DNA unwinding of blunt-ended, 3′− or 5′-tailed DNA with similar efficiency (32). To compare the relative activities of both enzymes, we selected 5′-tailed DNA, which is required for unwinding by Dna2 E675A. Remarkably, we show that Sgs1 was only slightly (∼30%) better in unwinding of the 2.7-kbp-long dsDNA than Dna2 E675A (Fig. 2 A and B), and similar results were obtained in kinetic experiments (Fig. 2C and Fig. S4). We thus conclude that Dna2 E675A is almost as vigorous a DNA helicase as Sgs1.
To further characterize the unwinding capacity of Dna2 E675A, we tested its ability to unwind long stretches of dsDNA. We have digested bacteriophage λ DNA with HindIII and 32P-labeled the restriction fragments. This procedure created linear DNA molecules of up to 23 kbp in length with 3-nt-long 5′ overhangs. Dna2 E675A could efficiently unwind DNA fragments of all lengths and produced a pattern of ssDNA molecules that comigrated with the heat-denatured substrate (Fig. 2 D and E). The reaction was remarkably efficient; 1.2 nM Dna2 E675A unwound ∼50% of the DNA substrate, which was present at a concentration corresponding to 2.4 nM DNA ends. Furthermore, the 23-kbp fragment was unwound only ∼twofold less efficiently than the 2.0-kbp fragment, showing that the length of duplex DNA only marginally affects the unwinding efficiency in the presence of S. cerevisiae RPA. Unlike E. coli SSB, human RPA could substitute cognate S. cerevisiae RPA, and, as expected, the reaction was completely dependent on ATP (Fig. S5). We conclude that S. cerevisiae Dna2 E675A is a very vigorous DNA helicase, capable of unwinding tens of kilobases of dsDNA in length in a reaction dependent on RPA and ATP.
Single Molecule Experiments Reveal High DNA Unwinding Processivity of Dna2 E675A.
To gain insight into the processivity and the rate of DNA unwinding by Dna2 E675A, we carried out single-molecule magnetic tweezers experiments (Fig. 3 A and B). As substrate we used a 6.6-kbp-long dsDNA fragment that contained 0.6-kbp dsDNA tails with multiple digoxigenin or biotin modifications at either end to allow its binding to the surface of an anti-digoxigenin–coated fluidic cell and to streptavidin-coated magnetic beads. The construct contained a 40-nt 5′-terminated ssDNA flap to allow the loading of Dna2. A pair of magnets above the fluidic cell was used to stretch single bead-tethered DNA constructs, and video microscopy was used to read out the DNA end-to-end distance in real time (33).
Fig. 3.
Single molecule experiments reveal highly processive DNA unwinding by Dna2 E675A. (A) Sketch of the DNA construct and (B) the magnetic tweezers assay. (C) Representative DNA unwinding events by Dna2 E675A (F = 25 ± 2 pN, SD). DNA lengthening was observed only after addition of the enzyme, RPA, and ATP in the reaction buffer (gray box). (D) Histogram of rates for unwinding 500 bp of DNA (F = 25 ± 2 pN, SD). Unwinding trajectories (n = 14) were split into successive 500-bp segments, and the mean unwinding rate was determined from the slope of a linear fit to each segment. The mean unwinding rate was 38 ± 4 bp⋅s−1 (SE). (E) Removal of free Dna2 E675A from solution during a DNA unwinding reaction. The reaction was initiated by adding the enzyme in the presence of RPA and ATP (left gray box). After several hundred unwound bps, ∼50 µl of ATP and RPA in reaction buffer were flushed through the fluidic cell (cell volume 30 µl) (blue boxes). The procedure was repeated four times during one unwinding event.
When adding Dna2 E675A in the presence of RPA and ATP, rapid DNA unwinding was observed that resulted in an apparent increase in the DNA length (Fig. 3 B and C). This increase is due to the larger extension of RPA-coated ssDNA in comparison with dsDNA (34, 35). No significant DNA lengthening (>100 bp) was observed in the absence of either RPA, ATP, or the 5′-terminated flap (but in presence of Dna2), in agreement with our previous data showing that RPA, ATP, and a 5′ ssDNA tail were required for the unwinding of long lengths of DNA by the Dna2 helicase. As expected, no DNA lengthening was found when RPA was added in the absence of the helicase at the applied forces. The observed DNA lengthening is thus due to DNA unwinding by Dna2 E675A.
Unwinding rates were found to be highly variable (Fig. 3 C and D) as found also for other DNA translocases (36). Although some events displayed a rather constant unwinding rate of up to 120 bp⋅s−1 over distances of several kbp, also much slower events (down to 15 bp⋅s−1) were observed with similar frequency. The average unwinding rate over 500 bp of DNA was found to be 38 ± 4 bp⋅s−1 (SE) at a force of 25 pN (Fig. 3D). Slow events often displayed multiple unwinding rate changes or stalls, with periods of fast DNA unwinding in between. However, rate changes and stalls were characteristic of fast unwinding events as well (Fig. S6A). Because we measured DNA unwinding under an external tension, we also investigated how the unwinding process is influenced by different forces. Because the unwinding rates were variable from molecule to molecule, we applied different forces during a single unwinding run of a molecule (Fig. S6B). The unwinding rate was found to decrease ∼fivefold when lowering the force from 30 to 10 pN. Nonetheless DNA unwinding of several kbp was found also at the lower forces.
An average unwinding distance of 4.2 ± 0.5 kbp (SE, n = 25) was obtained (Fig. 3C). Activity was terminated after unwinding several kbp or the complete 6.1-kbp DNA substrate (F = 25 pN). Partially also the loss of the magnetic bead was observed. DNA unwinding activity commenced after highly variable lag times ranging from 0 to 800 s with a mean of 85 ± 31 s (SE, n = 25) (Fig. 3C). Variable lag times and the abrupt termination of helicase reactions before unwinding of the full-length substrate, suggested that, in most cases, the reaction was catalyzed by a single Dna2 E675A complex. Thus, Dna2 E675A could be a processive DNA helicase. To investigate processivity in further detail, we repeatedly flushed out Dna2 E675A (by adding buffer containing ATP and RPA only) just after an unwinding event had been initiated. Each flush corresponded to ∼1.7 flow cell volumes. DNA unwinding was not affected by removing free Dna2 E675A from the solution either during or after the flush (Fig. 3E). Adding Dna2 E675A back in after the unwinding stopped did not initiate further unwinding. Our results strongly suggest that a single enzyme complex drives the observed long-range DNA unwinding. In summary, these measurements show that Dna2 E675A is a highly processive helicase capable of unwinding kbp-sized DNA fragments in a single run.
Dna2 E675A Possesses Vigorous DNA-Dependent Atpase Activity That Is Stimulated by RPA.
The unexpected DNA helicase activity of Dna2 E675A prompted us to characterize its capacity to hydrolyze ATP. To this point, we used a spectrophotometric assay, which measures ATPase activity based on a reaction coupled to the oxidation of NADH (37, 38). To obtain kinetic parameters for ATP hydrolysis, we first varied ATP concentration in the presence of Y-structure DNA as a cofactor (Fig. S7). The curve showing the dependence of the ATPase activity on ATP concentration was hyperbolic, and a fit to the Michaelis–Menten equation yielded Vmax = 21 ± 2 μM·min−1 and Km = 130 ± 24 μM. The ATPase was stimulated by RPA, with Vmax = 24 ± 2 μM·min−1 and Km = 56 ± 10 μM (Fig. S7). Next, we used poly(dT) as cofactor, which is ssDNA devoid of secondary structure, and varied its concentration (Fig. 4A). The fit to the data yielded Vmax = 9.6 ± 1 μM·min−1 and Km = 164 ± 44 nM. Supplementing the reactions with RPA promoted the rate of ATP hydrolysis and resulted in Vmax = 22 ± 2 μM·min−1 and Km = 181 ± 33 nM.
Fig. 4.
Dna2 E675A shows DNA-dependent ATPase activity that is stimulated by RPA. (A) Rate of ATP hydrolysis and its dependence on the DNA concentration. The reactions contained Dna2 E675A (4 nM), ATP (1 mM), RPA where indicated (150 nM), and the indicated concentrations of poly(dT). (B) Apparent ATP turnover number and its dependence on various DNA structures (1 μM nucleotides). The reactions contained Dna2 E675A (3 nM) and ATP (1 mM). Error bars, SE, n = 2.
Next, we compared the ATPase activity in the presence of various DNA cofactors. The greatest ATPase activity was observed in the presence of 5′-tailed DNA (Fig. 4B). The apparent kcat, which is the apparent ATP turnover number, was ∼85 s−1, which is comparable with the kcat of Sgs1 ∼82 s−1 on the same DNA structure (32). Nearly as effective was the Y-structure dsDNA, with kcat ∼79 s−1. Other DNA substrates were substantially less effective; ssDNA stimulated about 2.6-fold less than 5′-tailed DNA, and 3′-tailed DNA and dsDNA stimulated 3.5- or 6.1-fold less efficiently, respectively (Fig. 4B). The capacity of the DNA structures to promote ATP hydrolysis by Dna2 E675A corresponded to the unwinding preference. The 5′-tailed and Y-structure DNA substrates were the preferred substrates for DNA unwinding by Dna2 E675A (Fig. 1E), and they were also the most efficient in stimulating its ATPase activity (Fig. 4B). Almost no ATPase activity was observed in the absence of DNA, showing that the ATPase activity of Dna2 E675A is DNA-dependent (Fig. 4B). As expected, the ATPase-deficient Dna2 K1080E variant showed no ATPase activity (Fig. 4B). In summary, these parameters establish that Dna2 E675A is a strong ATPase that has a high affinity for both ATP and DNA.
Nuclease Activity Within Dna2 Limits Its Unwinding Capacity.
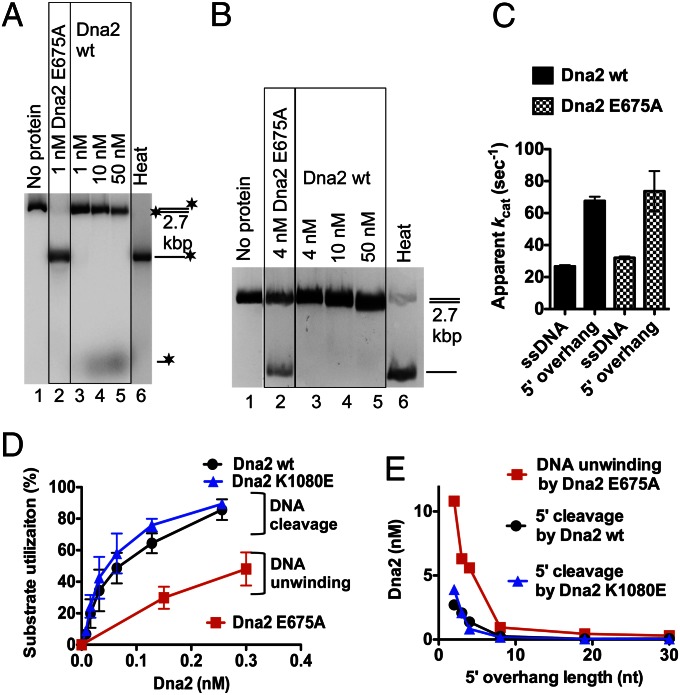
Having established that Dna2 E675A variant lacking the nuclease activity is a vigorous DNA helicase, we next wanted to study the interplay of the nuclease with the helicase within the wild-type protein. The nuclease of Dna2 is ssDNA-specific but may act on dsDNA upon spontaneous denaturation/melting of dsDNA ends (39). We show in Fig. 5A that, whereas 1 nM Dna2 E675A was sufficient to completely unwind a 2.7-kbp dsDNA substrate (lane 2), the same concentration of wild-type Dna2 had no detectable activity (lane 3). When we used 10- to 50-fold higher wild-type Dna2 concentration (10 and 50 nM), only limited DNA degradation was observed, as indicated by the release of the radioactive label (Fig. 5A, lanes 4 and 5), or mobility shift of unlabeled DNA stained with ethidium bromide (Fig. 5B). DNA degradation by wild-type Dna2 was largely restricted to the vicinity of the DNA ends (compare panels A and B in Fig. 5). Thus, wild-type Dna2 apparently lacked the vigorous helicase activity observed in the nuclease-dead E675A variant. This observation suggests either that the helicase/ATPase domain of the wild-type protein is compromised, or that the nuclease of Dna2 inhibits its own helicase activity. To differentiate between these two possibilities, we compared the initial rates of ATP hydrolysis between the wild-type and the nuclease-dead Dna2 E675A proteins. We show that both polypeptides display similar levels of ATPase activity (Fig. 5C), indicating that the ATPase/helicase domain in the wild-type protein is active and therefore likely properly folded. This result suggested that the nuclease of wild-type Dna2 limits the engagement of its helicase. We next set out to study this phenomenon in more detail.
Fig. 5.
The nuclease activity of Dna2 interferes with its helicase activity. (A) A representative experiment comparing the activities of wild-type Dna2 and nuclease-dead E675A variant on 5′-tailed 2.7-kbp dsDNA substrate 32P-labeled at the 3′ end (1 nM). Reactions were incubated in the presence of S. cerevisiae RPA (0.4 μM). *, position of the 32P label. Heat, heat-denatured substrate. (B) A representative experiment comparing the activities of wild-type Dna2 and nuclease-dead E675A variant on 5′-tailed 2.7-kbp dsDNA substrate (3.8 nM). Reactions were incubated for 30 min in the presence of S. cerevisiae RPA (1.68 μM). A 1% agarose gel was stained with ethidium bromide. Heat, heat-denatured substrate. (C) Comparison of apparent ATP turnover numbers of Dna2 wild-type and Dna2 E675A variant. The reactions contained the indicated DNA structures (1 μM nucleotides), Dna2 wild type or E675A (3 nM) and ATP (1 mM). Error bars, SE, n = 2. (D) Comparison of nuclease and/or helicase activities of Dna2 wild-type, K1080E, and E675A variants, respectively, based on substrate utilization. All assays contained RPA (22.5 nM). Error bars, SE, n = 2. (E) Dependence of Dna2 activities on the length of the 5′ ssDNA overhang. Dna2 concentrations required for 50% of maximal DNA cleavage (by Dna2 wild type or Dna2 K1080E) or 50% of maximal DNA unwinding (by Dna2 E675A) were plotted against the length of the 5′ ssDNA overhang. The data were obtained by analysis of the results such as from Fig. S8.
Because the nuclease activity of Dna2 preferentially degrades 5′ ssDNA overhangs, and these overhangs are required for DNA unwinding by the Dna2 E675A variant (Fig. 1E), we asked whether the nuclease of wild-type Dna2 might cleave the 5′ ssDNA tails before they can be accessed by the helicase. This activity could explain the apparent lack of DNA unwinding activity of wild-type Dna2. To this point, we set out to compare enzyme concentrations required for the unwinding and/or the nucleolytic degradation of oligonucleotide-based DNA substrates. Specifically, we compared the nuclease activity of Dna2 wild type and Dna2 K1080E with the helicase activity of Dna2 E675A. All these enzyme variants were prepared using an identical procedure, and we noted that independent preparations of the same enzyme variant led to consistent levels of specific activity (Materials and Methods). We observed that, first, the nuclease activities of both wild-type and helicase-dead K1080E enzymes were indistinguishable based on substrate utilization (Fig. 5D). The lack of the helicase activity therefore did not affect the initial 5′ end cleavage by Dna2. Second, the nuclease activity was ∼sixfold more frequent than the helicase activity based on substrate utilization: although 50 pM wild-type and helicase-dead K1080E Dna2 cleaved ∼50% of the 5′ tails within 30 min, as much as 300 pM of nuclease-dead Dna2 E675A was required to unwind 50% of the DNA substrate during the same time period (Fig. 5D). These results show that the wild-type protein is more likely to degrade a 5′ ssDNA tail, rather than to use it to initiate DNA unwinding.
We next wondered whether varying the length of the 5′ ssDNA tail might differentially affect the nuclease or the helicase activities of Dna2. To this point, we analyzed wild-type Dna2, E675A, and K1080E variants on duplex DNA substrates containing 5′ ssDNA tails of 0- to 30-nt in length. Both nuclease and helicase activities preferred longer ssDNA tails (Fig. S8 A–C). However, irrespectively of the length of the ssDNA tail, ∼four- to sixfold higher enzyme concentration was required for DNA unwinding by Dna2 E675A than for the nucleolytic cleavage by either wild type or Dna2 K1080E (Fig. 5E). The nuclease activity was thus clearly dominant in the wild-type protein. In summary, our data show that the nuclease of Dna2 limits its unwinding capacity.
Nuclease Activity of Wild-Type Dna2 Interferes with Its Stable Binding to DNA.
Nuclease-dead Dna2 E675A efficiently binds 5′-tailed DNA (Kd ∼5 nM) and binds dsDNA with ∼eightfold lower efficiency (Kd ∼40 nM, Fig. 6 A and C; see also Fig. 1F). In contrast, wild-type Dna2 rapidly cleaves the 5′ tail and binds the resulting structure with affinity comparable with dsDNA (Kd ∼40 nM, Fig. 6 B and C), showing that the nuclease activity interferes with DNA binding. We have also used ssDNA blocked with biotin-bound streptavidin on both ends as a substrate for electrophoretic mobility shift assays. Blocked ends are known to inhibit both the ssDNA-specific nuclease as well as the helicase activity of Dna2 (21, 22). Using this uncleavable DNA, we show that both wild-type Dna2 and the E675A variant bound this structure with similar affinity (Kd ∼8 nM), indicating that wild-type Dna2 is capable of strong DNA binding (Fig. 6 D–F). Finally, we observed that, whereas the rate of ATP hydrolysis by Dna2 E675A was relatively constant in the presence of 5′-tailed DNA as a cofactor, it rapidly decreased in case of wild-type Dna2 (Fig. 6G). In agreement with the data presented in Fig. 6 A–C, we believe that the nuclease activity of the wild-type protein rapidly degrades the ssDNA tail during the course of the reaction, producing duplex or nearly duplex DNA structures that no longer stimulate its ATPase activity (Fig. 6H).
Fig. 6.
The nuclease activity of Dna2 inhibits its binding to DNA. (A) Electrophoretic mobility shift assays were performed with a range of Dna2 E675A concentrations and 32P-labeled 30-nt-long 5′-tailed DNA or fully dsDNA (both 1 nM). The panel shows representative 6% polyacrylamide gels. *, position of the 32P label. (B) Electrophoretic mobility shift assay as in A, but with wild-type Dna2. (C) Quantitation of DNA binding assays such as from A and B. Error bars, SE, n = 2. (D) Electrophoretic mobility shift assays were performed with a range of Dna2 E675A concentrations and 32P-labeled 70-nt-long ssDNA substrate (1 nM). The DNA substrate contained biotin near both of its ends, which was bound to streptavidin where indicated. Shown is a representative 6% polyacrylamide gel. *, position of the 32P label; S, position of biotin-bound streptavidin. (E) Electrophoretic mobility shift assay as in D, but with wild-type Dna2. (F) Quantitation of the assays such as from D and E. Error bars, SE, n = 2. (G) Kinetics of ATP hydrolysis by wild-type Dna2 and Dna2 E675A (both 4 nM). The reactions contained 30-nt-long 5′ overhang substrate (1 μM nucleotides). Error bars, SE, n = 2. (H) Model explaining the kinetics of ATP hydrolysis by wild-type Dna2 on a 5′-tailed substrate. Initially, the overhang substrate stimulates the ATPase activity of wild-type Dna2. The nuclease activity then degrades the ssDNA tail, which results in a substrate that no longer efficiently stimulates the ATPase activity.
Taken together, these data explain why the nuclease activity of Dna2 strongly inhibits its unwinding capacity. We show that the nuclease degrades 5′ ssDNA tails, which in turn inhibits DNA binding and thus the unwinding capacity of Dna2. Previously, it was believed that the nuclease of Dna2 obscures the detection of the products of its limited unwinding activity (22). The data presented here suggest a different scenario, in which the nuclease activity prevents the helicase activity from engaging its respective substrate.
Helicase Activity of Wild-Type Dna2 Promotes Its Capacity to Degrade dsDNA.
Up to this point, we demonstrated that the nuclease of wild-type Dna2 inhibits its unwinding capacity. By cleaving ssDNA tails that serve as entry points for the helicase, the nuclease impedes DNA binding and thus the initiation of dsDNA unwinding. In subsequent experiments, we set out to find conditions to detect the unwinding capacity of the wild-type enzyme.
Using 5′-tailed dsDNA substrate 32P-labeled at the ssDNA tail, the activity of wild-type Dna2 was indistinguishable from that of the helicase-dead K1080E variant. The helicase activity did not stimulate cleavage of the 5′-terminated ssDNA tail, and we could detect no DNA unwinding intermediates (Fig. S9 A and B). Similarly to experiments presented in Fig. 5 D and E, the helicase activity thus had no apparent effect on the behavior of Dna2. We reasoned that Dna2 binds and translocates on the tailed oligonucleotide, which gets then preferentially cleaved, and this cleavage may limit the detection of DNA unwinding intermediates. We next labeled the bottom oligonucleotide that does not contain the overhang (see diagram at the top of Fig. 7A), and analyzed the activity of wild-type, helicase-dead K1080E, and nuclease-dead Dna2 E675A variants. The nuclease of both wild type and K1080E degraded the 5′-terminated DNA overhang, resulting in species that comigrated with the tail-less dsDNA marker (Fig. 7 A and B). Importantly, we could also clearly detect ssDNA in reactions with wild-type Dna2 (Fig. 7A, lanes 3–6). In contrast, ssDNA was nearly undetectable in reactions with the helicase-dead K1080E variant; the minimal amount of ssDNA we observed was likely the result of the nuclease acting on the top strand upon spontaneous thermal melting of the DNA substrate in the presence of RPA (Fig. 7B). The ssDNA was apparent at wild-type Dna2 concentrations as low as 16–32 pM (Fig. 7A), which was also the minimal protein concentration required to detect DNA unwinding by the nuclease-dead Dna2 E675A variant (Fig. 7C). These results, together with the ATPase assays (Fig. 5C), indicate that wild-type Dna2 likely possesses the same level of helicase activity as the nuclease dead Dna2 E675A variant.
Fig. 7.
Wild-type Dna2 possesses DNA helicase activity. (A) Processing of 5′-tailed DNA by wild-type Dna2. The bottom oligonucleotide was 32P-labeled at the 5′ end. The reactions contained RPA (22.5 nM). Heat, heat-denatured DNA substrate. *, position of the 32P label. The panel shows a representative 10% polyacrylamide gel. (B) Experiment as in panel A, but with helicase-dead Dna2 K1080E variant. (C) Experiment as in panel A, but with nuclease-dead Dna2 E675A variant.
The experiments in Fig. 7 A and B also showed that the helicase activity of Dna2 promotes degradation of dsDNA. The degradation of DNA by the helicase-deficient Dna2 K1080E variant was mostly limited to the 5′-terminated ssDNA overhang, as the species comigrating with tail-less DNA was the dominant product across a wide range of enzyme concentrations (Fig. 7B). In contrast, wild-type Dna2 could more efficiently enter and degrade dsDNA as well, as witnessed by the appearance of small-molecular-weight DNA species, denoted as degraded DNA (Fig. 7A).
To further investigate the role of the Dna2 helicase in degradation of dsDNA, we carried out additional experiments with a 5′-tailed substrate that was 3′-labeled at the dsDNA end (see diagram at the top of Fig. 8A). As above, the helicase activity of wild-type Dna2 promoted degradation of dsDNA (Fig. 8 A and B). We show that dsDNA degradation requires the presence of the 5′-terminated ssDNA tail, as tail-less dsDNA was not cleaved at similar enzyme concentrations (Fig. S10A). Furthermore, the dsDNA degradation was dependent on RPA, as no significant 3′ end cleavage product was observed in the absence of RPA, with both wild-type Dna2 or the helicase-dead K1080E variant (Fig. S10B). RPA therefore stimulates the degradation of dsDNA by both wild-type and helicase-dead Dna2 K1080E, probably by melting the dsDNA ends. The dsDNA degradative capacity is then further enhanced by the helicase activity of Dna2 (Fig. 8 A and B).
Fig. 8.
The helicase activity of wild-type Dna2 moderately promotes its capacity to degrade dsDNA. (A) A representative polyacrylamide gel (10%) showing the activity of wild-type Dna2 and K1080E variant on 3′-labeled 5′-ssDNA tailed substrate (1 nM). *, position of the 32P label. All reactions contained RPA (22.5 nM). (B) Quantitation of data such as from panel A. Error bars, SE, n = 2. (C). Degradation of 2.7-kbp dsDNA with 4-nt-long 5′ ssDNA overhangs by wild-type Dna2 and helicase dead Dna2 K1080E variant in the presence of various ATP concentrations, as indicated. The reactions contained 3.8 nM DNA and were supplemented with RPA (1.68 μM). The reaction products were separated on 1% agarose gel and stained with ethidium bromide. Panel shows a representative experiment.
Finally, we investigated the degradation of plasmid-sized tailed DNA on agarose gels stained with ethidium bromide. Limited degradation of DNA near its ends resulted in change of mobility (Fig. 8C). In the absence of ATP, a condition favored by the Dna2 nuclease (4), both wild-type and helicase-dead Dna2 K1080E variant degraded DNA to a similar extent. Upon supplementing the reaction with ATP, which lowered the free magnesium concentration required by the nuclease and activated the helicase, DNA degradation by the wild-type enzyme was promoted whereas DNA degradation by the K1080E variant was inhibited. Thus, in the presence of ATP, the helicase of Dna2 clearly promotes degradation of dsDNA. The extent of DNA degradation is, however, limited in comparison with the extent of DNA unwound by the equivalent concentration of the nuclease-dead Dna2 E675A mutant (Fig. 5 A and B).
In summary, our data reveal an unusual interplay of the nuclease and helicase activities of Dna2. The nuclease activity strongly reduces the ability of Dna2 to unwind DNA. However, the inhibition of the DNA unwinding capacity is not total, and we show that the helicase activity may promote nucleolytic degradation of dsDNA. The ssDNA-specific nuclease activity thus controls the unwinding capacity and the ability of the enzyme to process and degrade dsDNA. We propose that modulation of the interplay between the nuclease and helicase activities in vivo might represent a regulatory mechanism for Dna2 to fine-tune its behavior to carry out specific cellular functions. Conditions that promote the nuclease activity would result in Dna2 degradation limited to ssDNA tails whereas inhibition of the nuclease activity would allow the enzyme to process also dsDNA (Fig. 9 and Discussion).
Fig. 9.

Model of the interplay of helicase and nuclease activities of Dna2. In the presence of vigorous nuclease activity, the helicase capacity is inhibited and the DNA degradation is limited to ssDNA (Left). Moderate inhibition of the nuclease activity might allow the enzyme to degrade dsDNA (Center). Complete inactivation of nuclease activity, such as in nuclease-dead Dna2 E675A variant, turns the enzyme into a vigorous DNA helicase (Right). See text for details.
Discussion
The Dna2 nuclease-helicase functions in multiple fundamental cellular processes ranging from lagging-strand DNA synthesis, replication fork stability, and dsDNA break repair, to the repair of damaged DNA (6, 18, 29, 39). Recombinant Dna2 possesses a potent nuclease activity that appears to be essential for all Dna2 functions, but the 5′–3′ DNA helicase activity of the enzyme was described as weak. The observed limited unwinding capacity led to the speculation that the motor function of Dna2 might act as an ssDNA translocase that removes secondary structures from ssDNA rather than to unwind dsDNA (23). We noticed that recombinant Dna2 was very unstable and sensitive to the omission of reducing agents during purification. Because Dna2 was recently shown to contain an iron–sulfur cluster (30, 31), we speculated that it might be prone to inactivation by oxidation. Indeed, our current purification protocol yielded a highly active enzyme with strong nuclease and helicase activities.
Unexpectedly, biochemical characterization of the wild-type, nuclease-dead, and helicase-dead enzyme variants generated in the course of this study revealed that Dna2 possesses a vigorous but cryptic helicase activity. Mutational inactivation of the Dna2 nuclease unleashes this helicase activity. Nuclease-dead Dna2 E675A variant is capable of catalytic unwinding of oligonucleotide-based DNA substrates at subnanomolar enzyme concentrations (Fig. 1). Furthermore, it shows functional interaction with RPA, which allows it to unwind tens of kilobases of dsDNA in length at low nanomolar enzyme concentrations (Fig. 2). The helicase and ATPase activities of Dna2 E675A compare favorably with the RecQ family Sgs1 helicase (Figs. 2 and 4), which is possibly the most vigorous eukaryotic helicase characterized to date (32). Single molecule experiments revealed that DNA unwinding by Dna2 E675A is highly processive (Fig. 3).
The Dna2 nuclease inhibits this vigorous unwinding capacity; although the ATPase activity of wild-type Dna2 is comparable with that of the nuclease-dead Dna2 E675A variant, the former protein is inefficient in dsDNA unwinding (Fig. 5). The Dna2 helicase requires a free 5′ terminus to load onto to initiate DNA unwinding. We show that the nuclease activity of wild-type Dna2 is more likely to degrade such structures rather than use them for DNA unwinding (Figs. 5 and 6). Dna2 thus represents a remarkable example of an enzyme in which one biochemical property (nuclease) negatively regulates the other (helicase) by competing for the same substrate (5′-tailed DNA).
The nuclease-mediated inhibition of the unwinding capacity was not total, and we were able to observe a fraction of unwound DNA in reactions with wild-type Dna2 as well (Fig. 7). As a result, the helicase activity of wild-type Dna2 promoted the degradation of dsDNA to a limited extent (Figs. 7 and 8). We therefore speculate that modulation of the nuclease activity might regulate the global activity of Dna2. In this scenario, moderate inhibition of the nuclease activity might promote dsDNA unwinding and, consequently, also degradation of dsDNA. Conversely, stimulation of the nuclease would inhibit the helicase, which would restrict the nucleolytic activity of the enzyme to ssDNA (Fig. 9). The regulatory mechanism proposed here might therefore restrict the unwinding capacity only to situations when it is required. It is of interest that overexpression of nuclease-dead Dna2 proteins had a dominant negative effect in dna2-1 mutants with impaired both helicase and nuclease functions of Dna2 under certain growth conditions (20). Similarly, overexpression of nuclease-dead human DNA2 was more detrimental than overexpression of both nuclease and helicase-dead construct (40). This result, together with our data showing that the nuclease activity limits the helicase function, implies that hyperactive and unregulated Dna2 helicase is toxic, and highlights the importance of restricting the helicase activity of Dna2 in vivo.
How could the activity of Dna2 be regulated in vivo? Obvious candidates for potential regulators are interactors and/or posttranslational modifications. During DNA replication, Dna2 forms a complex with Rad27 whereas, during dsDNA break repair, it interacts with Sgs1 (5, 12). It is thus possible that these two proteins affect the balance between the helicase and nuclease activities. Furthermore, the activity of human DNA2 was found to be stimulated by acetylation (41). The effect of acetylation on the relative activities of the helicase and nuclease functions is unknown. Similarly, Dna2 is also phosphorylated in vivo, which regulates its recruitment to DNA and controls its function on stalled replication forks (18, 42). It will be of interest to investigate how these modifications affect the biochemical behavior of Dna2. The Dna2 polypeptide contains a large (∼405-amino acid) N-terminal domain, which mediates the interaction with RPA, but which was also found to interact with a region of Dna2 located between the helicase and nuclease domains (43). Based on this intramolecular interaction, it has been proposed that the N-terminal domain might have a regulatory role, in that its position might affect the biochemical activities of Dna2 (43). Finally, it is also possible that the interplay between the nuclease and helicase activities of Dna2 might be controlled via the redox states of its iron–sulfur cluster (30, 31).
Dna2 physically and functionally interacts with the Sgs1 helicase in DNA end resection to initiate homologous recombination (12). The Sgs1–Dna2 heterodimer is functionally similar to the resection machinery of E. coli, the RecBCD factor. Both complexes possess two helicases and a single nuclease activity, and both must load on free DNA ends, yet degrade DNA endonucleolytically (12). Similarly, the Sgs1–Dna2 complex could be a bipolar helicase, with each subunit translocating on opposite strands but in the same overall direction (12). The unwinding capacity of the heterodimer cannot be inhibited by 5′ cleavage, as Sgs1 is likely the lead helicase that translocates with a 3′ to 5′ polarity. A vigorous motor activity of Dna2 would be important here, as only rapidly moving Dna2 could “keep up” with the Sgs1 helicase. We also point out that the motor activity of Dna2 was reported dispensable for DNA end resection in an earlier study (9). In complementation experiments, the expression of wild-type and Dna2 variants was driven from a plasmid in the latter work, and higher than physiological Dna2 levels may have masked the involvement of the Dna2 helicase. Nevertheless, the role of the Dna2 helicase in DNA end resection remains unclear and deserves further attention.
In RecBCD, both RecB and RecD motors precede the RecB nuclease domain to feed ssDNA into the RecB nuclease site in a highly coordinated manner (44). The RecC subunit, however, contains a defunct nuclease domain, which is interestingly situated ahead of the RecD motor (44–46). Our biochemical data presented here might imply that the Dna2 nuclease is similarly placed ahead of the Dna2 helicase although detailed structural analysis will be required to test this hypothesis. In Bacillus subtilis, the processing of dsDNA ends is catalyzed by the AddAB complex, which in contrast contains one helicase motor and two nuclease domains (47). The AddB subunit is however clearly related to Dna2, and both proteins contain an iron–sulfur cluster (30, 31). Data presented here together with previous work (12, 47, 48) demonstrate that the interplay of helicase and nuclease activities regulates dsDNA end processing in eukaryotes as well.
Materials and Methods
DNA Substrates.
The oligonucleotides used for the preparation of the Y-structure, dsDNA, ssDNA, 19-nt 5′-tailed and 19-nt 3′-tailed ssDNA substrates have been described previously (32). The oligonucleotide used to prepare the 30-nt 5′-tailed ssDNA substrate was PC 92, GGTACTCAAGTGACGTCATAGACGATTACATTGCTAGGACATGCTGTCTAGAGACTATCGC. The oligonucletides were 32P-labeled either at the 5′terminus with [γ-32P]ATP and T4 polynucletide kinase (New England Biolabs), or at the 3′ end with [α-32P]cordycepin-5′-triphosphate and terminal transferase (New England Biolabs) according to the manufacturer’s instructions. Unincorporated nucleotides were removed using MicroSpin G25 columns (GE Healthcare). pUC19 dsDNA and bacteriophage λ dsDNA were digested with HindIII and purified by phenol-chloroform extraction and ethanol precipitation. The linearized dsDNA was then 3′-labeled with [α-32P]dATP and Klenow fragment of DNA polymerase I (New England Biolabs). Unincorporated nucleotides were removed using MicroSpin G25 columns (GE Healthcare). Streptavidin-blocked oligonucleotide was prepared by incubating streptavidin (15 nM) with the DNA substrate in reaction buffer for 15 min before addition of other recombinant proteins and initiation of the reaction.
Recombinant Proteins.
Sgs1 and RPA proteins were expressed and purified as described (32, 49). SSB was a gift from S. Kowalczykowski (University of California, Davis) and human RPA from Stephanie Bregenhorn (University of Zurich). Wild-type Dna2 as well as Dna2 E675A and K1080E variants were expressed from a modified pGAL:DNA2 (20) vector that contained amino (N)-terminal Flag and HA tags and a C-terminal His6 tag, in the S. cerevisiae strain WDH668 (50). Yeast cells (4L) were grown to approximately OD ∼0.6 in a standard synthetic medium, lacking uracil, and supplemented with glycerol (3% vol/vol) and lactic acid (2% vol/vol) as carbon sources. Expression of Dna2 was induced with galactose, the cells were lysed, and Dna2 was bound to Ni2+-NTA agarose (Qiagen) as described previously (12). Dna2 was eluted with 250 mM imidazole. The fractions containing proteins were pooled, diluted 1:8 with de-gassed dilution buffer (25 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 10% (vol/vol) glycerol, 0.5 mM β-mercaptoethanol, 10 μg⋅ml−1 leupeptin, 1 mM phenylmethylsulphonyl fluoride and protease-inhibitor mixture (Sigma, P8340, diluted 1:1,000)) and incubated batch-wise with M2 anti-FLAG affinity resin (0.5 mL; Sigma) for 45 min. The resin was then washed with degassed wash buffer (25 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 10% (vol/vol) glycerol, 1 mM β-mercaptoethanol), and Dna2 was eluted with wash buffer supplemented with 4xFLAG Peptide (200 μg⋅ml−1; Sigma). Fractions containing protein were pooled, and small aliquots were frozen in liquid nitrogen and stored at −80 °C. The final protein concentration was estimated by densitometry by comparison with dilution series of broad range protein marker (BioRad) on 10% (wt/vol) polyacrylamide gel stained with Coomassie blue. The protein yields were ∼150–250 μg and concentration ∼100–400 nM for Dna2 wild-type, K1080E, and E675A variants. The specific activities of independent enzyme preparations varied by about ±15% with respect to a mean value.
Helicase and Nuclease Assays.
The experiments were performed in a 15-μl volume in 25 mM Tris-acetate (pH 7.5), 2 mM magnesium acetate, 1 mM ATP, 1 mM DTT, 0.1 mg/mL BSA (New England Biolabs), 1 mM phosphoenolpyruvate, 16 U/mL pyruvate kinase, DNA substrate, and recombinant proteins, as indicated. Unless indicated otherwise, the reactions were incubated for 30 min at 30 °C and carried out and analyzed as described previously (32). We noted that recombinant Dna2 was unusually sensitive to protein dilution, and supplementing the reactions with BSA was often required to observe enzymatic activity.
Magnetic Tweezers Assay.
The DNA construct was prepared by digesting plasmid pNLrep with BamHI, BsrGI, and Nt.BbvCI, filling part of the the gap created by the nicking enzyme with an DNA oligomer (oliomer sequence: 5′-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCAGCTAGCCTCAGCCTACAATCACC) and ligating the biotin (BsrGI digested) and digoxigenin-modified (BamHI digested) tails as described (53, 52). Magnetic tweezers experiments were carried out as described before (33). Helicase experiments were done at room temperature using the same buffer as the bulk helicase assays (but lacking the ATP recycling system) using 1 nM Dna2 E675A and 22 nM RPA. RPA itself at this concentration was found to slowly unwind dsDNA (4 bp⋅s−1) by polymerizing on ssDNA at forces of 35 pN. Experiments were therefore always performed at lower forces where this behavior was not observed. To convert DNA length changes during unwinding at a given force into a corresponding number of unwound bp, force-extension measurements on the bare DNA construct and on the unwound RPA-coated construct were used.
ATPase and Electrophoretic Mobility Shift Assays.
These experiments were performed as described previously (32, 37, 38).
Supplementary Material
Acknowledgments
We thank Stephen Kowalczykowski (University of California, Davis) for help during the initial phase of this project and for the recombinant SSB protein. We thank Judith Campbell (California Institute of Technology) for the Dna2 expression construct and Stephanie Bregenhorn (University of Zurich) for human RPA. We acknowledge Pavel Janscak, Elda Cannavo, Lepakshi, Josef Jiricny (all from University of Zurich), Cosimo Pinto (ETH Zurich), and Mark Dillingham (University of Bristol) for discussions and helpful comments on the manuscript. This work was supported by Swiss National Science Foundation Professorship PP00P3 133636.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300390110/-/DCSupplemental.
References
- 1.Kuo C, Nuang H, Campbell JL. Isolation of yeast DNA replication mutants in permeabilized cells. Proc Natl Acad Sci USA. 1983;80(21):6465–6469. doi: 10.1073/pnas.80.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budd ME, Campbell JL. A yeast gene required for DNA replication encodes a protein with homology to DNA helicases. Proc Natl Acad Sci USA. 1995;92(17):7642–7646. doi: 10.1073/pnas.92.17.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budd ME, Choe WC, Campbell JL. DNA2 encodes a DNA helicase essential for replication of eukaryotic chromosomes. J Biol Chem. 1995;270(45):26766–26769. doi: 10.1074/jbc.270.45.26766. [DOI] [PubMed] [Google Scholar]
- 4.Bae SH, et al. Dna2 of Saccharomyces cerevisiae possesses a single-stranded DNA-specific endonuclease activity that is able to act on double-stranded DNA in the presence of ATP. J Biol Chem. 1998;273(41):26880–26890. doi: 10.1074/jbc.273.41.26880. [DOI] [PubMed] [Google Scholar]
- 5.Budd ME, Campbell JL. A yeast replicative helicase, Dna2 helicase, interacts with yeast FEN-1 nuclease in carrying out its essential function. Mol Cell Biol. 1997;17(4):2136–2142. doi: 10.1128/mcb.17.4.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae SH, Bae KH, Kim JA, Seo YS. RPA governs endonuclease switching during processing of Okazaki fragments in eukaryotes. Nature. 2001;412(6845):456–461. doi: 10.1038/35086609. [DOI] [PubMed] [Google Scholar]
- 7.Bae SH, Seo YS. Characterization of the enzymatic properties of the yeast dna2 Helicase/endonuclease suggests a new model for Okazaki fragment processing. J Biol Chem. 2000;275(48):38022–38031. doi: 10.1074/jbc.M006513200. [DOI] [PubMed] [Google Scholar]
- 8.Kao HI, Veeraraghavan J, Polaczek P, Campbell JL, Bambara RA. On the roles of Saccharomyces cerevisiae Dna2p and Flap endonuclease 1 in Okazaki fragment processing. J Biol Chem. 2004;279(15):15014–15024. doi: 10.1074/jbc.M313216200. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134(6):981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455(7214):770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22(20):2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cejka P, et al. DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature. 2010;467(7311):112–116. doi: 10.1038/nature09355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu H, et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature. 2010;467(7311):108–111. doi: 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimonkar AV, et al. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 2011;25(4):350–362. doi: 10.1101/gad.2003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe W, Budd M, Imamura O, Hoopes L, Campbell JL. Dynamic localization of an Okazaki fragment processing protein suggests a novel role in telomere replication. Mol Cell Biol. 2002;22(12):4202–4217. doi: 10.1128/MCB.22.12.4202-4217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesur I, Campbell JL. The transcriptome of prematurely aging yeast cells is similar to that of telomerase-deficient cells. Mol Biol Cell. 2004;15(3):1297–1312. doi: 10.1091/mbc.E03-10-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng L, et al. Human DNA2 is a mitochondrial nuclease/helicase for efficient processing of DNA replication and repair intermediates. Mol Cell. 2008;32(3):325–336. doi: 10.1016/j.molcel.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, et al. The intra-S phase checkpoint targets Dna2 to prevent stalled replication forks from reversing. Cell. 2012;149(6):1221–1232. doi: 10.1016/j.cell.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Peng G, et al. Human nuclease/helicase DNA2 alleviates replication stress by promoting DNA end resection. Cancer Res. 2012;72(11):2802–2813. doi: 10.1158/0008-5472.CAN-11-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budd ME, Choe Wc, Campbell JL. The nuclease activity of the yeast DNA2 protein, which is related to the RecB-like nucleases, is essential in vivo. J Biol Chem. 2000;275(22):16518–16529. doi: 10.1074/jbc.M909511199. [DOI] [PubMed] [Google Scholar]
- 21.Kao HI, Campbell JL, Bambara RA. Dna2p helicase/nuclease is a tracking protein, like FEN1, for flap cleavage during Okazaki fragment maturation. J Biol Chem. 2004;279(49):50840–50849. doi: 10.1074/jbc.M409231200. [DOI] [PubMed] [Google Scholar]
- 22.Balakrishnan L, Polaczek P, Pokharel S, Campbell JL, Bambara RA. Dna2 exhibits a unique strand end-dependent helicase function. J Biol Chem. 2010;285(50):38861–38868. doi: 10.1074/jbc.M110.165191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae SH, et al. Coupling of DNA helicase and endonuclease activities of yeast Dna2 facilitates Okazaki fragment processing. J Biol Chem. 2002;277(29):26632–26641. doi: 10.1074/jbc.M111026200. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, et al. Isolation of human Dna2 endonuclease and characterization of its enzymatic properties. Nucleic Acids Res. 2006;34(6):1854–1864. doi: 10.1093/nar/gkl102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda-Sasa T, Polaczek P, Campbell JL. Single strand annealing and ATP-independent strand exchange activities of yeast and human DNA2: Possible role in Okazaki fragment maturation. J Biol Chem. 2006;281(50):38555–38564. doi: 10.1074/jbc.M604925200. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Choe W, Campbell JL. Identification of the Xenopus laevis homolog of Saccharomyces cerevisiae DNA2 and its role in DNA replication. J Biol Chem. 2000;275(3):1615–1624. doi: 10.1074/jbc.275.3.1615. [DOI] [PubMed] [Google Scholar]
- 27.Masuda-Sasa T, Imamura O, Campbell JL. Biochemical analysis of human Dna2. Nucleic Acids Res. 2006;34(6):1865–1875. doi: 10.1093/nar/gkl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budd ME, Campbell JL. The pattern of sensitivity of yeast dna2 mutants to DNA damaging agents suggests a role in DSB and postreplication repair pathways. Mutat Res. 2000;459(3):173–186. doi: 10.1016/s0921-8777(99)00072-5. [DOI] [PubMed] [Google Scholar]
- 29.Formosa T, Nittis T. Dna2 mutants reveal interactions with Dna polymerase alpha and Ctf4, a Pol alpha accessory factor, and show that full Dna2 helicase activity is not essential for growth. Genetics. 1999;151(4):1459–1470. doi: 10.1093/genetics/151.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeeles JT, Cammack R, Dillingham MS. An iron-sulfur cluster is essential for the binding of broken DNA by AddAB-type helicase-nucleases. J Biol Chem. 2009;284(12):7746–7755. doi: 10.1074/jbc.M808526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pokharel S, Campbell JL. Cross talk between the nuclease and helicase activities of Dna2: Role of an essential iron-sulfur cluster domain. Nucleic Acids Res. 2012;40(16):7821–7830. doi: 10.1093/nar/gks534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cejka P, Kowalczykowski SC. The full-length Saccharomyces cerevisiae Sgs1 protein is a vigorous DNA helicase that preferentially unwinds holliday junctions. J Biol Chem. 2010;285(11):8290–8301. doi: 10.1074/jbc.M109.083196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klaue D, Seidel R. Torsional stiffness of single superparamagnetic microspheres in an external magnetic field. Phys Rev Lett. 2009;102(2):028302. doi: 10.1103/PhysRevLett.102.028302. [DOI] [PubMed] [Google Scholar]
- 34.De Vlaminck I, et al. Torsional regulation of hRPA-induced unwinding of double-stranded DNA. Nucleic Acids Res. 2010;38(12):4133–4142. doi: 10.1093/nar/gkq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dessinges MN, Lionnet T, Xi XG, Bensimon D, Croquette V. Single-molecule assay reveals strand switching and enhanced processivity of UvrD. Proc Natl Acad Sci USA. 2004;101(17):6439–6444. doi: 10.1073/pnas.0306713101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller DN, Raymer DM, Kottadiel VI, Rao VB, Smith DE. Single phage T4 DNA packaging motors exhibit large force generation, high velocity, and dynamic variability. Proc Natl Acad Sci USA. 2007;104(43):16868–16873. doi: 10.1073/pnas.0704008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreuzer KN, Jongeneel CV. Escherichia coli phage T4 topoisomerase. Methods Enzymol. 1983;100:144–160. doi: 10.1016/0076-6879(83)00051-8. [DOI] [PubMed] [Google Scholar]
- 38.Kowalczykowski SC, Krupp RA. Effects of Escherichia coli SSB protein on the single-stranded DNA-dependent ATPase activity of Escherichia coli RecA protein: Evidence that SSB protein facilitates the binding of RecA protein to regions of secondary structure within single-stranded DNA. J Mol Biol. 1987;193(1):97–113. doi: 10.1016/0022-2836(87)90630-9. [DOI] [PubMed] [Google Scholar]
- 39.Kang YH, Lee CH, Seo YS. Dna2 on the road to Okazaki fragment processing and genome stability in eukaryotes. Crit Rev Biochem Mol Biol. 2010;45(2):71–96. doi: 10.3109/10409230903578593. [DOI] [PubMed] [Google Scholar]
- 40.Duxin JP, et al. Okazaki fragment processing-independent role for human Dna2 enzyme during DNA replication. J Biol Chem. 2012;287(26):21980–21991. doi: 10.1074/jbc.M112.359018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balakrishnan L, Stewart J, Polaczek P, Campbell JL, Bambara RA. Acetylation of Dna2 endonuclease/helicase and flap endonuclease 1 by p300 promotes DNA stability by creating long flap intermediates. J Biol Chem. 2010;285(7):4398–4404. doi: 10.1074/jbc.M109.086397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, et al. Cell cycle regulation of DNA double-strand break end resection by Cdk1-dependent Dna2 phosphorylation. Nat Struct Mol Biol. 2011;18(9):1015–1019. doi: 10.1038/nsmb.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bae SH, et al. Tripartite structure of Saccharomyces cerevisiae Dna2 helicase/endonuclease. Nucleic Acids Res. 2001;29(14):3069–3079. doi: 10.1093/nar/29.14.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dillingham MS, Kowalczykowski SC. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol Mol Biol Rev. 2008;72(4):642–671. doi: 10.1128/MMBR.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rigden DJ. An inactivated nuclease-like domain in RecC with novel function: Implications for evolution. BMC Struct Biol. 2005;5:9. doi: 10.1186/1472-6807-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singleton MR, Dillingham MS, Gaudier M, Kowalczykowski SC, Wigley DB. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature. 2004;432(7014):187–193. doi: 10.1038/nature02988. [DOI] [PubMed] [Google Scholar]
- 47.Yeeles JT, Gwynn EJ, Webb MR, Dillingham MS. The AddAB helicase-nuclease catalyses rapid and processive DNA unwinding using a single Superfamily 1A motor domain. Nucleic Acids Res. 2011;39(6):2271–2285. doi: 10.1093/nar/gkq1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saikrishnan K, et al. Insights into Chi recognition from the structure of an AddAB-type helicase-nuclease complex. EMBO J. 2012;31(6):1568–1578. doi: 10.1038/emboj.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kantake N, Sugiyama T, Kolodner RD, Kowalczykowski SC. The recombination-deficient mutant RPA (rfa1-t11) is displaced slowly from single-stranded DNA by Rad51 protein. J Biol Chem. 2003;278(26):23410–23417. doi: 10.1074/jbc.M302995200. [DOI] [PubMed] [Google Scholar]
- 50.Solinger JA, Lutz G, Sugiyama T, Kowalczykowski SC, Heyer WD. Rad54 protein stimulates heteroduplex DNA formation in the synaptic phase of DNA strand exchange via specific interactions with the presynaptic Rad51 nucleoprotein filament. J Mol Biol. 2001;307(5):1207–1221. doi: 10.1006/jmbi.2001.4555. [DOI] [PubMed] [Google Scholar]
- 51.Luzzietti N, Knappe S, Richter I, Seidel R. Nicking enzyme-based internal labeling of DNA at multiple loci. Nat Protoc. 2012;7(4):643–653. doi: 10.1038/nprot.2012.008. [DOI] [PubMed] [Google Scholar]
- 52.Luzzietti N, et al. Efficient preparation of internally modified single-molecule constructs using nicking enzymes. Nucleic Acids Res. 2011;39(3):e15. doi: 10.1093/nar/gkq1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.