Abstract
Establishment and maintenance of apico-basolateral trafficking pathways are critical to epithelial homeostasis. Loss of polarity and trafficking fidelity are thought to occur as a consequence of transformation; however, here we report that selective mistrafficking of the epidermal growth factor receptor (EGFR) ligand epiregulin (EREG) from the basolateral to the apical cell surface drives transformation. Normally, EREG is preferentially delivered to the basolateral surface of polarized Madin-Darby canine kidney cells. EREG basolateral trafficking is regulated by a conserved tyrosine-based basolateral sorting motif in its cytoplasmic domain (YXXΦ: Y156ERV). Both Y156 and V159 are required for basolateral sorting of EREG, because Y156A and V159G substitutions redirect EREG to the apical cell surface. We also show that basolateral sorting of EREG is adaptor protein 1B–independent. Apical mistrafficking of EREG has a distinctive phenotype. In contrast to transient EGFR tyrosine phosphorylation after basolateral EREG stimulation, apical EREG leads to prolonged EGFR tyrosine phosphorylation, which may be related, at least in part, to a lack of negative regulatory Y1045 phosphorylation and subsequent ubiquitylation. Notably, Madin-Darby canine kidney cells stably expressing apically mistrafficked EREG form significantly larger, hyperproliferative, poorly differentiated, and locally invasive tumors in nude mice compared with WT EREG-expressing cells.
Keywords: epithelial transformation, growth factor trafficking, receptor tyrosine kinase, protein sorting
Under normal physiological conditions, binding of endogenous ligand to EGF receptor (EGFR) is required for initiation of signal transduction. In polarized epithelial cells, EGFR is thought to be restricted to the basolateral surfaces (1). All seven EGFR ligands are produced as type 1 transmembrane proteins (2). Newly synthesized ligands are inserted into the plasma membrane, whereupon they undergo ectodomain cleavage to release soluble growth factor. We previously studied the biosynthesis and trafficking of EGF, TGF-α (TGFA), and amphiregulin (AREG) in polarized Madin-Darby canine kidney (MDCK) cells (3–5). These ligands have distinct motifs that govern their basolateral sorting (6–8). Of clinical relevance is a germline mutation in the basolateral sorting motif of EGF in individuals with isolated renal hypomagnesemia (9). In this autosomal recessive disorder, loss of EGF delivery to the basolateral surface impairs EGFR activity in the distal convoluted tubules, which is required for efficient magnesium absorption.
Here we undertook the study of another EGFR ligand, epiregulin (EREG). EREG is proteolytically cleaved by a disintegrin and a metalloprotease 17 (ADAM17) to release soluble ligand that binds to and activates ERBB1/EGFR and ERBB4/Her4 (2, 10, 11). Expression of EREG in adults is restricted (12–14), but EREG is overexpressed in a number of human breast and colorectal cancer cell lines and tumors (15–19). EREG also has been implicated as a “metastasis driver” in breast cancer cells selected to metastasize to the lung (20).
Given the importance of EREG in normal physiology and disease, we undertook studies to delineate the trafficking of EREG to the cell surface in polarized MDCK cells. We found that EREG is delivered directly to the basolateral surface. During these studies, we uncovered a tyrosine-based YXXΦ basolateral sorting motif in the 29-aa cytoplasmic domain of EREG that acts at the level of biosynthetic delivery. Moreover, mistrafficking of EREG to the apical surface of MDCK cells results in large, hyperproliferative, locally invasive tumors, which may be related, at least in part, to sustained EGFR signaling by apical EREG. We propose that apical mistrafficking of EREG crystallizes an apical EGFR signaling complex that may be uncoupled from basolateral regulatory restraints.
Results and Discussion
EREG Localizes to the Basolateral Cell Surface: Characterization of Its Basolateral Sorting Motif.
To examine cell surface trafficking of EREG in polarized epithelial cells, we stably expressed a full-length human EREG coding sequence with a carboxyl-terminal EGFP tag in MDCK-II cells (hereafter referred to as MDCK cells). Polarized MDCK cells showed predominantly basolateral localization of the EREG-EGFP chimera (Fig. 1A, Left). GFP fluorescence was detected along the lateral membrane with punctate staining in the subapical region that did not colocalize with the apical marker gp135. Selective cell surface biotinylation confirmed that more than 90% of EREG-EGFP was localized to the basolateral cell surface (Fig. 1A, Center). mCherry-tagged and untagged EREG also localized to the basolateral cell surface (Fig. S1 A and B).
Fig. 1.
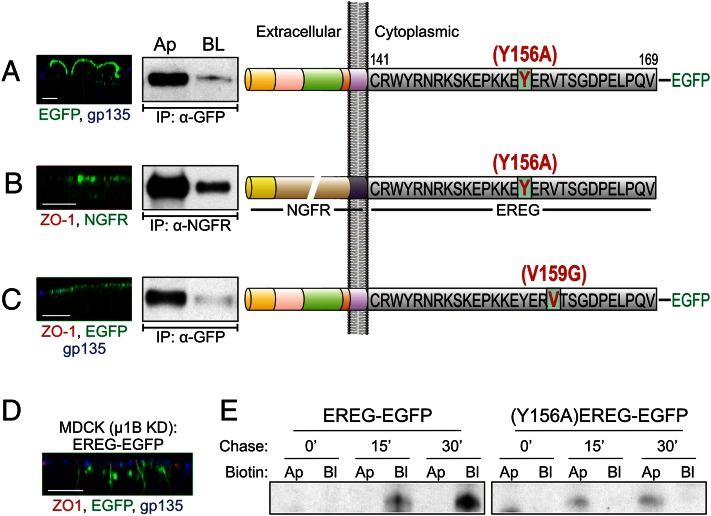
Cytoplasmic domain of EREG contains a dominant-acting basolateral sorting motif. (A) (Right) Domain organization of human EREG. The 169-aa pre-propeptide consists of a 29-aa signal peptide, a 46-aa mature region, a juxtamembrane region, a 21-aa transmembrane domain, and a 29-aa cytoplasmic domain. (Left) Polarized MDCK cells stably expressing an EREG-EGFP chimera were fixed and immunostained for ZO-1 (red) and gp135 (blue). Confocal projections in xz planes are shown. (Center) Selective cell surface biotinylation of EREG-EGFP labeled on apical (Ap) and basolateral (BL) cell surfaces. Total protein levels confirm equal loading. A similar display is shown in B–D. (B) MDCK cells stably expressing an EREG∆CT-EGFP construct that lacks the terminal 25 residues of the EREG cytoplasmic domain were processed as in A. (C) The cytoplasmic domain of an apical protein, NGFR, was replaced with the cytoplasmic domain of EREG; the resulting chimera (NGFR-EREG CT) was stably expressed in polarized MDCK cells, processed, and displayed as in A. (D) MDCK cells stably expressing successive 5-aa truncations of the EREG cytoplasmic domain (CT1–4) were processed to assess steady-state localization of EREG. (Scale bars: 10 µm.)
On deletion of the terminal 25 of 29 residues of the EREG cytoplasmic domain (EREG∆CT-EGFP), nearly complete relocalization of EREG from the basolateral to the apical cell surface was seen (Fig. 1B, Left, immunofluorescence; Center, biotinylation). Such a reversal of sorting was not previously observed after disruption of the basolateral sorting motifs of TGFA and AREG (6, 8), suggesting the presence of a recessive apical signal in the extracytoplasmic region of EREG. Extracellular glycosylation is a known apical sorting signal (21), and we found that EREG is N-glycosylated at asparagine 47 (Fig. S2 A and B). However, chemical (tunicamycin) or genetic (N47A/D) abrogation of glycosylation did not affect apical trafficking of mutant EREG, indicating that N47 glycosylation is not the EREG apical sorting signal (Fig. S2 C and D).
We next tested whether the EREG cytoplasmic domain was sufficient for basolateral localization. Nerve growth factor receptor (NGFR/p75NTR) is an apical protein whose localization is dependent on O-glycosylation in the extracellular juxtamembrane region (Fig. S3) (22). When the cytoplasmic domain of NGFR was replaced with the EREG cytoplasmic domain, the resulting chimeric protein (NGFR-EREG CT) was localized exclusively to the basolateral cell surface by immunofluorescence and selective cell surface biotinylation (Fig. 1C). Thus, the cytoplasmic domain of EREG is necessary and sufficient for its basolateral localization, and the EREG basolateral sorting motif is dominant over the NGFR apical sorting motif.
To identify the basolateral sorting motif in the cytoplasmic domain of EREG, we sequentially removed five amino acids from the EREG cytoplasmic domain (Fig. 1D). GFP fluorescence and biotin labeling were observed at the basolateral cell surface after removal of five (CT1) and 10 (CT2) amino acids (Fig. 1D). However, removal of an additional five amino acids (CT3) resulted in reversion of both GFP fluorescence and biotin labeling from the basolateral to apical cell surfaces (Fig. 1D). Subsequent truncations (CT4 and ∆CT) also localized to the apical cell surfaces (Fig. 1D). These results show that residues 155–159 (EYERV) contain essential basolateral sorting information.
EREG Biosynthetic Delivery Is Regulated by Its YXXΦ Motif.
Residues 155–159 (EYERV) contain a YXXΦ motif that in epithelial cells is most often recognized by the clathrin adaptor protein 1B (AP-1B) and AP-2 for basolateral delivery and endocytic sorting, respectively (23). Both tyrosine 156 (Tyr156) and valine 159 (Val159) residues of EREG are conserved across all known species (Fig. S4A). A Tyr156-to-alanine (Y156A) EREG mutant localized to the apical cell surface, as determined by immunofluorescence and selective cell surface biotinylation (Fig. 2A). Likewise, an analogous Y156A substitution in the NGFR-EREG CT chimera also led to apical localization of the chimera, the normal location of WT NGFR (Fig. 2B).
Fig. 2.
Basolateral localization of EREG is AP-1B–independent but depends on a YXXΦ-based basolateral motif for regulation of its biosynthetic delivery. (A) EREG-EGFP constructs were mutated at Tyr-156 to alanine. (Right) The resulting (Y156A)EREG-EGFP was stably expressed and examined in polarized MDCK cells. (Left) Confocal xz projections showing EREG (green) and gp135 (blue) localization. (Center) Selective cell surface biotinylation. (B) The Y156A mutation was introduced into the NGFR-EREG CT chimera shown in Fig. 1C; the resulting chimera [NGFR-(Y156A)EREG CT] was stably expressed in polarized MDCK cells. Confocal projections in the xz plane (Left) and selective cell surface biotinylation (Center) are shown. (C) EREG-EGFP constructs were mutated at Val159 to Gly (occupying the Φ position in the YXXΦ motif); the resulting (V159G)EREG-EGFP was stably expressed and examined in polarized MCDK cells. Confocal and biotinylation data from these cells are shown as in A. (D) Parental MDCK cells with stable µ1B knockdown were transfected with EREG-EGFP constructs and polarized on Transwell filters. The xz confocal projections show GFP fluorescence (green) along with immunostaining for ZO-1 (red) and gp135 (blue). (E) Polarized EREG-EGFP and (Y156A)EREG-EGFP MDCK cells were pulse-labeled for 20 min and then chased for the indicated times. After the pulse-chase, cells were biotinylated on either the apical (Ap) or basolateral (BL) cell surfaces and lysed. Lysates were sequentially immunoprecipitated with GFP antibody, followed by affinity purification with streptavidin agarose beads. Lysates were subsequently resolved on SDS/PAGE (8–12% gradient) and dried, and autoradiographs were developed. These radiographs show the newly synthesized cell surface EREG species at the indicated chase times.
Both Y and Φ residues are required for interaction with the µ subunits of the AP adaptor complexes (23). We confirmed the requirement of the conserved distal Val residue occupying the Φ position for EREG basolateral sorting. A V159G substitution also led to predominant apical localization of EREG (Fig. 2C). V159A substitution was largely basolateral, likely because it retained the hydrophobicity required at the Φ position (Fig. S4B). Charged substitutions with lysine and aspartate are predominantly apical (Fig. S4 B and C). Taken together, these results show that Val159 is required for EREG basolateral sorting.
Medium subunit 1B (μ1B) is the cargo recognition component of the AP-1B heterotetramer, and cells lacking µ1B are AP-1B–deficient (24). EREG localized to the basolateral cell surface in µ1B knockdown MDCK cells (Fig. 2D) and µ1B null Lilly Laboratories cell porcine kidney 1 (LLC-PK1) cells (Fig. S5 A and B). Thus, EREG basolateral sorting is AP-1B–independent. To determine whether newly synthesized WT EREG and Y156A EREG are delivered directly to the basolateral and apical cell surfaces, respectively, we performed metabolic labeling pulse-chase analysis with selective cell surface biotinylation. Newly synthesized WT EREG was delivered to the basolateral cell surface, and the mutant was delivered to the apical cell surface, within 15 min of chase, indicating that Y156 is required for basolateral biosynthetic delivery of EREG (Fig. 2E). In addition, the rapid delivery of EREG argues against the involvement of AP-2 at the level of endocytic sorting. Nonetheless, these findings do not rule out a role for AP-1B during recycling or roles for other AP family members, including AP-1A and AP-4, which have been shown to regulate basolateral sorting in MDCK cells (25–27).
Sustained Phosphorylation of EGFR by Apical EREG.
EGFR is generally thought to be restricted to the basolateral cell surface such that in an intact monolayer, an apically released ligand will not have access to basolateral EGFRs (1, 28, 29). However, we found low, but detectable, levels of endogenous EGFR at the apical cell surface by selective cell surface biotinylation of polarized MDCK cells (Fig. 3A). Furthermore, in response to apical EREG stimulation, we observed weak but sustained levels of EGFR tyrosine phosphorylation. In contrast, we found strong, yet transient, EGFR tyrosine phosphorylation after basolateral EREG stimulation (Fig. 3B). Interestingly, the ratio of EGFR tyrosine phosphorylation after apical and basolateral EREG stimulation reflects the relative abundance of EGFR at the two surfaces (compare Fig. 3B with 3A). This suggests that apical and basolateral pools of EGFR may be activated by apical or basolateral EREG, respectively, but they differ in their ability to attenuate the signal after activation. Intriguingly, EREG-induced Y1045 EGFR phosphorylation was not detected after apical stimulation, whereas it was present after basolateral stimulation (Fig. 3C). Y1045-EGFR phosphorylation is recognized by the E3 ligase casitas B-lineage lymphoma proto-oncogene (CBL), which then targets EGFR for K63-mediated lysosomal degradation (30). We also observed ubiquitylation of EGFR after basolateral EREG stimulation, but not after apical EREG stimulation (Fig. 3C). Although we cannot exclude the possibility that the absence of Y1045 phosphorylation after apical EREG stimulation may reflect a low abundance of apical EGFR, this absence does provide one possible explanation for prolonged EGFR phosphorylation. Alternatively, differential availability of other negative regulators (e.g., LRIG1, ERRFI1/MIG6, and PTPN1/PTP1B) at the apical compartment versus the basolateral compartment may contribute to prolonged EGFR phosphorylation after the addition of apical EREG (31, 32).
Fig. 3.
Sustained EGFR activation by apical EREG. (A) (Upper) Polarized MDCK cells were biotinylated on apical (Ap) or basolateral (BL) cell surfaces, lysed and immunoprecipated for EGFR, resolved on SDS/PAGE, blotted, and probed with HRP-streptavidin. (Lower) Total EGFR levels. (B) Polarized MDCK cells were stimulated with 100 ng/mL EREG on apical (Upper) or basolateral (Lower) cell surfaces for the indicated times. Cells were then lysed and immunoprecipated for EGFR, resolved on SDS/PAGE, blotted, and probed for phosphotyrosine (4G10). (C) Polarized MDCK cells were stimulated with 100 ng/mL EREG for the indicated times. (Top) Lysates from these cells were resolved on SDS/PAGE, blotted, and probed for pY1045 EGFR. (Upper Middle) Lysates from these cells were immunoprecipated for EGFR, resolved on SDS/PAGE, blotted, and probed for ubiquitin. (Lower Middle) Membranes from the preceding row were reprobed for total EGFR levels. (Bottom) Equivalent amounts of lysates from these cells were resolved on SDS/PAGE, blotted, and probed for tubulin.
Apical Mistrafficking of EREG Results in Enhanced Transformation in Vitro and in Vivo.
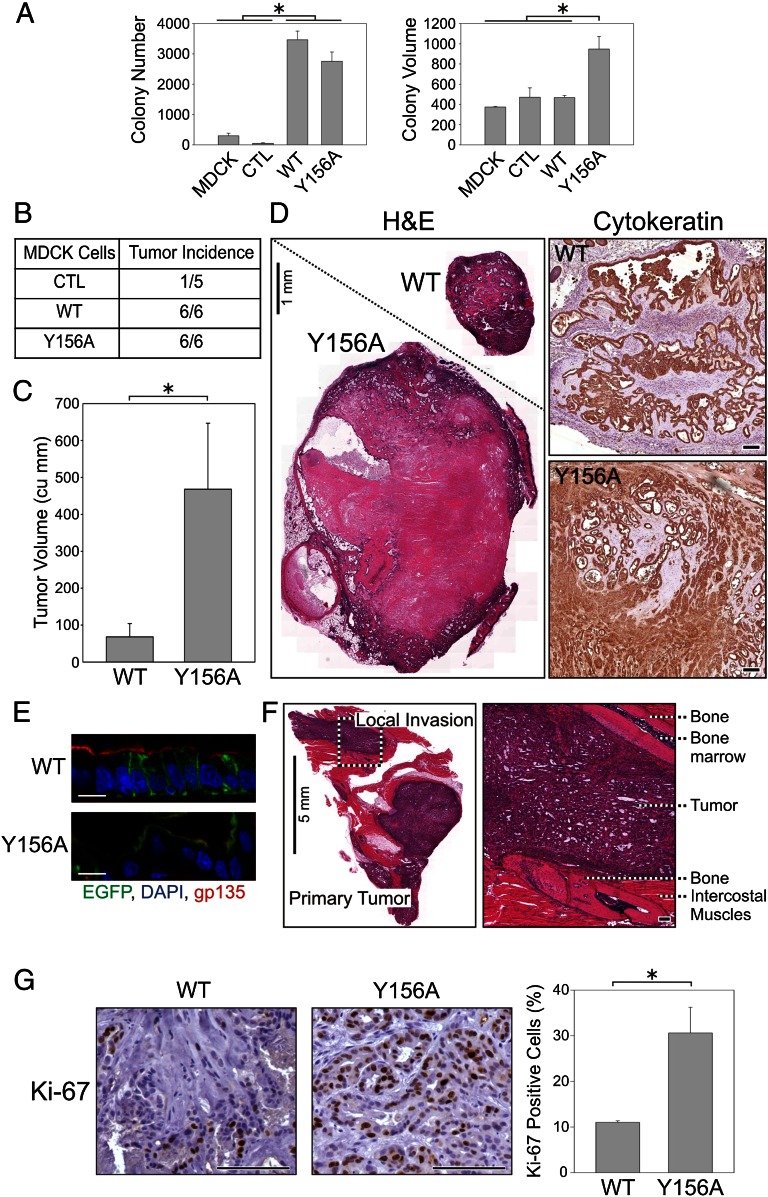
Given the role of EREG in cancer (20) and our observation of sustained EGFR phosphorylation by apical EREG, we examined the neoplastic consequences of apical EREG mistrafficking. Both WT and apical Y156A mutant EREG-expressing MDCK cells formed 10-fold more colonies compared with parental MDCK cells or MDCK cells expressing EGFP alone (Fig. 4A, Left). Whereas the number of colonies formed by WT and mutant EREG-expressing cells was similar, the mutant colonies were significantly larger than the WT colonies (Fig. 4A, Right).
Fig. 4.
Apically missorted EREG mutants lead to enhanced transformation in vitro and in vivo. (A) A total of 10,000 MDCK cells stably expressing the indicated constructs were embedded in soft agar and allowed to grow for 15 d. (Left) Colony numbers: MDCK, 297 ± 85; GFP = CTL, 54 ± 13; EREG-EGFP = WT, 3,477 ± 287; (Y156A)EREG-EGFP = Y156A, 2,757 ± 303. (Right) Colony volume: WT, 535 ± 35; Y156A, 947 ± 125 (n = 3, mean colony number or volume ± SD). (B and C) Ten million MDCK cells stably expressing indicated constructs were injected s.c. into nude mice. At 4 mo after injection, the number of palpable tumors was scored (B), and the volume of tumors positive for GFP fluorescence was quantified by ultrasound imaging and expressed as mean tumor volume (cu mm) ± SEM; n = 6 (C). WT, 68 ± 36; Y156A, 468 ± 178. (D) (Left) H&E staining of representative formalin-fixed, paraffin-embedded sections from WT and Y156A EREG tumors shown at equal magnification. (Right) An equivalent region from each tumor magnified to an equal extent; cytokeratin staining confirms the epithelial origin of tumors. (Scale bars: Left, 1 mm; Right, 100 µm.) (E) GFP fluorescence (green) shows the basolateral and apical localization of WT and Y156A EREG; cryosections are counterstained for nuclei (DAPI, blue) and apical surface (gp135, red). (Scale bars: 10 µm.) (F) H&E staining of (Y156A)EREG-EGFP-expressing tumors shows local invasion to intercostal muscles. (Left) Gross tumor morphology interspersed between bones and cartilages from the rib cage. (Right) Magnified section of an invasive tumor flanked by two ribs. (Scale bars: Left, 5 mm; Right, 100 µm.) (G) Formalin-fixed, paraffin-embedded sections from MDCK tumors expressing EREG-EGFP (WT) and (Y156A)EREG-EGFP (Y156A) were stained for Ki-67, a marker of cycling cells. For each tumor, at least three nonnecrotic regions were analyzed (totaling more than 1,000 nuclei). (Left) Representative sections. (Right) For quantification, three WT tumors and five Y156A tumors were analyzed; data are plotted as mean ± SEM of the percentage of Ki-67–positive nuclei: WT, 11.0 ± 0.49; Y156A, 30.6 ± 5.64. (Scale bars: 100 µm.) *Statistically significant difference; P = 0.025, two-tailed unpaired t test.
We next tested the ability of these cells to form tumors after s.c. injection into nude mice. Although both WT and Y156A mutant EREG-expressing cells formed tumors (Fig. 4B), the apical EREG-expressing tumors were approximately sevenfold larger than the WT EREG-expressing tumors, as quantified by ultrasound imaging (Fig. 4C and Fig. S6). Both WT and Y156A tumors stained positive for cytokeratin, confirming their epithelial origin (Fig. 4D, Insets). Within differentiated regions of the tumors, WT EREG and Y156A mutant EREG were present at the basolateral and apical cell surfaces, respectively, as detected by GFP fluorescence (Fig. 4E and Fig. S7). Y156A mutant EREG tumors exhibited a solid, densely packed growth pattern with central necrosis, indicative of poorly differentiated tumors. Y156A mutant tumors demonstrated cystically dilated glandular structures of varying size, often multilayered, and exhibiting a papillary growth pattern, with tumor cells projecting into the lumen. Tumor budding, with single cells and small clusters at the leading edge, was seen at the tumor margins. In contrast, WT tumors exhibited well-formed glandular structures without necrosis (Fig. 4D, Insets). Of note, three of six apical Y156A mutant EREG-expressing tumors invaded the intercostal muscles, and one tumor invaded perineurally (Fig. 4F and Fig. S8).
To investigate why the mutant EREG tumors were so much larger than WT EREG tumors, we compared proliferative (Ki-67), apoptotic (caspase-3), and angiogenic (von Willebrand factor and CD31) indices between WT and mutant tumors. Of these parameters, significant differences were seen only in proliferation, with Ki-67 immunoreactivity approximately threefold greater in mutant tumors compared with WT tumors (Fig. 4G). Both WT and Y156A mutant EREG cell lines secreted ∼10 ng/mL/day of EREG (Fig. S9); thus, the differences in tumor size cannot be attributed to differences in EREG levels. Similar levels of EREG (up to 6.3 ng/mL) have been detected in tumor biopsy specimens from patients with colorectal cancer (18).
More than 90% of human tumors are carcinomas, arising from epithelial cells that are organized as polarized monolayers, with apical and basolateral cell surfaces composed of distinct proteins and lipids (33, 34). EGFR is an important regulator of epithelial architecture and function (35). In polarized epithelial cells, EGFR signaling is tightly controlled by its largely restricted localization to the basolateral cell surface, ligand availability, and coordinated activity of inducible negative regulators that are frequently disrupted in carcinomas (2, 36, 37). In this report, we identify an additional mode of EGFR regulation—the fidelity of ligand trafficking—which, when perturbed, results in transformation. Thus, instead of the usual loss-of-function phenotype associated with trafficking defects (38), EREG mistrafficking to the apical surface results in a gain-of-function transformation phenotype. Of interest, mutations in EREG (R147stop) that would eliminate the basolateral sorting motif have been found in human tumors (Fig. S10) (39). Thus, disruption of polarized trafficking of the EGFR ligand, EREG, in epithelial cells is sufficient to cause transformation and invasion.
Materials and Methods
Reagents and Antibodies.
All chemicals were purchased from Sigma-Aldrich unless stated otherwise. Galardin was purchased from Calbiochem. EZ-Link Sulfo-NHS-LC-biotin was purchased from Pierce Biotechnology. All cell culture reagents were purchased from Gibco Laboratories. Fluorescent secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. EGF and EREG were purchased from R&D Systems. The gp135 mouse monoclonal antibody was a kind gift from James R Goldenring, Vanderbilt University, Nashville, TN. Rabbit polyclonal GFP and ZO-1 antibodies, protein G agarose, and rhodamine-phalloidin were purchased from Invitrogen. Monoclonal antibodies to p75/NGFR extracellular domain (ME20.4) and ubiquitin (P4D1) were purchased from Santa Cruz Biotechnology. Anti-cytokeratin (AE1/AE3) antibodies were purchased from Millipore.
Cell Culture.
Parental MDCK cells and µ1B knockdown MDCK cells were obtained from Enrique Rodriguez-Boulan, Weill Cornell Medical College, New York (25, 38). MDCK cells stably expressing ZO-1-GFP were a kind gift from Alan S. Fanning, University of North Carolina, Chapel Hill, NC (40). All cell lines were maintained in DMEM containing 10% (vol/vol) bovine growth serum (BGS), nonessential amino acids, l-glutamine, and penicillin/streptomycin.
Polarized Epithelial Cell Culture.
A total of 100,000 MDCK cells were seeded on the inner chamber of 12-mm Transwell filters (polycarbonate, 0.4 µm pore size; Corning) in DMEM (10% BGS), with fresh medium added every other day. Cells were considered polarized when the transepithelial electrical resistance exceeded 200 Ω/cm2, as measured with the Millicell Electrical Resistance System (Millipore); this usually occurred at day 4–5 after seeding.
EREG Cloning and Expression.
EREG was cloned from cDNA prepared from LM2-4175 cells (a lung metastatic MDA-MB-231 derivative) in frame with EGFP coding region in pEGFP-N1 vector (20). Other mutations and chimeras (truncations and substitutions) were subsequently derived from this construct. Untagged EREG was cloned in the pIRES2-EGFP plasmid upstream of the IRES site. The NGFR-EREG CT chimera was constructed by an overlapping PCR method and cloned into the upstream IRES site in pIRES2-EGFP. These constructs were then transfected into the indicated cells and selected for 10 d (G418; 1 mg/mL) and cloned by flow cytometry using GFP fluorescence. Clones stably expressing EREG constructs were maintained in 300 µg/mL G418, which was removed during the experiments unless stated otherwise.
EREG Polyclonal Antibody.
Peptide (CRWYRNRKSKEPKKEYERVT) from the EREG cytoplasmic domain was synthesized for immunization in rabbits. Subsequent immunization in rabbits and affinity purification of sera was performed by Covance. These antibodies were used at a 1:1,000 dilution for immunofluorescence, at a 1:5,000 dilution for Western blot analysis, and at 2 µL/mg protein for immunoprecipitation.
Cell Lysis, Immunoprecipitation, and Immunoblotting.
Cells were lysed for 30 min on ice in buffer containing 50 mM Hepes (pH 7.5), 150 mM NaCl, 1% (vol/vol) Triton X-100, 1 mM EDTA, 10% (vol/vol) glycerol, and 10 mM sodium pyrophosphate, with 2 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM PMSF, 5 µg/mL leupeptin, 5 µg/mL pepstatin, and 10 µg/mL aprotinin added fresh. Lysates were precleared at 18,000 × g for 15 min at 4 °C in a tabletop centrifuge. Protein concentration was determined using a BCA Protein Assay Kit (Pierce). For Western blot analysis, samples were mixed with 2× Laemmli buffer [5% (vol/vol) β-mercaptoethanol] and run on 10% SDS/PAGE for EREG and 7.5% SDS/PAGE for EGFR, unless indicated otherwise. For immunoprecipitation, lysates were precleared with protein G agarose beads overnight, after which fresh beads and appropriate antibodies were added in the immunoprecipitation dilution buffer [HNTG: 200 mM Hepes (pH 7.5), 600 mM NaCl, 40% (vol/vol) glycerol, and 0.4% Triton X-100] and incubated for 4 h at 4 °C under gentle agitation. Beads were then washed three times in 600 µL of HNTG buffer and resuspended in 30 µL of 2× Laemmli buffer. Samples were boiled for 5 min, run on SDS/PAGE gels, and then transferred onto nitrocellulose membranes. All membranes were blocked with 5% (wt/vol) milk in Tris-buffered saline containing 0.1% Tween20 (TBST), except for the biotinylation blots, which were blocked with 5% (wt/vol) BSA in TBST. Primary and secondary antibodies were diluted in blocking buffer. Stripping of antibodies from the membranes was performed by incubating them for 45 min at 50 °C in 65 mM Tris (pH 6.8) containing 2% (wt/vol) SDS and 0.8% β-mercaptoethanol. Membranes were then washed at least 10 times in TBST and blocked for subsequent immunoblotting.
Selective Cell Surface Biotinylation.
MDCK cells polarized on 12-mm Transwell filters were washed three times with cold PBS containing 0.1 mM CaCl2 and 1.0 mM MgCl2 (PBS-CM). Cold biotin working solution was freshly prepared at a concentration of 0.5 mg/mL in cold PBS-CM from a 200-mg/mL stock dissolved in anhydrous DMSO. This solution was then added to either the apical side (0.5 mL) or the basolateral side (1.5 mL) of the filters and incubated for 20 min at 4 °C. Fresh biotin was again added for another 20 min, followed by quenching with five washes of PBS-CM containing 0.2% BSA and 100 mM glycine at 4 °C. After two additional washes with PBS-CM at 4 °C, filters were cut out of the Transwell inserts and placed in Eppendorf tubes containing 500 µL of lysis buffer. After gentle rotation for 30 min at 4 °C, filters were removed from the tubes, and lysates were precleared and processed for immunoprecipitation.
Metabolic Labeling, Pulse-Chase, and Selective Cell Surface Biotinylation.
[35S]-translabel (Met-Cys) was purchased from MP Biochemicals. A total of 500,000 MDCK cells were seeded on 24-mm Transwell filters and allowed to polarize, after which they were subjected to metabolic labeling. In brief, filters were washed three times with DMEM (-cys, -met) at 37 °C. Then 100-µL puddles of DMEM (-cys, -met) containing [35S]-translabel (1 mCi/mL) were placed on Parafilm, with filters placed on top, and incubated for 20 min at 37 °C in a humidified chamber. The label was washed with DMEM (10% BGS) containing a 10-fold excess of l-cysteine and l-methionine at 18 °C, and then chased with DMEM/BGS for the indicated times at 37 °C. The chase was stopped by chilling cells in PBS (0.2% BSA), and filters were lysed or subjected to selective cell surface biotinylation as described above. Lysates were immunoprecipitated for GFP as described above, and after four washes in HNTG buffer, the beads were eluted after boiling for 5 min in 30 µL of 2× Laemmli buffer or in 50 µL of 10% (wt/vol) SDS solution. SDS eluates were collected in a separate tube and diluted with HNTG buffer to a total volume of 500 µL. Then 40 µL of streptavidin agarose beads were added to these samples, followed by incubation for 4 h at 4 °C. Samples were then washed, eluted, and run on SDS/PAGE (8–12% gradient) as described above. Gels were later fixed in methanol:water:acetic acid (50:40:10) for 30 min, dried, and exposed to autoradiography films at −80 °C for several days.
Immunofluorescence and Confocal Microscopy.
Cells were polarized on 12-mm Transwell filters, washed three times with cold PBS, and fixed with 4% paraformaldehyde in PBS for 30 min at 4 °C. Cells were again washed three times with cold PBS on ice, and then permeabilized in immunofluorescence (IF) buffer (0.1% BSA and 0.1% Triton X-100 in PBS) overnight at 4 °C. All subsequent steps were carried out at room temperature in IF buffer. Permeabilized cells were blocked with 5% normal donkey serum for 1 h, followed by incubation with primary antibody for 1 h. Filters were washed three times every 15 min with IF buffer and subsequently incubated with secondary antibody for 1 h. Filters were then washed three times in IF buffer every 15 min, cut out from the Transwell inserts, and mounted in Prolong Gold mounting medium. Laser scanning confocal microscopy was performed with a Zeiss LSM 510/710 inverted confocal microscope. Samples were scanned using a 63× objective, with Z slices obtained at 0.5-µm intervals. Contents of the image windows were exported as JPEG files using Zeiss LSM Image Browser version 4.2.0.121. Images were processed using Adobe Photoshop CS4, version 11.0.2.
Soft Agar Colony Formation Assay.
For this assay, 1 mL of 0.8% soft agar in DMEM 10% BGS was layered in each well of a six-well dish. A total of 10,000 cells in 1 mL of 0.4% soft agar in DMEM (10% BGS) were seeded per well and allowed to grow for 15 d. Each line was plated in triplicate. Colonies were counted using GelCount (Oxford Optronix) with identical acquisition and analysis settings.
Ultrasound Measurements.
The Vevo770 system (VisualSonics) was used to collect tumor ultrasound imaging data. Three-dimensional ultrasound data of the tumor xenografts of anesthetized mice were collected in B-mode with a 30-MHz ultrasound transducer. Data were analyzed with Amira 5.2.0 (Visage Imaging). Tumor margins in several xy projections were marked manually. Tumor density and morphology were readily distinguishable from surrounding normal tissue (Fig. S6). The software interpolated intermediate slices and integrated over the entire scan to quantify total tumor volume.
Supplementary Material
Acknowledgments
We acknowledge the support of Vanderbilt University's Cell Imaging, Translational Pathology, and Flow Cytometry Shared Resources. We thank James N. Higginbotham for assistance with FACS, Frank Revetta for assistance with immunohistochemistry, Zhao Ping for nude mice injections, H. Charles Manning for ultrasound imaging, Gregory D. Ayers for statistical analyses, and Ramona Graves Deal for assistance with the soft agar assay. We also thank Emily J. Poulin for editorial assistance and Graham Carpenter for helpful suggestions and critical appraisal of the manuscript. This work was supported by National Cancer Institute (NCI) RO1 CA 46413 (to R.J.C.) and NCI P50 95103 GI Special Program of Research Excellence (to M.K.W. and R.J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305508110/-/DCSupplemental.
References
- 1.Playford RJ, et al. The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut. 1996;39(2):262–266. doi: 10.1136/gut.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284(1):2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 3.Brown CL, Coffey RJ, Dempsey PJ. The proamphiregulin cytoplasmic domain is required for basolateral sorting, but is not essential for constitutive or stimulus-induced processing in polarized Madin-Darby canine kidney cells. J Biol Chem. 2001;276(31):29538–29549. doi: 10.1074/jbc.M102114200. [DOI] [PubMed] [Google Scholar]
- 4.Coffey RJ, Jr, et al. Acceleration of mammary neoplasia in transforming growth factor alpha transgenic mice by 7,12-dimethylbenzanthracene. Cancer Res. 1994;54(7):1678–1683. [PubMed] [Google Scholar]
- 5.Dempsey PJ, Meise KS, Yoshitake Y, Nishikawa K, Coffey RJ. Apical enrichment of human EGF precursor in Madin-Darby canine kidney cells involves preferential basolateral ectodomain cleavage sensitive to a metalloprotease inhibitor. J Cell Biol. 1997;138(4):747–758. doi: 10.1083/jcb.138.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dempsey PJ, Meise KS, Coffey RJ. Basolateral sorting of transforming growth factor-alpha precursor in polarized epithelial cells: Characterization of cytoplasmic domain determinants. Exp Cell Res. 2003;285(2):159–174. doi: 10.1016/s0014-4827(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 7.Li C, et al. Myristoylated Naked2 escorts transforming growth factor alpha to the basolateral plasma membrane of polarized epithelial cells. Proc Natl Acad Sci USA. 2004;101(15):5571–5576. doi: 10.1073/pnas.0401294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gephart JD, et al. Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic. 2011;12(12):1793–1804. doi: 10.1111/j.1600-0854.2011.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groenestege WM, et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest. 2007;117(8):2260–2267. doi: 10.1172/JCI31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komurasaki T, Toyoda H, Uchida D, Morimoto S. Epiregulin binds to epidermal growth factor receptor and ErbB-4 and induces tyrosine phosphorylation of epidermal growth factor receptor, ErbB-2, ErbB-3 and ErbB-4. Oncogene. 1997;15(23):2841–2848. doi: 10.1038/sj.onc.1201458. [DOI] [PubMed] [Google Scholar]
- 11.Sahin U, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164(5):769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J-Y, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 13.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14(8):1147–1161. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 14.Toyoda H, Komurasaki T, Uchida D, Morimoto S. Distribution of mRNA for human epiregulin, a differentially expressed member of the epidermal growth factor family. Biochem J. 1997;326(Pt 1):69–75. doi: 10.1042/bj3260069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thøgersen VB, et al. A subclass of HER1 ligands are prognostic markers for survival in bladder cancer patients. Cancer Res. 2001;61(16):6227–6233. [PubMed] [Google Scholar]
- 16.Shigeishi H, et al. Expression of epiregulin, a novel epidermal growth factor ligand associated with prognosis in human oral squamous cell carcinomas. Oncol Rep. 2008;19(6):1557–1564. [PubMed] [Google Scholar]
- 17.Baba I, et al. Involvement of deregulated epiregulin expression in tumorigenesis in vivo through activated Ki-Ras signaling pathway in human colon cancer cells. Cancer Res. 2000;60(24):6886–6889. [PubMed] [Google Scholar]
- 18.Khambata-Ford S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25(22):3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs B, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2009;27(30):5068–5074. doi: 10.1200/JCO.2008.21.3744. [DOI] [PubMed] [Google Scholar]
- 20.Gupta GP, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446(7137):765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 21.Scheiffele P, Peränen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378(6552):96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- 22.Yeaman C, et al. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol. 1997;139(4):929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonifacino JS, Dell’Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145(5):923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fölsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99(2):189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 25.Gravotta D, et al. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA. 2007;104(5):1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvajal-Gonzalez JM, et al. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci USA. 2012;109(10):3820–3825. doi: 10.1073/pnas.1117949109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmen T, Höning S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol. 2002;4(2):154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 28.Vermeer PD, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422(6929):322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 30.Mohapatra B, et al. Protein tyrosine kinase regulation by ubiquitination: Critical roles of Cbl-family ubiquitin ligases. Biochim Biophys Acta. 2013;1833(1):122–139. doi: 10.1016/j.bbamcr.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fry WH, Kotelawala L, Sweeney C, Carraway KL., 3rd Mechanisms of ErbB receptor negative regulation and relevance in cancer. Exp Cell Res. 2009;315(4):697–706. doi: 10.1016/j.yexcr.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomic S, et al. Association of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells: Phosphatidic acid activates receptor dephosphorylation by PTP1C. J Biol Chem. 1995;270(36):21277–21284. doi: 10.1074/jbc.270.36.21277. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Gerl MJ, et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J Cell Biol. 2012;196(2):213–221. doi: 10.1083/jcb.201108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Threadgill DW, et al. Targeted disruption of mouse EGF receptor: Effect of genetic background on mutant phenotype. Science. 1995;269(5221):230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 36.Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 37.Powell AE, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149(1):146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dempsey PJ, Coffey RJ. Basolateral targeting and efficient consumption of transforming growth factor-alpha when expressed in Madin-Darby canine kidney cells. J Biol Chem. 1994;269(24):16878–16889. [PubMed] [Google Scholar]
- 39.Memorial Sloan Kettering Cancer Center 2013. cBio Cancer Genomics Portal: tumor samples TCGA-D3-A2JF, TCGA-EE-A20C, and TCGA-D1-A17Q (TCGA provisional). Available at http://www.cbioportal.org. Accessed March 15, 2013.
- 40.Utepbergenov DI, Fanning AS, Anderson JM. Dimerization of the scaffolding protein ZO-1 through the second PDZ domain. J Biol Chem. 2006;281(34):24671–24677. doi: 10.1074/jbc.M512820200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.