Abstract
The middle ear ossicles are only rarely preserved in fossil hominins. Here, we report the discovery of a complete ossicular chain (malleus, incus, and stapes) of Paranthropus robustus as well as additional ear ossicles from Australopithecus africanus. The malleus in both early hominin taxa is clearly human-like in the proportions of the manubrium and corpus, whereas the incus and stapes resemble African and Asian great apes more closely. A deep phylogenetic origin is proposed for the derived malleus morphology, and this may represent one of the earliest human-like features to appear in the fossil record. The anatomical differences found in the early hominin incus and stapes, along with other aspects of the outer, middle, and inner ear, are consistent with the suggestion of different auditory capacities in these early hominin taxa compared with modern humans.
The middle ear ossicles have historically played a prominent role in paleontological studies because the appearance of the three bone ossicular chain is considered a defining feature of the emergence of mammals (1, 2). The evolutionary transformation of the malleus and incus, which once formed part of the lower jaw, represents a profound modification of both the feeding and auditory apparatuses and had important implications for the sensory ecology of early mammals (3), including primates (4). However, surprisingly little is known of the auditory ossicles in our early human ancestors because they are among the rarest hominin fossils recovered (5–11). Nevertheless, their study holds great potential as an avenue of inquiry into the evolutionary relationships among fossil taxa, as well as aspects of their sensory perception.
In humans, the embryological origins of each of the three ear bones have been thoroughly studied. These tiny bones are fully formed at birth (12, 13) and, unlike other bones of the skeleton, generally do not remodel after about the first year of life (14). The ear ossicles then, in some ways, remain “relic” embryonic bones throughout life, and their evolutionarily conservative nature makes them particularly suitable for phylogenetic analysis (15–17). Comparative genomic studies have revealed changes during the course of our evolutionary history in several genes related to the development of the auditory structures (18) and hearing (19), and previous studies of the inner ear in early hominins have provided insights into their taxonomic relationships and locomotion (20).
A few anatomical differences in the ear ossicles of fossil hominins have been reported previously (6–11). Among early hominins, the incus in Paranthropus robustus (SK 848) was argued to show a highly derived articular facet morphology, revealing profound differences from living hominids (7, 21). In contrast, the stapes of Australopithecus africanus (Stw 151) appears similar to African apes in showing generally small metric dimensions, including the size of the footplate (8). We report here on a complete right ossicular chain (malleus, incus, and stapes) (Table 1) that was removed from a P. robustus specimen (SKW 18) recovered from Swartkrans (South Africa) (22) (SI Appendix, SI Text S1). This represents an exceptional case of preservation in the human fossil record because to date only two late Pleistocene Neanderthal specimens are reported to preserve a complete ossicular chain (10, 11). In addition, a left malleus and partial right stapes were removed from a specimen attributed to Australopithecus africanus (Stw 255) from Sterkfontein (South Africa) (SI Appendix, SI Text S1). These discoveries allow for a direct comparison of the ear ossicles between these two early hominin taxa, and for comparison with previously reported early hominin specimens (Table 1).
Table 1.
South African early hominin ear ossicles
Malleus
The malleus shows some differences between A. africanus and P. robustus in the morphological details (Fig. 1). A. africanus shows an inferiorly inflected manubrium tip and an anterior (gracile) process, for attachment of the anterior ligament on the malleus. In contrast, P. robustus shows a straight manubrium and no anterior process. Both of these features are variable in living humans, but an anterior process is normally present in extant African and Asian great apes (SI Appendix, Fig. S1). In addition, a distinct crest is often found on the superior aspect of the neck of the malleus in extant African apes and humans, and the early hominin taxa show different morphologies in this trait. In A. africanus, the crest is restricted to the superior aspect of the neck, as in the majority of chimpanzees and gorillas, whereas P. robustus shows a longer crest that curves around below the articular facet, like the majority of living humans (Fig. 1 and SI Appendix, Fig. S1). Perhaps more relevant phylogenetically, the head of the malleus is generally flattened in the anteroposterior (A-P) direction in extant hominids, and this flattening is also seen in A. africanus. In contrast, P. robustus shows a rounder, more globular head, with the A-P and superioinferior dimensions being approximately equal. Despite some variation, extant hominids generally show an A-P flattened mallear head. In P. robustus, the globular mallear head contributes to a generally inflated appearance of both the malleus and incus (see below) in this taxon, and may represent a derived condition.
Fig. 1.

The SKW 18 malleus (Upper) in posterior (Left), superior (Center), and anterior (Right) views, and the Stw 255 malleus (Lower) in anterior (Left), superior (Center), and posterior (Right) views. Note the morphology of the crest on the superior neck (white arrows), the inflection of the manubrium tip in Stw 255, and the rounded, globular shape to the head in SKW 18. (Scale bar: 5 mm.)
Metrically, the malleus in P. robustus is slightly larger than that in A. africanus, but both taxa generally show smaller dimensions than the living great apes (SI Appendix, Table S1). The most striking metrical difference seen in the early hominin taxa is the very high (open) angle (150.9°) between the manubrium and the head/corpus in P. robustus, falling above the range of variation in extant hominids. Compared with the other great apes (SI Appendix, Fig. S1), the main metrical differences in the human malleus are related to a shortening and thickening of the manubrium and (to maintain articulation of the ossicular chain) a concomitant lengthening of the corpus (SI Appendix, Table S1). Indices that combine these measurements generally show little overlap between African and Asian apes, on the one hand, and humans, on the other (SI Appendix, Table S1, and Fig. 2). In all of the most diagnostic measurements, mainly related to the corpus and manubrium dimensions, both the early hominin mallei clearly resemble their human counterparts. Discriminant function analysis (DFA) of the malleus variables (SI Appendix, SI Text S2 and Table S2) in extant hominids classifies both A. africanus and P. robustus with humans with a posterior probability >0.999 (i.e., >99.9% classification).
Fig. 2.
Scatterplot of the malleus manubrium robusticity index vs. the manubrium/corpus index. Both the early hominin mallei clearly show the modern human pattern of a relatively short, thick manubrium and relatively long corpus.
Incus
The presence of a notch (related to the attachment of the posterior incudal ligament) along the lower margin of the short process is a variable feature in humans (23) but is generally absent among African and Asian great apes. P. robustus shows some variation in its expression of this anatomical variant, with SK 848 lacking any sign of a notch and SKW 18 showing the presence of a shallow and wide notch (Fig. 3). The short process of the incus of P. robustus (SK 848) has been described as slender and cylindrical with a markedly concave superior border (7). The articular facet in SK 848 has been suggested to show a different (highly derived) orientation and morphology relative to that seen in African apes and humans (7). The discovery of a second P. robustus incus (SKW 18) reveals a degree of variation in these features (Fig. 3), and, contrary to previous suggestions, the orientation and morphology of the articular facet in both P. robustus incudi can be accommodated within the variation seen in extant hominids (SI Appendix, SI Text S3 and Fig. S2). In addition, the body of the incus shows an inflated appearance (unlike humans) in both SK 848 (7) and SKW 18, and this may be related to the rounded, more globular mallear head in this taxon.
Fig. 3.

The SK 848 (Upper) and SKW 18 (Lower) early hominin incudi in lateral (Left), anterior (Center), and medial (Right) views. (Scale bar: 5 mm.)
Among the P. robustus incudi, SK 848 is slightly larger than SKW 18 in its preserved dimensions (SI Appendix, Table S5). The greatest metric distinctions among extant hominids involve an increase in the functional length of the long process and a more open angle between the long and short processes in humans compared with African and Asian great apes. The low value for the incus angle (51.0°) and the short functional length in SKW 18 are closest to the chimpanzee means (SI Appendix, Table S5), and a scatterplot of these variables shows P. robustus falling among chimpanzees (Fig. 4). DFA of the incus variables (SI Appendix, SI Text S2 and Table S3) in extant hominids classifies SKW 18 with chimpanzees (posterior probability of >0.999).
Fig. 4.
Scatterplot of the incus functional length vs. the angle between the short and long processes. P. robustus clearly shows a short functional length and a relatively closed angle, falling within the chimpanzee range of variation.
Stapes
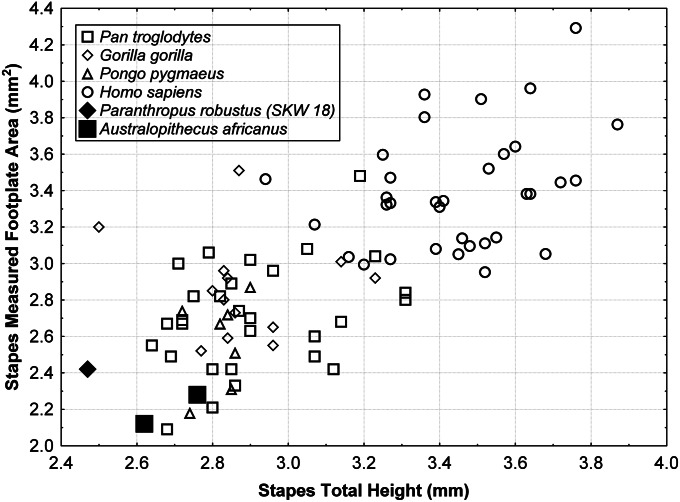
In contrast to the malleus and incus, stapes morphology is fairly similar across extant hominids (SI Appendix, Fig. S3). Metric dimensions in all of the early hominin stapedes, including the size of the footplate, are relatively small compared with living humans (SI Appendix, Table S6). The footplate area in SKW 18 (2.42 mm2) is similar to the values reported for several early hominin specimens (8). Although the two most complete early hominin stapedes (SKW 18 and Stw 151) (Fig. 5) are metrically very similar, Stw 255 differs in showing a relatively taller head and narrower obturator foramen (SI Appendix, Table S6). The distinctions between the stapes of modern humans and the other great apes are primarily related to size differences, with modern humans being larger in nearly all dimensions, and the small stapes dimensions in both the early hominin taxa resemble African and Asian apes (Fig. 6). DFA of the stapes variables (SI Appendix, SI Text S2 and Table S4) in extant hominids classifies P. robustus (SKW 18) and one A. africanus individual (Stw 151) with chimpanzees (posterior probabilities of 0.88 and 0.99, respectively), whereas a second A. africanus individual (Stw 255) is classified with gorillas (posterior probability of 0.68).
Fig. 5.

The SKW 18 (Left), Stw 255 (Center), and Stw 151 (Right) early hominin stapes in superior view. (Scale bar: 5 mm.)
Fig. 6.
Scatterplot of stapes total height vs. footplate area. The metric variation among extant hominids seems to be largely size related, and the small dimensions in both the early hominin taxa resemble African and Asian great apes.
Discussion
Although the phylogenetic polarity of many of these features needs to be more firmly established, the malleus of A. africanus resembles living hominids somewhat more closely than does that of P. robustus. The inflated mallear head and incus body in P. robustus may represent derived features in this taxon (7), perhaps related to their common embryological origins (12), and this is consistent with previous suggestions of autapomorphic traits in P. robustus (7, 21, 24). Nevertheless, despite some differences between the ossicles attributed to A. africanus and P. robustus, their most remarkable feature is the strong degree of similarity in most of their morphological details and metric dimensions.
Although some ossicular dimensions are correlated with body size across mammals (25), within the extant hominids there are clear shape differences that distinguish the malleus and incus in humans from those of African and Asian great apes, regardless of variation in body size (SI Appendix, Tables S1 and S5). Both early hominin taxa are characterized by human-like proportions in the malleus manubrium and corpus (Fig. 2) despite a body size that is similar to chimpanzees (26). In contrast, P. robustus maintained the short functional length and low angle between the long and short processes seen in the incudi of the African and Asian great apes (Fig. 4), and these likely represent primitive features. The stapes appears to be evolutionarily the most conservative bone among the auditory ossicles, perhaps reflecting its phylogenetically older status (1), and the anatomical variation in the stapes among extant hominids does seem to mainly reflect size (rather than shape) differences. Nevertheless, the variation in stapes dimensions is not clearly related to body size differences across extant hominid taxa, because most stapes dimensions in African and Asian great apes are similar (SI Appendix, Table S6) despite a considerable range in body size. The small metric dimensions in the early hominin stapes resemble the African and Asian great apes most closely.
The human-like malleus in both A. africanus and P. robustus represents a shared, derived characteristic (synapomorphy), most likely inherited from their last common ancestor. Discovery of additional fossil ear ossicles from even earlier hominin taxa would make it possible to identify more precisely when this human-like malleus first appeared in the hominin evolutionary lineage. Although the precise reasons behind these changes in malleus proportions are currently unclear, the functional length of the malleus (manubrium length) shows a strong correlation with the area of the tympanic membrane across haplorhines, including humans (27, 28). The tight developmental and functional relationship with the tympanic membrane (28–30) suggests the shortened manubrium in modern humans may be related with the smaller dimensions of the tympanic membrane compared with the African and Asian great apes (28, 31, 32).
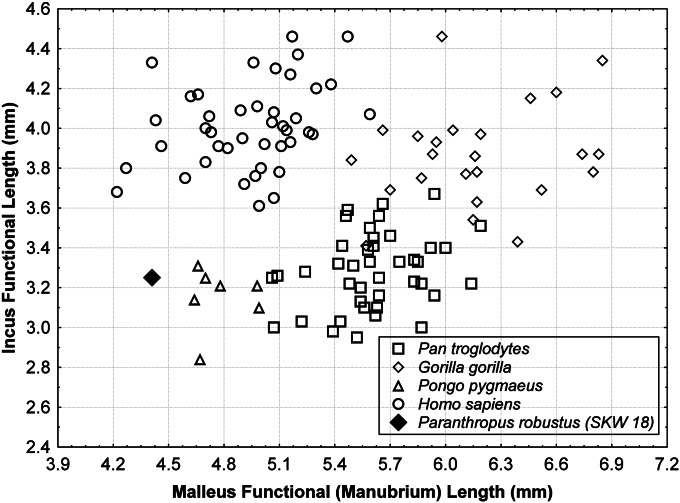
The functional lengths of the malleus and incus (and the associated lever ratio) and the stapes footplate area are important physiological variables in modeling audition (31, 33–36). Individually, each of these dimensions is strongly correlated with several measures of auditory sensitivity in primates (35). The unique combination of a human-like malleus and ape-like incus (Fig. 7) yields a lever ratio in P. robustus (1.36) that falls between the mean values for humans (1.23) and African and Asian great apes (1.52–1.71) (Table 2). More importantly, a short incus functional length (as in P. robustus) is consistent with the presence of a decrease in auditory sensitivity (a “notch” in the audiogram) in the midrange frequencies across primates (35). Such a notch characterizes the audiograms of the vast majority of haplorhine taxa tested to date, including chimpanzees, but is generally not found in humans (36, 37). This is one of the most salient distinctions in the hearing pattern of humans compared with other primates. Although the relationship between individual auditory structures and hearing performance is complex (28, 33–35, 38), the suggestion of an auditory difference in early hominins based on the ear ossicles is further supported by additional anatomical differences noted previously in their outer, middle, and inner ear (8, 21, 28, 39), some of which have clear auditory implications (40). These anatomical differences in the early hominin ear highlight the possibility of reconstructing their auditory capacities, as has been done for archaic members of the genus Homo (33, 36), an approach that promises to reveal new insights into the sensory ecology of early hominins.
Fig. 7.
Scatterplot of malleus vs. incus functional lengths (i.e., lever ratio). Note the position of P. robustus, which combines a human-like malleus and an ape-like incus.
Table 2.
Malleus/incus lever ratio in Paranthropus robustus compared with living hominids
| Species* | Malleus, manubrium length, mean ± SD, range | Incus, functional length, mean ± SD, range | Malleus/incus, lever ratio, mean ± SD, range |
| Paranthropus robustus (SKW 18) | 4.41 | 3.25 | 1.36 |
| Pan troglodytes (n = 41) | 5.61 ± 0.27 | 3.29 ± 0.19 | 1.71 ± 0.11 |
| 5.06–6.19 | 2.98–3.69 | 1.46–1.96 | |
| Gorilla gorilla (n = 24) | 6.17 ± 0.40 | 3.87 ± 0.25 | 1.60 ± 0.13 |
| 5.49–6.85 | 3.41–4.46 | 1.34–1.86 | |
| Pongo pygmaeus (n = 7) | 4.77 ± 0.15 | 3.15 ± 0.15 | 1.52 ± 0.09 |
| 4.64–4.99 | 2.84–3.31 | 1.41–1.64 | |
| Homo sapiens (n = 42) | 4.94 ± 0.31 | 4.01 ± 0.22 | 1.23 ± 0.08 |
| 4.22–5.59 | 3.61–4.46 | 1.02–1.39 |
Hominid samples restricted to individuals preserving both the malleus and incus.
Methods
Samples of auditory ossicles representing the extant hominids (SI Appendix, Table S7) were studied to provide a firm comparative context within which to interpret the anatomical details and metric dimensions of the early hominin ossicles. The comparative analysis includes the largest samples of ear ossicles reported to date for the African apes and orangutans, as well as a sample of contemporary Homo sapiens. All measurements were collected on scaled, digital photographs of the individual bones, once these were removed from the tympanic cavity, according to previously defined techniques (6, 28, 41, 42). Several methodological issues related to standardization of measurement definitions for both the malleus and incus have recently been clarified (6), and these measurement protocols are used in the present study. The measurement definitions for the malleus and incus are provided in ref. 6, whereas that for the stapes are provided in SI Appendix, Table S8 and Fig. S4. Regarding the stapes, Masali (41) established three reference axes (X, Y, and Z) corresponding to the anterior and posterior crura and the footplate, respectively. Three angles between the crura and footplate (α, β, and γ) were also defined. The angle α is formed by the X and Y axes (anterior and posterior crura). The angle β is formed by the X and Z axes (anterior crus and footplate). The angle γ is formed by the Y and Z axes (posterior crus and footplate). These reference axes and angles (defined as angles A, B, and C in SI Appendix, Table S8) have been used in the present study.
Supplementary Material
Acknowledgments
We thank C. Dean for comments on a previous version of this paper and the following individuals and institutions for providing access to specimens: F. Thackeray, S. Potze (Ditsong National Museum of Natural History), P. V. Tobias, B. Zipfel (University of the Witwatersrand), B. Latimer, Y. Haile-Selassie, L. Jellema (Cleveland Museum of Natural History), T. Greiner (New York Chiropractic College), I. Tattersall, G. García (American Museum of Natural History), T. Daeschler, N. Gilmore (Academy of Natural Sciences of Philadelphia), and J. Cabot (Estación Biológica Doñana). A portion of this research was funded by the Leakey Foundation, Ministerio de Ciencia e Inovación of the Government of Spain Project CGL2009-12703-C03-03, and the Ray A. Rothrock ’77 Fellowship at Texas A&M University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303375110/-/DCSupplemental.
References
- 1.Carroll R. Vertebrate Paleontology and Evolution. New York: Freeman; 1988. p. 698. [Google Scholar]
- 2.Meng J, Wang Y, Li C. Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature. 2011;472(7342):181–185. doi: 10.1038/nature09921. [DOI] [PubMed] [Google Scholar]
- 3.Rosowski J. Hearing in transitional mammals: Predictions from the middle-ear anatomy and hearing capabilities of extant mammals. In: Webster D, Fay R, Popper A, editors. The Evolutionary Biology of Hearing. New York: Springer; 1992. pp. 615–632. [Google Scholar]
- 4.Masterton B, Heffner H, Ravizza R. The evolution of human hearing. J Acoust Soc Am. 1969;45(4):966–985. doi: 10.1121/1.1911574. [DOI] [PubMed] [Google Scholar]
- 5.Arensburg B, Nathan H. A propos de deux osselets de l’oreille moyenne d’un néandertaloïde trouvés a Qafzeh (Israel) L’Anthropologie (Paris) 1972;76(3–4):301–308. [Google Scholar]
- 6.Quam R, Rak Y. Auditory ossicles from southwest Asian Mousterian sites. J Hum Evol. 2008;54(3):414–433. doi: 10.1016/j.jhevol.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Rak Y, Clarke RJ. Ear ossicle of Australopithecus robustus. Nature. 1979;279(5708):62–63. doi: 10.1038/279062a0. [DOI] [PubMed] [Google Scholar]
- 8.Moggi-Cecchi J, Collard M. A fossil stapes from Sterkfontein, South Africa, and the hearing capabilities of early hominids. J Hum Evol. 2002;42(3):259–265. doi: 10.1006/jhev.2001.0524. [DOI] [PubMed] [Google Scholar]
- 9.Quam R, Martinez I, Arsuaga JL. Middle Pleistocene auditory ossicles from the Sierra de Atapuerca (Spain) Am J Phys Anthropol. 2006;42(Supp):149. [Google Scholar]
- 10. Heim JL (1982) Les Enfants Neandertaliens de La Ferrassie [The Neandertal Children from La Ferrassie] (Masson, Paris). French.
- 11.Maureille B. A lost Neanderthal neonate found. Nature. 2002;419(6902):33–34. doi: 10.1038/419033a. [DOI] [PubMed] [Google Scholar]
- 12.Frenz D, McPhee J, van de Water T. Structural and functional development of the ear. In: Jahn A, Santos-Sacchi J, editors. Physiology of the Ear. 2nd Ed. San Diego: Singular; 2001. pp. 191–214. [Google Scholar]
- 13.Mallo M. Formation of the middle ear: Recent progress on the developmental and molecular mechanisms. Dev Biol. 2001;231(2):410–419. doi: 10.1006/dbio.2001.0154. [DOI] [PubMed] [Google Scholar]
- 14.Marotti G, Farneti D, Remaggi F, Tartari F. Morphometric investigation on osteocytes in human auditory ossicles. Ann Anat. 1998;180(5):449–453. doi: 10.1016/S0940-9602(98)80106-4. [DOI] [PubMed] [Google Scholar]
- 15.Wood B. Investigating human evolutionary history. J Anat. 2000;197(Pt 1):3–17. doi: 10.1046/j.1469-7580.2000.19710003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieberman D. Testing hypothesis about recent human evolution from skulls: Integrating morphology, function, development and phylogeny. Curr Anthropol. 1995;36(2):159–197. [Google Scholar]
- 17.Lieberman D. The Evolution of the Human Head. Cambridge, MA: Harvard Univ Press; 2011. p. 756. [Google Scholar]
- 18.Clark AG, et al. Inferring nonneutral evolution from human-chimp-mouse orthologous gene trios. Science. 2003;302(5652):1960–1963. doi: 10.1126/science.1088821. [DOI] [PubMed] [Google Scholar]
- 19.Scally A, et al. Insights into hominid evolution from the gorilla genome sequence. Nature. 2012;483(7388):169–175. doi: 10.1038/nature10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spoor F, Wood B, Zonneveld F. Implications of early hominid labyrinthine morphology for evolution of human bipedal locomotion. Nature. 1994;369(6482):645–648. doi: 10.1038/369645a0. [DOI] [PubMed] [Google Scholar]
- 21.Rak Y, Clarke RJ. Aspects of the middle and external ear of early South African hominids. Am J Phys Anthropol. 1979;51(3):471–474. doi: 10.1002/ajpa.1330510320. [DOI] [PubMed] [Google Scholar]
- 22.de Ruiter DJ, Steininger CM, Berger LR. A cranial base of Australopithecus robustus from the hanging remnant of Swartkrans, South Africa. Am J Phys Anthropol. 2006;130(4):435–444. doi: 10.1002/ajpa.20386. [DOI] [PubMed] [Google Scholar]
- 23.Arensburg B, Nathan H. Observations on a notch in the short (superior or posterior) process of the incus. Acta Anat (Basel) 1971;78(1):84–90. doi: 10.1159/000143578. [DOI] [PubMed] [Google Scholar]
- 24.Rak Y. The middle ear of Australopithecus robustus. Does it bear evidence of a specialized masticatory system? In: Corruccini R, Ciochon R, editors. Integrative Paths to the Past: Paleoanthropological Advances in Honor of F. Clark Howell. Englewood Cliffs, NJ: Prentice Hall; 1994. pp. 223–227. [Google Scholar]
- 25.Rosowski J. Outer and middle ears. In: Fay R, Popper A, editors. Comparative Hearing: Mammals. New York: Springer; 1994. pp. 172–247. [Google Scholar]
- 26.McHenry H, Coffing K. Australopithecus to Homo: Transformations in body and mind. Annu Rev Anthropol. 2000;29:125–146. [Google Scholar]
- 27.Coleman MN, Ross CF. Primate auditory diversity and its influence on hearing performance. Anat Rec A Discov Mol Cell Evol Biol. 2004;281(1):1123–1137. doi: 10.1002/ar.a.20118. [DOI] [PubMed] [Google Scholar]
- 28. Quam R (2006) Temporal bone anatomy and the evolution of acoustic capacities in fossil humans. PhD dissertation [Binghamton University (State University of New York), Binghamton, NY]
- 29.Mallo M, Schrewe H, Martin JF, Olson EN, Ohnemus S. Assembling a functional tympanic membrane: Signals from the external acoustic meatus coordinate development of the malleal manubrium. Development. 2000;127(19):4127–4136. doi: 10.1242/dev.127.19.4127. [DOI] [PubMed] [Google Scholar]
- 30.Puria S, Steele C. Tympanic-membrane and malleus-incus-complex co-adaptations for high-frequency hearing in mammals. Hear Res. 2010;263(1–2):183–190. doi: 10.1016/j.heares.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemilä S, Nummela S, Reuter T. What middle ear parameters tell about impedance matching and high frequency hearing. Hear Res. 1995;85(1–2):31–44. doi: 10.1016/0378-5955(95)00031-x. [DOI] [PubMed] [Google Scholar]
- 32.Masali M, Maffei M, Borgognini Tarli SM. Application of a morphometric model for the reconstruction of some functional characteristics of the external and middle ear in Circeo 1. In: Piperno M, Scichilone G, editors. The Circeo 1 Neandertal Skull: Studies and Documentation. Rome: Instituto Poligrafico e Zecca Dello Stato; 1991. pp. 321–338. [Google Scholar]
- 33.Martínez I, et al. Auditory capacities in Middle Pleistocene humans from the Sierra de Atapuerca in Spain. Proc Natl Acad Sci USA. 2004;101(27):9976–9981. doi: 10.1073/pnas.0403595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosowski J. Models of external- and middle-ear function. In: Hawkins H, McMullen T, Popper A, Fay R, editors. Auditory Computation. New York: Springer; 1996. pp. 15–61. [Google Scholar]
- 35.Coleman MN, Colbert MW. Correlations between auditory structures and hearing sensitivity in non-human primates. J Morphol. 2010;271(5):511–532. doi: 10.1002/jmor.10814. [DOI] [PubMed] [Google Scholar]
- 36.Quam R, et al. Studying audition in fossil hominins: A new approach to the evolution of language? In: Jackson M, editor. Psychology of Language. Hauppauge, NY: Nova Science Publishers; 2012. pp. 47–95. [Google Scholar]
- 37.Coleman MN. What do primates hear? A meta-analysis of all known nonhuman primate behavioral audiograms. Int J Primatol. 2009;30(1):55–91. [Google Scholar]
- 38.Aibara R, Welsh JT, Puria S, Goode RL. Human middle-ear sound transfer function and cochlear input impedance. Hear Res. 2001;152(1–2):100–109. doi: 10.1016/s0378-5955(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 39. Spoor F (1993) The comparative morphology and phylogeny of the human bony labyrinth. PhD dissertation (Utrecht University, Utrecht, The Netherlands)
- 40.Shaw EA. Transformation of sound pressure level from the free field to the eardrum in the horizontal plane. J Acoust Soc Am. 1974;56(6):1848–1861. doi: 10.1121/1.1903522. [DOI] [PubMed] [Google Scholar]
- 41.Masali M. Dati sulla variabilitá morfometrica e ponderale degli ossicini dell’udito nell’Uomo. Arch Ital Anat Embriol. 1964;69:435–446. [PubMed] [Google Scholar]
- 42.Masali M, Micheletti Cremasco M. Hoc alterum auditus organi ossiculum est: Ear ossicles in physical anthropology. Hum Evol. 2006;21(1):1–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.