Abstract
Chitin acts as a pathogen-associated molecular pattern from fungal pathogens whose perception triggers a range of defense responses. We show that LYSIN MOTIF DOMAIN-CONTAINING GLYCOSYLPHOSPHATIDYLINOSITOL-ANCHORED PROTEIN 2 (LYM2), the Arabidopsis homolog of a rice chitin receptor-like protein, mediates a reduction in molecular flux via plasmodesmata in the presence of chitin. For this response, lym2-1 mutants are insensitive to the presence of chitin, but not to the flagellin derivative flg22. Surprisingly, the chitin-recognition receptor CHITIN ELCITOR RECEPTOR KINASE 1 (CERK1) is not required for chitin-induced changes to plasmodesmata flux, suggesting that there are at least two chitin-activated response pathways in Arabidopsis and that LYM2 is not required for CERK1-mediated chitin-triggered defense responses, indicating that these pathways are independent. In accordance with a role in the regulation of intercellular flux, LYM2 is resident at the plasma membrane and is enriched at plasmodesmata. Chitin-triggered regulation of molecular flux between cells is required for defense responses against the fungal pathogen Botrytis cinerea, and thus we conclude that the regulation of symplastic continuity and molecular flux between cells is a vital component of chitin-triggered immunity in Arabidopsis.
Keywords: PAMP-triggered immunity, cell-to-cell communication
Plant defense responses comprise a matrix of events that define disease susceptibility. Primary defense responses involve the perception of pathogen- or microbe-associated molecular patterns (PAMPs and MAMPs, respectively) by pattern-recognition receptors (PRRs) exposed on the surface of the cell. For bacterial pathogens, the Arabidopsis receptor-like kinases FLAGELLIN SENSING 2 (FLS2) and ELONGATION FACTOR-TU RECEPTOR (EFR) recognize the PAMPs flagellin and elongation factor Tu, respectively, and the cell wall component chitin is detected by cell surface receptors during the recognition of fungal pathogens. PAMP-triggered responses are known to include calcium ion influx into the cytoplasm, a rapid increase in reactive oxygen species (ROS, known as the oxidative burst), activation of MAPK, and callose deposition (1). These responses serve to alter gene transcription, produce antimicrobial metabolites, and strengthen the cell wall, all of which reduce the pathogen’s ability to invade host cells and tissues.
The lysin motif (LysM) domain-containing protein CHITIN ELICITOR BINDING PROTEIN (CEBiP) was identified as a chitin PRR in rice (2) and interacts with the LysM receptor-like kinase CHITIN ELICITOR RECEPTOR KINASE 1 (OsCERK1 for rice, CERK1 for Arabidopsis) for both chitin perception and the transmission of chitin-triggered signals (3). Arabidopsis homologs for CEBiP and OsCERK1 are LYSIN MOTIF DOMAIN-CONTAINING GLYCOSYLPHOSPHATIDYLINOSITOL-ANCHORED PROTEIN 2 (LYM2, also known as AtCEBiP) and CERK1, respectively (4, 5). CERK1 was identified as a component of the chitin perception machinery in Arabidopsis; its ectodomain is capable of binding chitin (6–8), and the intracellular kinase domain is required for the induction of chitin-triggered defense responses such as oxidative burst and MAPK activation (4). The observation that CERK1 is a target for the bacterial effector AvrPtoB (9) and functions as part of a peptidoglycan receptor system (10) indicates that it also plays a role in responses triggered by pathogens that do not contain chitin. The receptor-like proteins LYM1 and LYM3 are close relatives of LYM2 and have been identified as additional components of the peptidoglycan receptor system in Arabidopsis (10); however, unlike CERK1, they do not bind chitin (11). LYM2 was identified in chitin pull-down assays, suggesting that it either binds chitin itself or is a component of a chitin-binding protein complex (7). Despite this affinity for chitin (11), there has been no direct evidence that LYM2 functions in chitin perception.
Many plant defense responses are considered cell-autonomous processes (12, 13). However, this conflicts with the presence of cytoplasmic connections [plasmodesmata (PD)] between neighboring cells and the production of diverse and potentially mobile small molecules such as ROS, calcium ions, nitric oxide, and a range of defense-related secondary metabolites (e.g., salicylic acid, jasmonic acid, and ethylene) as part of the defense reaction. To ensure cell-specific (or noncell-autonomous) activity of these small molecules, a mechanism by which cell-to-cell communication via PD is controlled after pathogen perception could be invoked. Although it is well-established that viruses move through PD to facilitate invasion of host cells and tissues (14, 15), little is known about the role PD play in infections by other biotic pathogens. The PD protein PLASMODESMATA LOCATED PROTEIN 5 (PDLP5) was recently found to be required for resistance against Pseudomonas maculicola and to be associated with the deposition of callose at PD in this context (16). Also, in the interaction between rice cells and the blast fungus Magnaporthe oryzae, invasion hyphae seek out PD as sites to cross the cell wall (17), and the effector PWL2 moves from cell-to-cell ahead of the infection front (18). Thus, cell-to-cell movement via PD appears to play a role in determining host susceptibility and pathogen virulence for nonviral biotic pathogens. However, our understanding of this role and the contribution it makes to resistance or susceptibility is yet to be defined.
Here we have identified LYM2 as a chitin PRR that mediates a decrease in molecular flux between cells in the presence of chitin. The work identifies a reduction in cell-to-cell connectivity via PD as an uncharacterized PAMP-triggered response that, for chitin, occurs independent of the known intracellular signaling pathways used in PAMP-triggered immunity (PTI). Importantly, lym2 mutant plants show altered susceptibility to a fungal pathogen, indicating that this pathway is a key component of PTI. This study significantly advances our understanding of the role of symplastic cell-to-cell communication during pathogen perception and its potential for regulating disease outcomes.
Results
PAMPs Chitin and flg22 Trigger a Reduction in PD Flux.
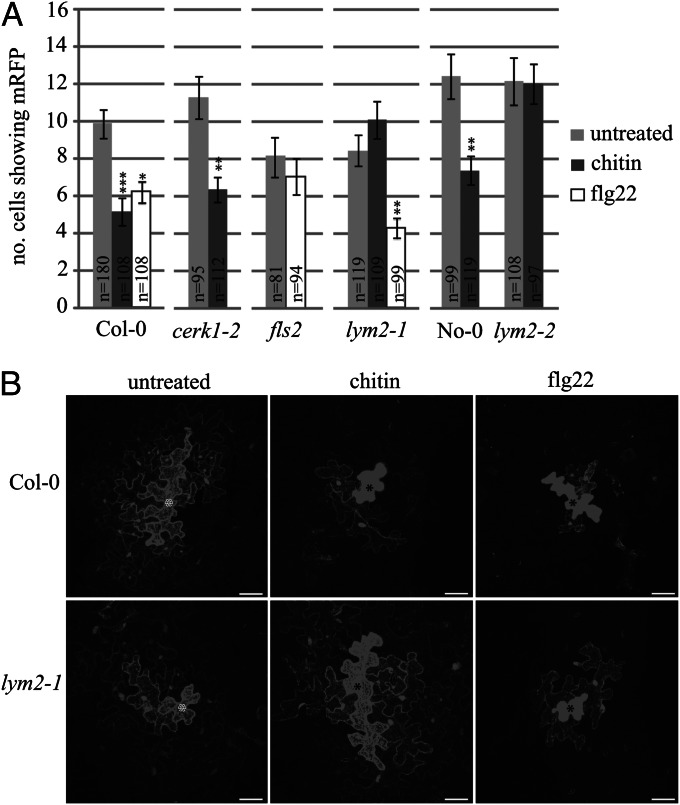
LYM2 was identified in the PD proteome (19), and therefore we hypothesized that it plays a specific role in the regulation of cell-to-cell connectivity. However, the dynamics of molecular flux through PD in the presence of chitin and other PAMPs had not been addressed. Therefore, we examined the effect of chitin and the flagellin-derived peptide flg22 on cell-to-cell movement of a constitutively expressed cytosolic marker by microprojectile bombardment of leaf tissue. This assay monitors the expression of fluorescent proteins such as GFP and monomeric red fluorescent protein (mRFP) in individual transformed cells, from where they can diffuse freely through PD and thus provide a measure of conductivity through the pore (20) (Fig. S1). Cobombardment of cDNAs for a cell-restricted protein [endoplasmic reticulum (ER)-localized mRFP (mRFPER)] with a cytosolic protein (GFP) confirmed our identification of the transformed cell within a patch of fluorescent cells (Fig. S1). Western blot analysis after PAMP treatment (Fig. S1) demonstrated that expression from the 35S promoter is not affected by chitin or flg22, allowing for a comparison of untreated and treated tissue. To determine the effect of PAMPs on intercellular flux, we bombarded leaves with mRFP and then treated tissue with either chitin or flg22. Both chitin and flg22 caused a significant reduction in cell-to-cell diffusion of mRFP relative to untreated tissue, demonstrating that molecular flux through PD in wild-type Arabidopsis plants is altered in the presence of both these PAMPs (Fig. 1).
Fig. 1.
PAMP-triggered reduction in molecular flux through PD. (A) Wild-type (Col-0 and No-0) plants and mutant lines (cerk1-2, fls2, lym2-1, and lym2-2) were bombarded with cDNA constructs capable of producing mRFP protein. Diffusion of mRFP to surrounding cells provided a measure of molecular flux through PD. Numbers of individual expression foci (n) are indicated on the bars. The lym2-2 mutant has No-0 as its parental control; all others were compared with Col-0. Error bars indicate SE. Asterisks indicate statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001. (B) Representative bombardment sites from Col-0 and lym2-1 leaves in untreated, chitin-treated, and flg22-treated tissue. Bombarded cells are indicated with an asterisk. (Scale bars, 20 μm.)
LYM2 and FLS2 Mediate PAMP-Triggered Reduction in PD flux.
The receptor-like kinases CERK1 and FLS2 are Arabidopsis PRRs for chitin and flagellin, respectively (6, 21). To critically test the influence of these PRRs and LYM2 on PD function, knockout lines in LYM2 were obtained in the Columbia (Col-0) (lym2-1) and Nossen (No-0) (lym2-2) backgrounds and compared with cerk1-2 and fls2 mutants. As observed in wild-type Col-0 plants, cerk1-2 mutant plants showed a reduction in mRFP diffusion after chitin treatment (Fig. 1), indicating that CERK1 is not necessary for chitin-triggered changes to molecular flux via PD. In contrast, diffusion of mRFP from bombardment sites was unchanged after chitin treatment in lym2-1 leaves (Fig. 1). Diffusion of mRFP in plants carrying the independent mutant allele lym2-2 also remained unchanged after chitin treatment, which differs from the response in No-0 wild-type plants (Fig. 1). Although flg22-treated lym2-1 leaves showed reduced diffusion of mRFP relative to untreated leaves (Fig. 1), fls2 leaves showed no change in mRFP diffusion in the presence or absence of flg22. These data implicate LYM2 and FLS2 independently in PD regulation after PAMP treatment.
LYM2 and CERK1 Act in Independent, Chitin-Responsive Signaling Pathways.
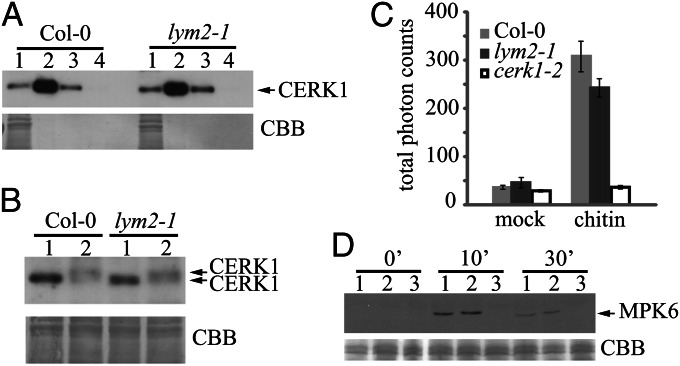
The receptor-like kinase CERK1 is required for chitin-triggered oxidative burst and MAPK activation (4, 7). To assess whether loss of LYM2 affects the chitin-binding capacity of CERK1, chitin pull-down assays using chitin beads were performed on extracts from Col-0 and lym2-1 plants. Antibody detection of CERK1 in the extracts, combined with elution with chitin oligomers, showed that binding of chitin by CERK1 is independent of LYM2 (Fig. 2A). Hence, CERK1 was captured and released by chitin equally for both extracts. Nevertheless, it remained possible that the pathway after activation by chitin may be affected. To address this, we monitored CERK1 phosphorylation, the chitin-induced oxidative burst, and MAPK activation in wild-type and lym2-1 plants. Phosphorylation of CERK1 is induced by the binding of chitin and, when monitored by changes in electrophoretic migration in gel-shift assays, was comparable in lym2-1 mutants and Col-0 (Fig. 2B). Thus, CERK1 phosphorylation does not require LYM2. Luminol-based detection of ROS in Col-0 leaves demonstrated a 10-fold increase in ROS after chitin treatment. A slightly smaller increase (six- to sevenfold) was observed in lym2-1, but this was not significantly different from Col-0. In contrast, chitin treatment of cerk1-2 showed no change in luminescence output over the untreated control (Fig. 2C). Chitin-triggered MAPK activation of MITOGEN-ACTIVATED PROTEIN KINASE 6 (MPK6) is dependent on CERK1 and was absent in cerk1-2 chitin-treated tissues. lym2-1 mutants showed phosphorylation of MPK6 similar to Col-0 at 0, 10, and 30 min after chitin treatment (Fig. 2D). In combination with the bombardment assays, these results indicate that LYM2 is not necessary for CERK1 activation and CERK1-mediated responses and vice versa, demonstrating that these two proteins respond to chitin independently.
Fig. 2.
CERK1 chitin-binding and chitin-induced responses are normal in lym2-1 mutants. (A) Total protein extracts (lane 1) from Col-0 and lym2-1 plants were applied to chitin beads and eluted with SDS-loading buffer (lane 2), chitin hexamers (lane 3), and water (lane 4). Eluted proteins were probed with anti-CERK1 antibodies. (B) Col-0 and lym2-1 plants were treated with water (lane 1) or chitin pentamers (lane 2), and total protein extracts were separated by SDS/PAGE. CERK1 was detected with anti-CERK1 antibodies in extracts from Col-0 and lym2-1 plants pretreated with chitin. lym2-1 mutants have normal oxidative burst and MAPK activation after chitin treatment. (C) Luminescence assay for ROS measured over 40 min after treatment of Col-0, lym2-1, and cerk1-2 mutants with water (mock) or chitin; n = 20 for all lines. Error bars indicate SE. (D) MAPK activation was monitored by immunodetection (Upper) of phosphorylated MPK6 0, 10, and 30 min after chitin treatment of Col-0 (lane 1), lym2-1 (lane 2), and cerk1-2 (lane 3) seedlings. Loading was visualized with Coomassie brilliant blue (CBB).
LYM2 Is a Plasma Membrane-Located Protein with PD Association.
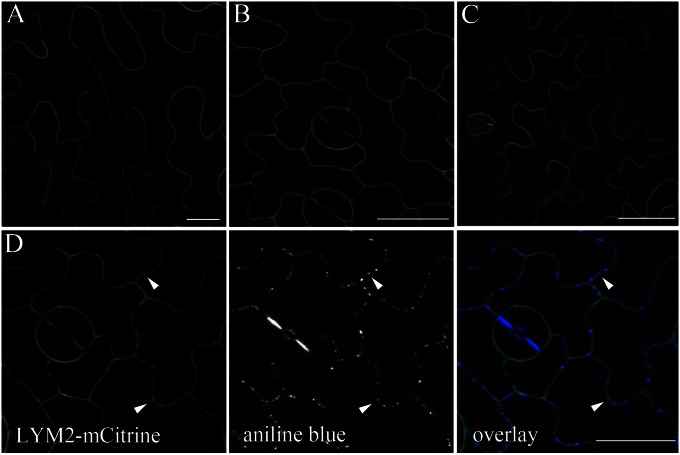
LYM2 is one member of a three-member protein family and was identified in protein extracts from purified PD (19). To determine their subcellular localization and considering that LYM1 and LYM2 are predicted GPI-anchored proteins (5), LYM1, LYM2, and LYM3 were cloned as translational fusions expressing the homologous proteins fused internally to fluorescent monomeric Citrine (mCitrine) in Arabidopsis. Fluorescence microscopy of transgenic lines revealed that LYM1-mCitrine (LYM1-mCit), LYM2-mCitrine (LYM2-mCit), and LYM3-mCitrine (LYM3-mCit) are located at the plasma membrane (Fig. 3); LYM3 was also observed in the ER. In contrast to LYM1-mCit and LYM3-mCit, LYM2-mCit was distributed unevenly in the plasma membrane. Patches of increased LYM2-mCit fluorescence corresponded with aniline blue-stained spots of PD-associated callose (Fig. 3). This was also observed when the construct was transiently expressed in Nicotiana benthamina leaves (Fig. S2). After our observation that FLS2 mediates flg22-triggered closure of PD, we carefully examined leaves of transgenic lines expressing FLS2-GFP (22) and found unevenly distributed signals in the plasma membrane. Similar to LYM2-mCit, bright domains of fluorescence corresponded with aniline blue-stained PD-associated callose (Fig. S2).
Fig. 3.
LYM proteins localize to the plasma membrane. Internal fusions of LYM1 (A), LYM2 (B), and LYM3 (C) to mCitrine show that each protein is located at the plasma membrane. (D) The fluorescence associated with LYM2 is uneven in the membrane; patches of increased fluorescence colocalize with aniline blue-stained, PD-associated callose (arrowheads). (Scale bars, 20 μm.)
LYM2 Is Required for Resistance to Botrytis cinerea.
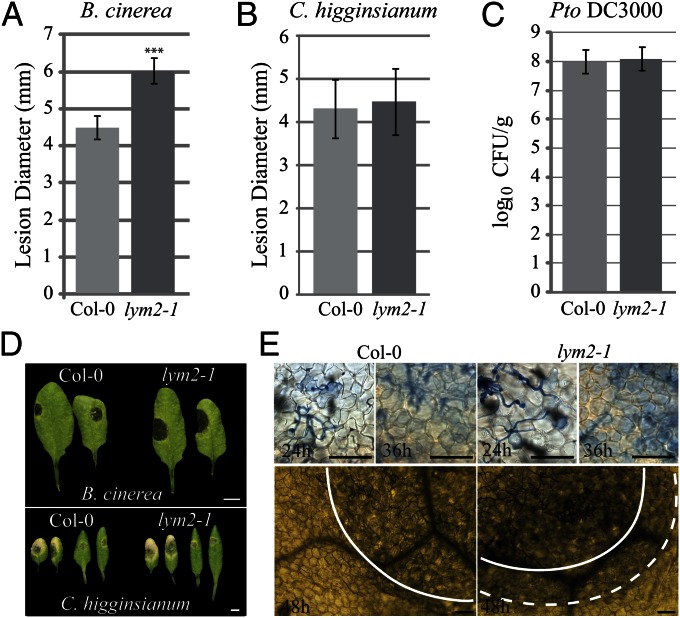
To ascertain whether or not chitin-induced regulation of cell-to-cell connectivity contributes to the development of infection, we assayed for increased resistance or susceptibility to two fungal pathogens and one bacterial pathogen in the lym2-1 line. Pathogenicity assays with B. cinerea demonstrated that relative to Col-0, lym2-1 developed larger disease lesions 3 days post inoculation (dpi) (Fig. 4 A and D). Trypan blue staining of inoculated leaves indicated that at the early stages of infection [24 and 32 hours post inoculation (hpi)], there was no difference between Col-0 and lym2-1 infection sites. At 24 hpi, epidermal cells beneath penetrating hyphae stained blue, indicating cell death. At 32 hpi, mesophyll cells beneath the infection site (defined microscopically by the presence of fungal hyphae on the leaf surface) showed evidence of cell death. In contrast, at 48 hpi, mesophyll cell death in lym2-1 leaves had spread beyond the infection site but was restricted beneath the infection site in Col-0 leaves (Fig. 4E).
Fig. 4.
lym2-1 mutants have increased susceptibility to B. cinerea but not C. higginsianum and Pto DC3000. B. cinerea (A) or C. higginsianum (B) was drop-inoculated on leaves of Col-0 or lym2-1 plants. Lesion diameter measured after 3 d for B. cinerea (D) or 5 d for C. higginsianum (D). (C) Col-0 and lym2-1 were spray-inoculated with Pto DC3000 and bacterial growth assessed as colony counts (log10 cfu/g) determined 2 dpi from pooled extracts (2 plants per count). Error bars indicate SD. Asterisks indicate statistical significance: ***P < 0.001. (E) Trypan blue-stained leaves infected with B. cinerea showed that at 24 and 36 hpi, infection sites appeared the same in Col-0 and lym2-1, showing a similar degree of cell death in the epidermis (24 h) and mesophyll (36 h). At 48 hpi, mesophyll cell death in Col-0 leaves was restricted to the site of infection (solid line), but in lym2-1 leaves, it was observed beyond this boundary (dotted line). [Scale bars, 0.5 cm (D) and 100 μm (E)].
Colletotrichum higginsianum employs a similar infection mechanism to M. oryzae: invasion hyphae cross between cells at PD (23). To examine the effect of a chitin-responsive PD regulator on this mode of intercellular spread, we performed pathogenicity assays with C. higginsianum. In contrast to the difference in susceptibility to B. cinerea, lesions (5 dpi) that developed after drop inoculation on lym2-1 mutant leaves were similar in size and appearance to those that developed on Col-0 leaves (Fig. 4 B and D). Lesions on leaves of cerk1-2 mutants also showed no difference to lesions on Col-0 (Fig. S3).
As our bombardment assays indicate that LYM2 responds to chitin but not to flg22, we hypothesized that LYM2 is not required for PTI against a pathogen that does not display chitin. To test this, we assayed for pathogenicity of Pseudomonas syringae pv. tomato DC3000 (Pto DC3000). Colony counts showed that there was no difference in bacterial growth on Col-0 and lym2-1 plants at 2 dpi (Fig. 4C).
Discussion
It is well established that the earliest defense responses to bacterial and fungal pathogens are triggered by the activity of different PRRs on binding to specific PAMPs. In rice, chitin perception employs the receptor-like protein CEBiP, but a similar function for its Arabidopsis homolog, LYM2, has been elusive despite evidence that LYM2 is a chitin-binding protein (7, 11). This study has identified that LYM2 has a specific role that mediates a reduction in molecular flux through PD in the presence of chitin. This implicates changes to cell-to-cell connectivity as an integral component of plant defenses against fungal pathogens.
Both chitin and flg22 elicit changes to intercellular flux. LYM2 mediates the chitin-triggered response, but the flg22-triggered response is mediated through its cognate PRR FLS2, demonstrating ligand specificity (Fig. 1). PAMP-triggered changes to molecular flux infer that PAMP responses include changes to symplastic domains and the movement of molecules between cells. Significantly, the chitin-induced reduction in PD flux observed in this study is independent of other chitin-triggered responses. lym2-1 mutant plants are incapable of chitin-triggered PD flux decreases but exhibit wild-type chitin-triggered MAPK activation, oxidative burst, CERK1 chitin-binding, and chitin-triggered CERK1 phosphorylation (Fig. 2). These results support previous work that demonstrated that chitin-triggered, CERK1-dependent increases in gene expression are normal in a lym1,2,3 triple knockout (11). Chitin-induced PD flux reduction occurs in cerk1-2 mutant plants, indicating that as LYM2 is not required for CERK1-mediated responses, neither is CERK1 required for LYM2-mediated responses (Fig. 1). These two proteins function in independent chitin-response pathways.
LYM2 is predicted to be a GPI-anchored receptor-like protein with no intracellular domains. Therefore, LYM2 activity must be restricted to the extracellular space or be mediated by another protein or proteins that activates an intracellular signaling pathway, leading to a reduction in PD aperture. Although not exclusively localized to PD, LYM2 does show increased association with the plasma membrane in the vicinity of PD. In combination with its extraction from purified PD (19), this indicates that it is resident in PD membranes. Whether or not LYM2 has an alternate function in the non-PD plasma membrane remains to be determined. The finding here, that FLS2 mediates flg22-triggered changes in cell-to-cell flux, suggests that there are also unconsidered facets to FLS2 activity. Similar to LYM2, FLS2 was observed in the vicinity of PD, and its regulation of PD flux suggests either a site-specific function for FLS2 or a downstream signaling component.
It would be expected that LYM2 is required for defense responses against fungal, but not bacterial, pathogens. Indeed, LYM2 is not required for defense against Pto DC3000. Our data indicate that although LYM2 is required for defense against the fungal pathogen B. cinerea, it does not contribute to interactions with C. higginsianum. It is possible that this difference arises from differences in lifestyle (B. cinerea is a necrotroph and C. higginsianum is a hemibiotroph) and infection mechanism, but we observed that cerk1-2 mutants are also equally susceptible to C. higginsianum compared with Col-0. This suggests that a general failure of chitin perception is responsible for the absence of a difference in susceptibility between Col-0 and lym2-1 mutants to C. higginsianum. It is possible that, similar to Cladosporium fulvum and M. oryzae, C. higginsianum is capable of evading chitin detection (24, 25).
Trypan blue staining of B. cinerea infections revealed that before the appearance of measurable necrotic lesions, mesophyll cell death had spread beyond the site of infection in lym2-1 mutants but remained restricted in Col-0. A similar difference in the spread of mesophyll cell death was observed in wrky33 mutants (26), which have enhanced expression of genes involved in the salicylic acid signaling pathway. Increased spread of cell death in lym2-1 may arise as a direct result of the inability of lym2-1 cells to regulate the flux of defense-associated signals that trigger cell death, such as salicylic acid.
We have determined that intercellular flux is reduced by the PAMPs chitin and flg22, and that this is required for the deployment of a full suite of defense responses against a fungal pathogen. The corollary of this finding is that signals must be transmitted between cells to trigger or suppress cellular defense responses. Given the plethora of small, defense-associated molecules involved in plant defense, it must be determined which of these have a role in intercellular signaling. With respect to the different mechanisms of chitin perception between rice and Arabidopsis, it remains to investigate chitin-triggered PD closure in rice and whether this is mediated by the CEBiP/OsCERK1 complex or by another member of the lysin motif-containing protein family (27). The purpose served by a reduction in cell-to-cell communication during defense responses remains to be explained, but through its identification and its protein mediator, we can now actively pursue deeper questions relating to PD function during plant–pathogen interactions.
Materials and Methods
Plant Material.
lym2-1 is SAIL_343_B03 in the Columbia (Col-0) background and lym2-2 is 11-4398-1 (Riken) in the Nossen (No-0) background. cerk1-2 is GABI_096F09 (4) in the Col-0 background. fls2 is SAIL_691_C04 in the Col-0 background. Plants were grown in short-day conditions (10 h light, 14 h dark) for all experiments.
DNA Constructs and Transgenic Plants.
LYM1-mCit, LYM2-mCit, and LYM3-mCit were generated by insertion of mCitrine downstream of the predicted signal peptide at positions 78, 72, and 75 bp, respectively. Gene fusions were generated by overlap PCR and then cloned by Gateway cloning (Invitrogen) into the pB7WG2.0 expression vector. These constructs were used to generate stably expressing Arabidopsis by floral dipping. mRFP was cloned into pB7WG2.0 for bombardment assays.
Chemicals.
Chitin oligosaccharides were purchased from Yaizu Suisankagaku, chitin magnetic beads from New England Biolabs, and chitin pentamers and hexamers from IsoSep. flg22 peptides were obtained from Peptron.
Cell Biology and Microscopy.
Confocal microscopy was performed on a Leica SP5 or a Zeiss LSM 510 Meta confocal microscope with a 25× water-dipping lens (HCX IRAPO 25.0 × 0.95 water), a 40× oil immersion lens (HCX PLAPO CS 40.0 × 1.25 OIL), or a 63× oil immersion lens (Plan-APOCHROMAT 63×/1.4 oil). mCitrine was excited with a 488- or 514-nm argon laser and collected at 525–560 nm. mRFP was excited with a DPSS laser and collected at 580–610 nm. For callose staining, aniline blue (0.1 mg/mL) was infiltrated into 4- to 5-wk-old leaves up to 2 h before imaging. The aniline blue fluorochrome was excited with a 405-nm laser and collected at 440–490-nm. Dual labeling with aniline blue and mCitrine was imaged by sequential scanning. For trypan blue staining, infected leaf material was boiled in trypan blue in lactophenol and cleared with chloral hydrate (28). Material was imaged on a Leica DM6000 microscope.
Microprojectile Bombardment.
Microprojectile bombardment assays were performed as described (20). Four- to 6-wk-old expanded leaves of relevant Arabidopsis lines were bombarded with gold particles coated with pB7WG2.0.mRFP, using a Bio-Rad Biolostic PDS-1000/He particle delivery system. Bombardment sites were imaged 24 h postbombardment by confocal microscopy. For PAMP treatment, 500 μg/mL chitin oligosaccharides or 100 nM flg22 was infiltrated into bombarded leaves 4 h postbombardment. For each treatment, data were collected from at least 3 independent bombardment events, each of which consisted of leaves from at least two individual plants. Statistical nonparametric Mann–Whitney analysis was performed using GraphPad InStat software.
Pull-Down and Gel-Shift Assays.
Total protein was extracted from Arabidopsis leaves as described previously (7). For each sample, 1 mg total protein was bound to chitin magnetic beads. Chitin-bound protein was eluted with SDS-loading buffer, 1 mM chitin hexamers, or water. Eluted proteins were probed with anti-CERK1 antibodies (9) by Western blot analysis. For gel-shift analysis, leaves were pretreated with 100 µg/mL chitin pentamers before protein extraction.
MAPK Activation.
MAPK activation assays were performed as described (29). Briefly, seeds were germinated on MS and 7-d-old seedlings were transferred to liquid culture. At 14 d, plants were treated with 500 μg/mL chitin oligosaccharides and harvested at 0, 10, and 30 min after chitin treatment. MAPK activation was determined by Western blot analysis with Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit monoclonal antibodies (Cell Signaling). Equal loading was verified by Coomassie blue staining.
Oxidative Burst.
ROS were elicited by treatment of leaf discs taken from 5-wk-old Arabidopsis plants with 100 μg/mL chitin or water and detected as described (9). Luminescence was detected for 40 min, using an intensified charge coupled device photon counting camera (Photek).
Pathogen Infection.
B. cinerea spores (2.5 × 105 spores/mL) were drop-inoculated on expanded leaves of 5-wk-old Arabidopsis plants, and developing disease lesions were measured 3 d postinoculation. Six leaves per plant were inoculated to provide 6 measurements per plant, and 3 replicate experiments, each containing 20 individuals, were performed.
C. higginsianum spores (2 × 106 spores/mL) were drop-inoculated on 4- to 5-wk-old Arabidopsis plants, and the diameter of necrotic lesions was measured 5 d postinoculation. Six leaves per plant were inoculated to provide 6 measurements per plant, and 3 replicate experiments, each containing 15 individuals, were performed.
P. syringae pv. tomato DC3000 was spray-inoculated on 5-wk-old Arabidopsis plants. Whole plants were harvested 2 d postinoculation. Two individual plants were combined for a single measurement, and 10 measurements were taken per replicate experiment. Three replicate experiments were performed.
Supplementary Material
Acknowledgments
The authors thank Chris Ridout and Cyril Zipfel for providing chitin oligosaccharides, flg22 peptides, and critical comments on the manuscript; and Henk-jan Schoonbeek, Yang Zhang, Heidrun Haweker, Jun Fan, Cecile Segonzac, and Freddy Boutrot for technical advice and assistance. The John Innes Centre is supported by the Biotechnology and Biological Science Research Council, and the S.R. laboratory is supported by the Gatsby Charitable Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
4Retired.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203458110/-/DCSupplemental.
References
- 1.Zipfel C. Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol. 2009;12(4):414–420. doi: 10.1016/j.pbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Kaku H, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA. 2006;103(29):11086–11091. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu T, et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 2010;64(2):204–214. doi: 10.1111/j.1365-313X.2010.04324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miya A, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104(49):19613–19618. doi: 10.1073/pnas.0705147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fliegmann J, et al. Biochemical and phylogenetic analysis of CEBiP-like LysM domain-containing extracellular proteins in higher plants. Plant Physiol Biochem. 2011;49(7):709–720. doi: 10.1016/j.plaphy.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Iizasa E, Mitsutomi M, Nagano Y. Direct binding of a plant LysM receptor-like kinase, LysM RLK1/CERK1, to chitin in vitro. J Biol Chem. 2010;285(5):2996–3004. doi: 10.1074/jbc.M109.027540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petutschnig EK, Jones AME, Serazetdinova L, Lipka U, Lipka V. The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. J Biol Chem. 2010;285(37):28902–28911. doi: 10.1074/jbc.M110.116657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, et al. Chitin-induced dimerization activates a plant immune receptor. Science. 2012;336(6085):1160–1164. doi: 10.1126/science.1218867. [DOI] [PubMed] [Google Scholar]
- 9.Gimenez-Ibanez S, et al. AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol. 2009;19(5):423–429. doi: 10.1016/j.cub.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Willmann R, et al. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc Natl Acad Sci USA. 2011;108(49):19824–19829. doi: 10.1073/pnas.1112862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinya T, et al. Functional characterization of CEBiP and CERK1 homologs in arabidopsis and rice reveals the presence of different chitin receptor systems in plants. Plant Cell Physiol. 2012;53(10):1696–1706. doi: 10.1093/pcp/pcs113. [DOI] [PubMed] [Google Scholar]
- 12.Dodds PN, Rathjen JP. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11(8):539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 13.Lipka U, Fuchs R, Kuhns C, Petutschnig E, Lipka V. Live and let die—Arabidopsis nonhost resistance to powdery mildews. Eur J Cell Biol. 2010;89(2-3):194–199. doi: 10.1016/j.ejcb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Calvino L, Faulkner C, Maule AJ. 2011. Plasmodesmata as active conduits for virus cell-to-Cell movement. Recent Advances in Plant Virology, ed Caranta C, Aranda MA, Tepfer M, Lopez-Moya JJ (Caister Academic Press, Norwich, UK), pp 47–74.
- 15.Benitez-Alfonso Y, Faulkner C, Ritzenthaler C, Maule AJ. Plasmodesmata: Gateways to local and systemic virus infection. Mol Plant Microbe Interact. 2010;23(11):1403–1412. doi: 10.1094/MPMI-05-10-0116. [DOI] [PubMed] [Google Scholar]
- 16.Lee J-Y, et al. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell. 2011;23(9):3353–3373. doi: 10.1105/tpc.111.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kankanala P, Czymmek K, Valent B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell. 2007;19(2):706–724. doi: 10.1105/tpc.106.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khang CH, et al. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell. 2010;22(4):1388–1403. doi: 10.1105/tpc.109.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Calvino L, et al. Arabidopsis plasmodesmal proteome. PLoS ONE. 2011;6(4):e18880. doi: 10.1371/journal.pone.0018880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol. 2008;6(1):e7. doi: 10.1371/journal.pbio.0060007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gómez-Gómez L, Boller T. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5(6):1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 22.Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell. 2012;24(10):4205–4219. doi: 10.1105/tpc.112.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao C-Y, et al. Characterization of three Colletotrichum acutatum isolates from Capsicum spp. Eur J Plant Pathol. 2012;133:599–608. [Google Scholar]
- 24.Mentlak TA, et al. Effector-mediated suppression of chitin-triggered immunity by magnaporthe oryzae is necessary for rice blast disease. Plant Cell. 2012;24(1):322–335. doi: 10.1105/tpc.111.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jonge R, et al. Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science. 2010;329(5994):953–955. doi: 10.1126/science.1190859. [DOI] [PubMed] [Google Scholar]
- 26.Birkenbihl RP, Diezel C, Somssich IE. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 2012;159(1):266–285. doi: 10.1104/pp.111.192641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, et al. Lysin motif-containing proteins LYP4 and LYP6 play dual roles in peptidoglycan and chitin perception in rice innate immunity. Plant Cell. 2012;24(8):3406–3419. doi: 10.1105/tpc.112.102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch E, Slusarenko A. Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell. 1990;2(5):437–445. doi: 10.1105/tpc.2.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutrot F, et al. Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proc Natl Acad Sci USA. 2010;107(32):14502–14507. doi: 10.1073/pnas.1003347107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.