Abstract
Growth and cell division in rod-shaped bacteria have been primarily studied in species that grow predominantly by peptidoglycan (PG) synthesis along the length of the cell. Rhizobiales species, however, predominantly grow by PG synthesis at a single pole. Here we characterize the dynamic localization of several Agrobacterium tumefaciens components during the cell cycle. First, the lipophilic dye FM 4-64 predominantly stains the outer membranes of old poles versus growing poles. In cells about to divide, however, both poles are equally labeled with FM 4-64, but the constriction site is not. Second, the cell-division protein FtsA alternates from unipolar foci in the shortest cells to unipolar and midcell localization in cells of intermediate length, to strictly midcell localization in the longest cells undergoing septation. Third, the cell division protein FtsZ localizes in a cell-cycle pattern similar to, but more complex than, FtsA. Finally, because PG synthesis is spatially and temporally regulated during the cell cycle, we treated cells with sublethal concentrations of carbenicillin (Cb) to assess the role of penicillin-binding proteins in growth and cell division. Cb-treated cells formed midcell circumferential bulges, suggesting that interrupted PG synthesis destabilizes the septum. Midcell bulges contained bands or foci of FtsA-GFP and FtsZ-GFP and no FM 4-64 label, as in untreated cells. There were no abnormal morphologies at the growth poles in Cb-treated cells, suggesting unipolar growth uses Cb-insensitive PG synthesis enzymes.
Keywords: agrobacterium cell cycle, bacterial cell growth, bacterial cell division, peptidoglycan synthesis
Much of our understanding of the growth and division of rod-shaped bacterial cells is based on investigations of distinct elongase and divisome protein complexes in the γ-proteobacterium Escherichia coli (1). The elongase adds peptidoglycan (PG) at discrete equally spaced sites along the cylindrical cell between the poles. After elongation is complete, divisome proteins assemble at the midcell in a specific temporal order where one component recruits the next via protein–protein interactions. A comprehensive study in the α-proteobacterium Caulobacter crescentus, however, revealed significant differences from the E. coli model in the timing of localization of cell-division proteins and the genetic requirements for localization (2).
Recent studies with another rod-shaped α-proteobacterium, Agrobacterium tumefaciens, revealed additional novel modes of bacterial growth and cell division (3, 4). First, a specific unipolar polysaccharide (UPP) is secreted from one pole and mediates attachment of cells to the substrate, and this activity is required for the formation of biofilms (3). Second, Agrobacterium (and other members of the Rhizobiales) grows by the addition of PG primarily at one end of the cell, the growth pole (4). After cell division, unipolar growth resumes in both daughter cells from the new cell poles generated at the division site. Notably, attachment and unipolar growth occur at opposite ends of the cell, as secretion of the UPP occurs from the old pole (4).
Agrobacterium is well-known for its ability to genetically transform plant cells (5) using a specific virulence type IV secretion system (vir-T4SS) that now serves as a model for T4SS (6) in other pathogenic bacteria that cause disease in human and animal cells (7). We showed that Agrobacterium assembles multiple periodically localized vir-T4SS around the entire perimeter of the bacterial cell (8–10). Our long-term goal, to understand how the vir-T4SS inserts itself across the bacterial cell envelope in the context of the bacterial cell cycle, requires that we first understand Agrobacterium-specific cell growth and division independent of induction of the vir-T4SS.
Toward this end, here we aimed to study the localization of well-known cell-growth and -division proteins in Agrobacterium. Our bioinformatics searches revealed that the genomes of Agrobacterium does not encode homologs of most of the E. coli elongase-specific components, such as proteins MreB, penicillin binding protein 2 (Pbp2), MreC, MreD, and RodZ. However, divisome proteins, such as FtsA and FtsZ (1), are well-conserved. Strikingly, GFP fusions to Agrobacterium-specific FtsA and FtsZ reveal distinct patterns of both polar and midcell localization that are dynamic, independent of each other at different times of the cell cycle, and change in a cell cycle-dependent manner. Further, labeling growing cells with the membrane-specific fluorescent dye FM 4-64 demonstrates that the membrane character of growing “new” poles is distinct from that of the old poles. Finally, treatment with sublethal doses of carbenicillin reveals differential PG synthesis sensitivity at the midcell versus the growth pole.
Results
Growth Poles and Old Poles of Agrobacterium Exhibit Differential Staining with the Lipophilic Dye FM 4-64.
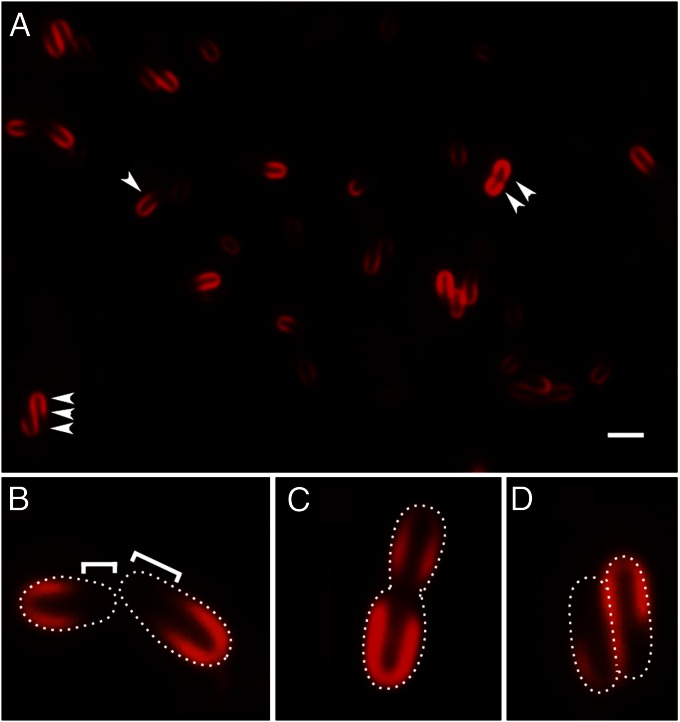
To enable easy monitoring of growing cells and changes in cell length during the cell cycle, we treated Agrobacterium with the lipophilic styryl fluorescent dye FM 4-64, which has been used extensively to uniformly label and image the outer boundaries of bacterial cells. Remarkably, Agrobacterium cells stained with FM 4-64 display three distinct asymmetric fluorescence patterns. The most common pattern is “horseshoe” cell labeling, where one pole stains intensely up to approximately two-thirds of the cell length (Fig. 1 A, single arrowhead and B); beyond this point, the intensity of membrane staining decreases substantially. Then, there are cells with FM 4-64 labeling at both ends and not at the midcell (Fig. 1A, double arrowhead and C). Finally, there are distinctive S-shaped labeling patterns (Fig. 1 A, triple arrowhead and D). These three patterns represent different stages of the Agrobacterium cell cycle, from early to late, respectively, as documented below (Figs. 2 and 3).
Fig. 1.
Differential labeling of old pole and growth pole by FM 4-64. (A) FM 4-64 labels older halves of cells (single arrowhead), both ends of a cell (double arrowhead), and S-shaped cell pairs (triple arrowheads). (Scale bar, 2 μm.) Close-ups of FM 4-64 labeling: (B) more intense labeling of old pole; brackets indicate unlabeled growth poles; (C) both poles labeled in a cell with midcell constriction; and (D) S-shaped cells just post cell division. White dots show the cell outlines.
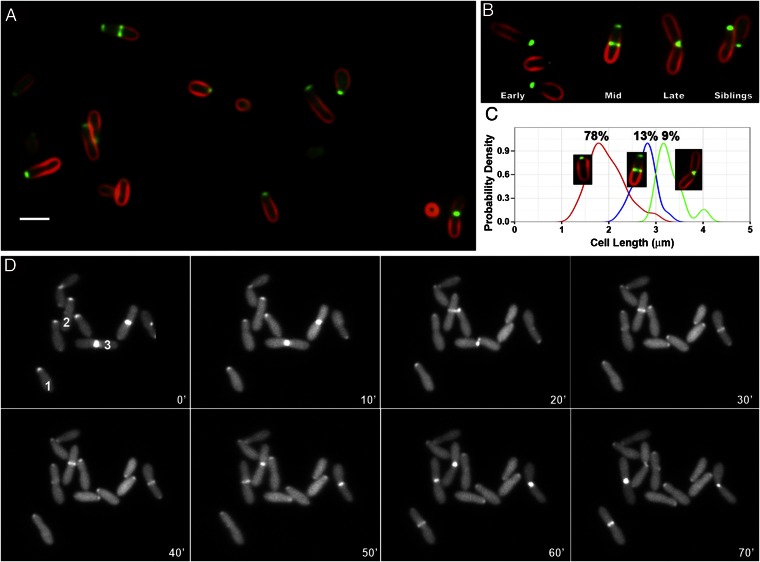
Fig. 2.
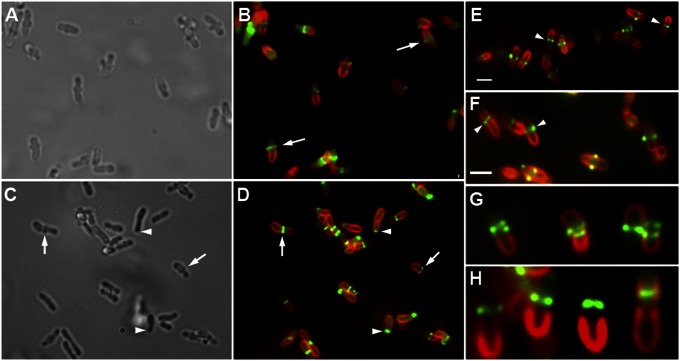
FtsA-GFP localization in Agrobacterium labeled with FM 4-64. (A) Fields of cells displaying unipolar, unipolar plus midcell, unipolar (in sibling cells with S-shaped FM 4-64 labeling), and midcell (in cells with midcell constriction) localization of FtsA-GFP. (Scale bar, 2 μm.) (B) Change in FtsA-GFP localization correlated with early, mid, and late times of cell-cycle progression. Siblings are a pair of daughter cells that remain in contact with each other after cell division. (C) Distribution of cell lengths for cells exhibiting unipolar localization of FtsA-GFP (Left Inset, red line), unipolar focus and midcell band (Center Inset, blue line), and midcell focus in cells with midcell constriction (Right Inset, green line). The lengths of cells with each localization pattern were measured, and these distributions were compared by calculating Gaussian kernel density estimates using the ggplot2 (25) package for R (26). To facilitate comparison, the density curves were each scaled to equal height. The frequencies of the different localization patterns are shown above each curve. (D) FtsA time-lapse microscopy of A. tumefaciens expressing IPTG-inducible FtsA-GFP. Bacteria were loaded into an ONIX live-cell imaging microfluidic flow chamber (CellASIC). Luria broth was continuously perfused through the chamber at room temperature. Cells were imaged every 10 min for 70 min on an Applied Precision Delta Vision Elite microscope. The three numbered cells (1, 2, 3) demonstrate the stages of FtsA-GFP localization throughout the cell cycle as described in the text.
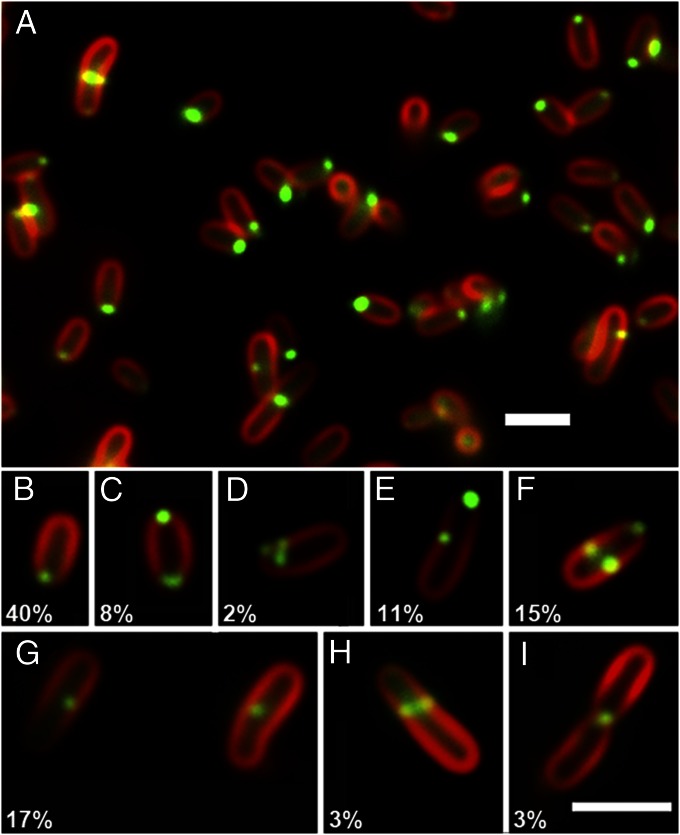
Fig. 3.
FtsZ-GFP localization in Agrobacterium labeled with FM 4-64. (A) Field of cells displaying different patterns of FtsZ-GFP localization shown in B–I. (B) Unipolar localization at the growth pole. (C) Bipolar foci. (D) Polar cluster of foci at growth pole. (E) Unipolar focus at growth pole and single focus near midcell. (F) Unipolar focus at growth pole and paired foci at midcell. (G) Midcell single focus. (H) Midcell band in cell with less intense FM 4-64 labeling at the growth pole and without midcell constriction. (I) Midcell focus in cell with midcell constriction and both poles labeling with FM 4-64. The relative percentages of the different localization patterns are shown in B–I. (Scale bars, 2 μm.)
Fig. 1 indicates that FM 4-64 predominantly labels one pole of the cell more than the other, and several observations suggest the old pole has higher affinity for FM 4-64. First, the slightly tapered ends of cells, indicative of the growth pole (4) (Fig. 2), do not label strongly with FM 4-64 (Fig. 1 A and B); these cells are also shorter in length. Second, in longer cells with a midcell constriction that appear just about to divide, FM 4-64 labeling is equally intense in both nascent division products, with little or no labeling at the midcell site of cell division (Fig. 1 A and C; see also Fig. 2B). Finally, freshly divided cells often stay in close proximity along their sides, with their growth poles oriented in opposite directions (see time-lapse images in Fig. 2D, and figures 3C and S2A in ref. 4). Siblings in this arrangement exhibit an S-shaped FM 4-64 labeling pattern (Fig. 1 A and D), with cells still attached to each other before separation, with stronger FM 4-64 labeling in the older halves of the cells and weak FM 4-64 labeling at presumptive new growth poles in both cells (see also Fig. 2B). This head-to-tail orientation, proximity of the adjacent sides of the siblings, and differential FM 4-64 staining produce the S-shaped fluorescence image.
To observe differential FM 4-64 labeling of old versus growing poles, we use a 5-min pulse with a dilute concentration of FM 4-64 (8 ng/μL); higher concentrations of FM 4-64 result in uniform labeling. To determine whether FM 4-64 stains the inner (IM) or outer membrane (OM) of Agrobacterium under our conditions, soluble GFP expression was induced to mark the cytoplasm. Before FM 4-64 staining, cells were pelleted and resuspended in 15% sucrose to induce plasmolysis. After sucrose treatment, the irregular appearance of GFP suggests the cytoplasm and associated IM have collapsed away from the rigid cell wall (11) (Fig. S1A). The pattern of FM 4-64 fluorescence in sucrose-treated cells (Fig. S1A) was indistinguishable from that of untreated cells (Fig. S1B), indicating that the FM 4-64 had labeled the OM. As there is no reason to suspect that the IM and OM would have different affinities for FM 4-64, it is likely that the OM is simply more accessible to labeling by FM 4-64. Labeling of the IM requires less efficient passage of FM 4-64 through the peptidoglycan layer. Thus, for the studies reported herein, we are primarily monitoring changes in the OM.
FtsA Localizes to Sites of both Polar Cell Growth and Cell Division.
Next, we examined the localization of GFP fusions to homologs of well-characterized E. coli cell-division proteins. We monitored both the actin homolog FtsA, which localizes to the Z ring at the midcell during division in E. coli, and the tubulin homolog FtsZ, which forms the Z ring (11, 12). Expression levels of the fusions, cloned in a low–copy-number plasmid, pSRKKm (13), were compared on Western blots probed with either antibodies to FtsZ or GFP. FtsZ-GFP accumulates to ∼10% of endogenous FtsZ levels, and FtsA-GFP accumulates to about 25% of the level of FtsZ-GFP (Fig. S2). Therefore, the expression of the fusion proteins did not significantly change the relative cellular concentrations of FtsZ and FtsA.
Surprisingly, Agrobacterium FtsA (Atu2087) fused to GFP exhibits four distinct patterns of localization (Fig. 2A). The most common localization pattern is a single focus of FtsA-GFP at one pole of the cell (Fig. 2 A and B, early). This localization of FtsA corresponds to the site of new polar growth, as it occurs in cells that are slightly tapered at one end, and such cells are generally shorter than the longer cells that occur just before cell division. The FtsA-GFP–labeled pole is further distinguished by the absence of FM 4-64 fluorescence. Second, FtsA-GFP is localized to one pole as well as in a band at the midcell (Fig. 2 A and B, mid) that likely corresponds to the Z ring (Fig. 3 A and I). In these cells, FM 4-64 staining decreases toward the unipolar focus of FtsA-GFP. The third pattern is a single, intense midcell region of FtsA-GFP fluorescence, whereas FM 4-64 stains the rest of the cell(s) as well as both poles (Fig. 2 A and B, late); such patterns likely represent cells just about to divide, as this labeling pattern occurs in longer cells that are constricted in the middle. The absence of polar FtsA localization and positive FM 4-64 labeling at both poles in these predivisional cells (Fig. 2B, late) indicates that the growth pole has been transformed into an old pole that now binds FM 4-64. Finally, Agrobacterium cells that have recently divided often remain in close proximity, with S-shaped FM 4-64 fluorescence as described above. FtsA-GFP localizes to the pole of each sibling daughter cell, and such poles again do not stain with FM 4-64 (Fig. 2B, siblings).
To directly test whether the different FtsA-GFP localization patterns correspond to progression through the cell cycle, the lengths of cells with different localization patterns were compared. Cells were categorized as those with FtsA-GFP localized to one pole, unipolar and midcell, or midcell with midcell constriction. Cell lengths were measured and a Gaussian kernel density estimate was plotted for cells of each FtsA-GFP localization category. Fig. 2C shows that cells with unipolar FtsA localization are the shortest (median, 1.9 μm), whereas cells with both unipolar and midcell FtsA localization are of intermediate length (median, 2.7 μm) and cells with only midcell FtsA and a midcell constriction are the longest (median, 3.2 μm). The frequencies of the different labeling patterns (in 150 cells) are shown above each curve. Nearly 80% of the cells display unipolar FtsA localization patterns, suggesting that unipolar growth makes up the bulk of the cell cycle.
Time-lapse imaging of Agrobacterium expressing FtsA-GFP confirms the correlation of FtsA-GFP localization with polar growth and cell division (Fig. 2D). Three numbered cells (1–3) demonstrate all of the stages of FtsA-GFP localization throughout the cell cycle. In cell 1, FtsA-GFP localizes at the elongating growth pole (0–50 min) and then changes to the midcell (60–70 min). In cell 2, FtsA-GFP is localized to the growth pole (0–10 min), changes to midcell localization (20–60 min), and is finally localized to the new growth poles of the sibling cells (70 min). In cell 3, FtsA-GFP is localized to the midcell (0–10 min), and after septation forms single foci at the new growth poles of sibling cells (20 min), where it remains as the sibling cells elongate (30–70 min).
Finally, cells expressing FtsA-GFP were pulse-labeled with Texas red-X succinimidyl ester (TRSE), which binds cell-surface proteins (4). Following short or long chases in media without TRSE, TRSE remained at the old pole, and FtsA-GFP was at the opposite end of the cell, namely the growth pole (Fig. S3 A and B).
FtsZ Exhibits a Complex Localization Pattern.
The tubulin homolog FtsZ is well-characterized with respect to its role in septation in E. coli and many other bacteria (reviewed in ref. 14). The genome of Agrobacterium contains three predicted ftsZ genes (Atu2086, Atu4215, and Atu4673). Here we studied Atu2086 because it encodes the longest protein and maps adjacent to ftsA (Atu2087) and ftsQ (Atu2088), which is identical to the chromosomal arrangement of ftsZ, ftsA, and ftsQ in E. coli. Furthermore, the predicted amino acid sequence of its encoded product, FtsZ2, contains an N-terminal domain, namely the tubulin domain, with the most significant homology (52% identity, 68% similarity) to FtsZ of E. coli and a highly conserved C terminus (64% identity, 93% similarity) containing 12 amino acids required for binding to FtsA (15). Fig. S4A shows an alignment of the amino acid sequences of the three predicted Agrobacterium FtsZ proteins compared with E. coli FtsZ, and the domain structure of these proteins is compared in Fig. S4B. FtsZ2 was previously named FtsZ1 and studied in another related α-proteobacterium, Rhizobium meliloti (16). Here, unless specified otherwise, FtsZ refers to Atu2086.
As with FtsA, the localization of Agrobacterium FtsZ fused to GFP is quite distinct from that observed in E. coli, and this localization is both similar to and more complex than FtsA-GFP localization. The frequencies of localization patterns were quantified in 400 cells expressing FtsZ-GFP and are noted in Fig. 3 B–I. Fig. 3A shows a representative field of view. As expected, FtsZ-GFP labels the constriction at the midcell in cells about to divide (Fig. 3I).
Surprisingly, Agrobacterium FtsZ-GFP formed single unipolar foci at the growth pole in 40% of the cells; as above, the growth pole is defined by the absence of FM 4-64 labeling (Fig. 3 A and B), and such polar FtsZ-GFP labeling occurred in shorter-growing cells. Most of the remaining patterns could be grouped into two categories related to cell-cycle progression. Approximately 21% of the cells had a unipolar focus of FtsZ-GFP and one or two additional foci (Fig. 3 C–E). These may represent transitory intermediates between the strictly unipolar focus and the Z ring. Thirty-two percent of the cells exhibited either a midcell band with a growth-pole focus (Fig. 3F) or a midcell focus (Fig. 3G) without a growth-pole focus, possibly reflecting stages immediately before Z-ring formation. Three percent of the cells have only an approximately midcell band of FtsZ-GFP as well as weaker growth-pole FM 4-64 labeling (Fig. 3H), suggesting polar growth is still active. Finally, 3% of the cells had only a single intense focus of FtsZ-GFP at the midcell constriction, indicating that septation had begun (Fig. 3I). Because the FtsZ-GFP pattern was unexpectedly complex, we assessed the localization of native FtsZ by immunofluorescence. In wild-type Agrobacterium, native FtsZ protein localizes in the same patterns observed for the FtsZ-GFP fusions, including midcell rings and polar and multiple foci localizations (Fig. S5).
Inhibition of PG Synthesis Affects Cell Morphology and Cell Polarity.
PG synthesis must be spatially and temporally regulated during the Agrobacterium cell cycle. Major PG synthesis must occur during unipolar growth and septum formation. Before cell division, the growth pole must be converted to an old pole by inhibition or removal of PG synthetic machinery and, after division, localized PG synthesis is reestablished in the new growth poles. Carbenicillin (Cb) blocks the activity of transpeptidase enzymes, so-called penicillin-binding proteins (PBPs), thereby inhibiting the linkage of new PG strands; this leads to weakening of the cell wall and ultimately cell lysis. Such severe outcomes occur with a lethal dose of Cb (100 μg/mL). To test the effect of perturbing PG synthesis during the Agrobacterium cell cycle, we grew cells in sublethal concentrations of Cb. Fig. S6 shows that cells maintain slow growth in the presence of 15–30 μg/mL Cb.
After 20 h of growth at 20 °C in minimal medium containing 30 μg/mL Cb, Agrobacterium cells exhibited a distinct morphology and FM 4-64 labeling patterns. More than half of the Cb-treated cells exhibited expansion at or near the midcell (Fig. 4A). Scanning electron microscopy shows that midcell expansion extends around the circumference of the cell to form single and double tire-like bulges (Fig. 4C) that occur at approximately equal frequency. Double bulges may reflect that PG synthesis (but lack of cross-linking due to Cb) occurs on either side of the presumptive septum. Single and smaller bulges may reflect an earlier stage of less extensive preseptal PG synthesis. These data support that PBPs are involved in PG synthesis at the midcell during cell division, as expected. Notably, the pattern of Cb-induced midcell bulging we observe in Agrobacterium is highly distinct from E. coli treated with the same sublethal concentration of Cb, where cells instead become very elongated (Fig. 4D). Thus, the architecture of the PG and/or the specific activities of PBPs are dissimilar between Agrobacterium and E. coli. Given that Agrobacterium elongates by unipolar growth, one might have expected to see an altered morphology of the growth pole following Cb treatment. However, no dramatic alterations were observed.
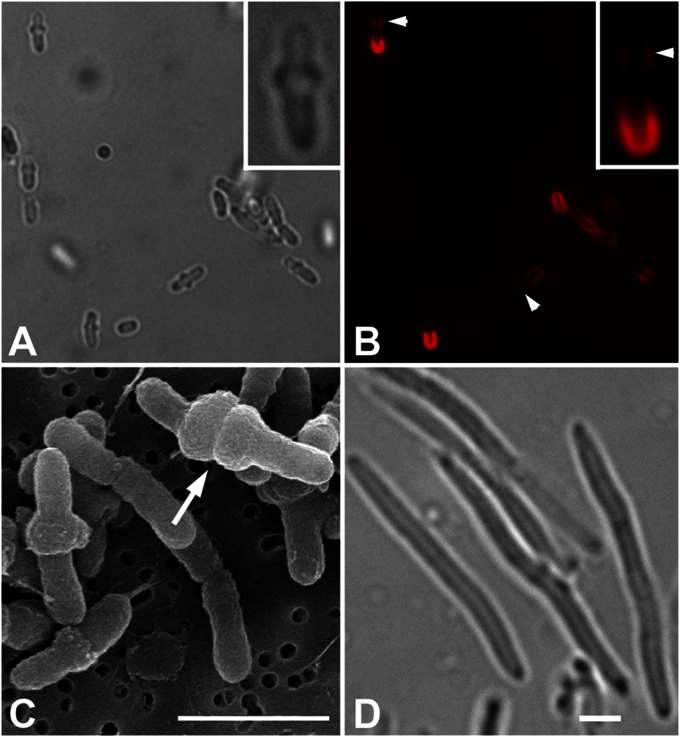
Fig. 4.
Morphology of A. tumefaciens and E. coli grown in carbenicillin. (A) Agrobacterium grown in Cb expands at the midcell (bright-field image). (B) Fluorescence image of A: Neither the region of midcell expansion nor the growth pole label with FM 4-64, whereas the region in-between does label (arrowheads). (A and B, Insets) Close-up of a cell with midcell expansion. (C) Scanning electron micrograph shows midcell expansion is circumferential and sometimes appears to be constricting (arrow). (D) E. coli elongate but do not expand at the midcell when grown in Cb. (Scale bars, 2 μm.)
Cb-treated cells exhibiting midcell expansion also displayed a characteristic bimodal pattern of FM 4-64 labeling that may reflect interrupted PG synthesis at the midcell. The old cell pole stains intensely with FM 4-64, as in untreated cells (Fig. 4B). The midregion corresponding to the region of Cb-induced bulging did not label with FM 4-64. Distal to this unstained midcell region is another area of FM 4-64 staining that is weaker than that at the old cell pole (Fig. 4B, arrowheads). The new growth pole, above this weaker staining area, does not stain with FM 4-64, as in non-Cb–treated cells.
FtsA and FtsZ Localization in Cb-Treated Cells.
In cells grown in Cb for 20 h, FtsA-GFP accumulated in the midcell expansion region either as a band or as a pair of foci (Fig. 5 A and B, arrows) representing different planes of focus. FtsA-GFP localization at the midcell in Cb-treated cells likely reflects its normal localization during septation and cell division. Unexpectedly, in 20-h Cb-treated cells, FtsA-GFP no longer localizes to the presumed growth poles (distinguished by lack of FM 4-64 labeling) as it does in untreated cells (Fig. 2). Because there were no FtsA-GFP polar foci, we examined cells early after Cb treatment. Midcell bulges and FtsA-GFP localization to this region became apparent in cells after 4 h of Cb treatment (Fig. 5 C and D, arrows). In elongating cells without midcell bulges, however, FtsA-GFP localized to a single focus at the growth pole (Fig. 5 C and D, arrowheads). Thus, FtsA localization to the poles or midcell is not affected by Cb treatment.
Fig. 5.
Localization of FtsA-GFP and FtsZ-GFP in Agrobacterium grown in the presence of carbenicillin. (A) Bright-field image shows cells with midcell expansions after 20 h of Cb treatment. (B) Fluorescence image of cells in A reveals that FtsA-GFP localizes to regions of midcell radial expansion (arrows) but no longer localizes to growth poles (identified by lack of FM 4-64 labeling). (C) Bright-field image shows cells with (arrows) and without (arrowheads) initiation of midcell expansion after 4.5 h of Cb treatment. (D) Fluorescence image of cells in C reveals that FtsA-GFP localizes to regions of midcell radial expansion (arrows), but in cells without midcell expansion it localizes to the growth pole (arrowheads). (E and F) FtsZ-GFP and FM 4-64 fluorescence in two panels of cells. Cells accumulate FtsZ-GFP in presumptive Z rings at midcell expansion regions (arrowheads) but do not accumulate FtsZ-GFP at growth poles (with weaker FM 4-64 labeling). (Scale bars, 2 μm.) (G and H) Galleries of Cb-treated Agrobacterium cells with additional presumptive Z rings (G) or with the Z ring positioned toward the former growth pole (H).
As with FtsA, FtsZ-GFP does not localize to the growth pole in 20-h Cb-treated cells (Fig. 5 E and F). However, FtsZ-GFP did form ring-like structures in midcell regions of expansion (Fig. 5 E and F, arrowheads). Cb treatment induced multiple Z rings in some cells (Fig. 5G). Whereas in untreated cells, the Z ring is located exactly at the midcell adjacent to the regions of the OM labeled with FM 4-64 (Fig. 3I), in Cb-treated cells, the ring was often positioned toward the growth pole in the region where FM 4-64 OM labeling is less intense (Fig. 5H). This may reflect the role of a Cb-sensitive transpeptidase in Z-ring placement similar to E. coli, where the position and orientation of the Z ring are perturbed if Pbp5 is inactive (17).
Inhibition of transpeptidase reactions by Cb likely reduces the mechanical strength of the PG layer. Indeed, we observed a few cells that produced spherical blebs labeled with FtsA-GFP or FtsZ-GFP that are likely membrane-bound cytoplasm that has been pushed by turgor pressure through a weakened area in the cell wall (Fig. S7). These cytoplasmic blebs primarily occur at the midcell, which is consistent with the hypothesis that most Cb-sensitive PG synthesis occurs near the midcell.
Discussion
Agrobacterium exhibits distinct asymmetry during cell growth. New growth occurs predominantly at one pole, designated the growth pole. In contrast, the opposite pole, designated the old pole, does not grow during the cell cycle, and under conditions that favor biofilm formation the old pole mediates attachment to the substrate (3, 4). Here we provide additional evidence for Agrobacterium cell polarity by characterizing dynamic changes in OM staining with the lipophilic dye FM 4-64 and in the localization of the cell-division proteins FtsA and FtsZ during the cell cycle. First, FM 4-64 does not label the OM at the growth pole as intensely as the OM at, and distal to, the old pole of the cell. Second, whereas FtsA and FtsZ localize predominantly to the site of cell division in numerous bacterial species (14, 18), in Agrobacterium these proteins localize primarily at the growth pole through much of the cell cycle and then at the Z ring just before cell division. Finally, PG synthesis at the septum is extremely sensitive to Cb, whereas PG synthesis at the growth pole is not.
The extensive PG synthesis that occurs at a single pole during unipolar growth may lead to concomitant disruption of membrane integrity during de novo OM synthesis so that FM 4-64 intercalation into these growing membranes is temporarily reduced. Or, it is formally possible that polar-membrane composition is altered during polar growth. Positively charged FM 4-64 has higher affinity for anionic phospholipids. Asymmetric FM 4-64 fluorescence may suggest that the concentration of anionic phospholipids in the polar membranes is dramatically decreased in the growing end of the cell so that neutral or positively charged phospholipids may be more abundant. Differences in membrane composition may be essential to recruit proteins essential for polar growth. For example, in Bacillus subtilis, helical lipid domains guide the helical localization of MinD (19), and polar localization of DivIVA may be mediated by physical cues in the geometry and shape of the membrane (20). Reduced FM 4-64 signal has also been reported for the division site of E. coli, an area of active PG synthesis (21).
In E. coli and B. subtilis, localization of FtsZ and FtsA have been predominantly studied in relation to their function at the divisome (14, 22). In C. crescentus, early cell-cycle unipolar FtsZ foci have been reported (2), although whether this localization is more than a postdivision remnant of the Z ring has not been resolved. FtsA in C. crescentus, however, is diffuse in the cytoplasm in the first half of the cell cycle, and then localizes to the Z ring until septation is complete (2).
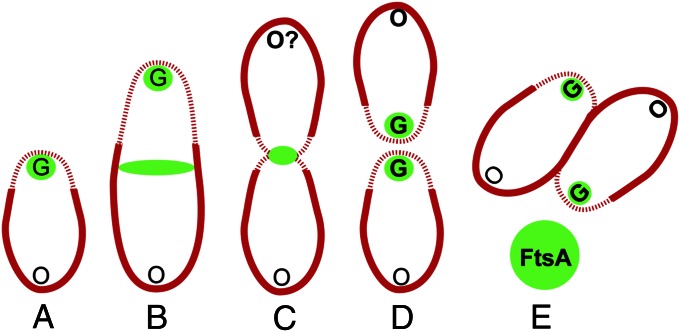
In contrast, FtsA-GFP and FtsZ-GFP in Agrobacterium are localized in discrete foci throughout most of the cell cycle. After cell division is complete, FtsA-GFP (Fig. 6A) is maintained in a unipolar focus at the growth poles of both daughter cells. Either this focus is a remnant of the divisome or a structure that forms at the completion of cell division. The growth-pole FtsA focus remains as the cell elongates. After a period of growth, FtsA-GFP accumulates in a band at the midcell (Fig. 6B). Cells forming this Z ring-like band retain the growth-pole localization of FtsA-GFP and asymmetric FM 4-64 staining, suggesting these cells are still growing. When the cell begins to constrict, FtsA-GFP is localized exclusively at the midcell and FM 4-64 staining is bipolar (Fig. 6C). The midcell localization of FtsA-GFP in constricted cells is consistent with a role in cell division, as in other bacteria (14, 23). Cytokinesis produces two siblings with FtsA localization at two new growth poles (Fig. 6 D and E). Seventy-five percent of the cells exhibit FtsZ-GFP localization that mimics FtsA-GFP, suggesting similar cell cycle-specific regulation of both localization patterns. However, FtsZ-GFP exhibits additional localization patterns (two foci, bipolar foci, and polar clusters of foci) not observed for FtsA-GFP; these additional patterns may be either intermediate stages in Z-ring assembly or other novel Agrobacterium functions of FtsZ.
Fig. 6.
FM 4-64 labeling and FtsA localization indicate pole identity and cell-cycle progression. An Agrobacterium cell begins with an old pole (O) and a growth pole (G). FM 4-64 fluorescence intensity is indicated by the thickness of the cell outline in red. FtsA localization is indicated in green. Original O and G poles, nonbold font. Poles created during the cell cycle or poles that acquire a new pole identity, bold font. FtsZ exhibits a similar dynamic localization pattern correlated with cell-cycle progression. After cell division is complete, FtsA-GFP (A) is maintained in a unipolar focus at the growth poles of both daughter cells. After a period of growth, FtsA-GFP accumulates in a band at the midcell (B). When the cell begins to constrict, FtsA-GFP is localized exclusively at the midcell and FM 4-64 staining is bipolar (C). Cytokinesis produces two siblings with FtsA localization at two new growth poles (D and E).
The response of Agrobacterium to treatment with sublethal concentrations of Cb provides insight into its distinctive cell growth and division. Early on, cells continue to elongate in the presence of Cb and the growth pole maintains its normal morphology with a unipolar focus of FtsA-GFP. Thus, polar growth is mediated by transpeptidases that are insensitive to Cb. Indeed, our bioinformatics searches revealed that Agrobacterium and related Rhizobiales species have more genes encoding ld-transpeptidases in their genome than other rod-shaped bacteria that predominantly encode dd-transpeptidases, and ld-transpeptidases are generally insensitive to β-lactam antibiotics (24). That PG biosynthesis in Agrobacterium is distinct is supported by its unique muropeptide profile (4). Also, in contrast to polar growth, midcell PG synthesis before division is significantly affected by Cb and results in striking tire-like expansions at this site. In fact, Agrobacterium carries two pbp3 genes encoding the β-lactam–sensitive dd-transpeptidase Pbp3, a required divisome component in all species studied to date (1). Presumably, glycan strands and their associated peptide side chains are made by the divisome PG-synthesizing complex but peptide cross-linking of these strands by Cb-sensitive PBP3 is inhibited, rendering the midcell wall less able to resist turgor pressure generated in the cytoplasm. Besides tire-like extrusions, we also observe large spherical blebs at the midcell in Cb-treated cells, consistent with the hypothesis that the midcell is the site of active (dd-transpeptidase–mediated) PG synthesis and the region primarily affected by Cb.
In summary, unipolar growth in Agrobacterium can be observed by three different molecular markers: localization of FtsA and FtsZ to the growing pole, and lack of FM 4-64 staining of membranes at this growth pole. These three markers have identical labeling characteristics at the septum, namely positive localization of FtsA and FtsZ and lack of labeling with FM 4-64. As PG synthesis is most active at the growth pole and in the presumptive “ends” of the newly forming daughter cells at the septum, these data imply that FM 4-64 does not label membranes at sites of active PG synthesis. Interestingly, treatment of cells with Cb further distinguishes PG synthesis at the growth pole versus the septum, as only septal synthesis is sensitive to Cb. These latter data suggest that polar PG synthesis is likely carried out by Cb-insensitive transpeptidases. Finally, our data suggest that Agrobacterium has evolved to usurp divisome proteins (FtsA and FtsZ) to also mediate unipolar budding-type growth.
Materials and Methods
Strains, Growth, and Microscopy.
Agrobacterium tumefaciens strain C58, containing nopaline pTiC58, was transformed with the GFP-fusion constructs described below. For induction with isopropyl β-d-1-thiogalactopyranoside (IPTG), an overnight culture (Luria broth, 28 °C) with appropriate antibiotics was diluted to an A600 of 0.1 in minimal AB medium (pH 5.5) with appropriate antibiotics and grown for 5 h at 19 °C; these conditions are more similar to the soil environment in which A. tumefaciens grows naturally as well as vir induction conditions (9). IPTG was added to a final concentration of 5 mM, and cultures were incubated at 19 °C for 20–24 h before imaging. After IPTG induction, cells were labeled with FM 4-64 at 8 ng/μL for 5 min and then concentrated by centrifugation to an A600 of 10 before observation. Microscopy and image processing were performed as previously described (9).
Cloning.
The sGFP sequence was amplified by PCR from pJZGFP (9) with primers that introduced 5′ NdeI and 3′ HindIII restriction sites. This product was cloned into pSRKKm (13) for tightly regulated IPTG induction using the NdeI and HindIII restriction sites to create pJZ205. Sequences encoding FtsA (Atu2087) and FtsZ (Atu2086) were amplified from A. tumefaciens genomic DNA with 5′ and 3′ primers that introduced 5′ and 3′ NdeI sites. Both products were cloned separately into pJZ205 using the NdeI restriction site. Clones were verified by DNA sequencing. pJZ207 encodes FtsZ-sGFP and pJZ208 encodes FtsA-sGFP.
Supplementary Material
Acknowledgments
We thank Drs. Steven Ruzin and Denise Schichnes (Biological Imaging Facility, University of California, Berkeley); and Drs. Sharik Khan and Stephen Farrand (University of Illinois, Urbana–Champaign) for kindly providing pSRKKM and Dr. Joe Lutkenhaus (University of Kansas Medical Center) for kindly providing anti-FtsZ (E. coli) antibody. J.A.-F. acknowledges support from National Institutes of Health Genetics Training Grant GM007127. This research was supported by two National Science Foundation grants (to P.C.Z.), MCB-0923840 (American Recovery and Reinvestment Act Grant) and MCB-1243360.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307241110/-/DCSupplemental.
References
- 1.den Blaauwen T, de Pedro MA, Nguyen-Distèche M, Ayala JA. Morphogenesis of rod-shaped sacculi. FEMS Microbiol Rev. 2008;32(2):321–344. doi: 10.1111/j.1574-6976.2007.00090.x. [DOI] [PubMed] [Google Scholar]
- 2.Goley ED, et al. Assembly of the Caulobacter cell division machine. Mol Microbiol. 2011;80(6):1680–1698. doi: 10.1111/j.1365-2958.2011.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlinson AD, Fuqua C. Mechanisms and regulation of polar surface attachment in Agrobacterium tumefaciens. Curr Opin Microbiol. 2009;12(6):708–714. doi: 10.1016/j.mib.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown PJB, et al. Polar growth in the Alphaproteobacterial order Rhizobiales. Proc Natl Acad Sci USA. 2012;109(5):1697–1701. doi: 10.1073/pnas.1114476109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelvin SB. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev. 2003;67(1):16–37. doi: 10.1128/MMBR.67.1.16-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fronzes R, Christie PJ, Waksman G. The structural biology of type IV secretion systems. Nat Rev Microbiol. 2009;7(10):703–714. doi: 10.1038/nrmicro2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zechner EL, Lang S, Schildbach JF. Assembly and mechanisms of bacterial type IV secretion machines. Philos Trans R Soc Lond B Biol Sci. 2012;367(1592):1073–1087. doi: 10.1098/rstb.2011.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilar J, Cameron TA, Zupan J, Zambryski P. Membrane and core periplasmic Agrobacterium tumefaciens virulence type IV secretion system components localize to multiple sites around the bacterial perimeter during lateral attachment to plant cells. mBio. 2011;2(6):e00218-11. doi: 10.1128/mBio.00218-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguilar J, Zupan J, Cameron TA, Zambryski PC. Agrobacterium type IV secretion system and its substrates form helical arrays around the circumference of virulence-induced cells. Proc Natl Acad Sci USA. 2010;107(8):3758–3763. doi: 10.1073/pnas.0914940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron TA, Roper M, Zambryski PC. Quantitative image analysis and modeling indicate the Agrobacterium tumefaciens type IV secretion system is organized in a periodic pattern of foci. PLoS One. 2012;7(7):e42219. doi: 10.1371/journal.pone.0042219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addinall SG, Lutkenhaus J. FtsA is localized to the septum in an FtsZ-dependent manner. J Bacteriol. 1996;178(24):7167–7172. doi: 10.1128/jb.178.24.7167-7172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma X, Ehrhardt DW, Margolin W. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc Natl Acad Sci USA. 1996;93(23):12998–13003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan SR, Gaines J, Roop RM, II, Farrand SK. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl Environ Microbiol. 2008;74(16):5053–5062. doi: 10.1128/AEM.01098-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutkenhaus J, Pichoff S, Du S. Bacterial cytokinesis: From Z ring to divisome. Cytoskeleton. 2012;69(10):778–790. doi: 10.1002/cm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Margolin W. Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J Bacteriol. 1999;181(24):7531–7544. doi: 10.1128/jb.181.24.7531-7544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margolin W, Long SR. Rhizobium meliloti contains a novel second homolog of the cell division gene ftsZ. J Bacteriol. 1994;176(7):2033–2043. doi: 10.1128/jb.176.7.2033-2043.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potluri L-P, de Pedro MA, Young KD. Escherichia coli low-molecular-weight penicillin-binding proteins help orient septal FtsZ, and their absence leads to asymmetric cell division and branching. Mol Microbiol. 2012;84(2):203–224. doi: 10.1111/j.1365-2958.2012.08023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson HP, Anderson DE, Osawa M. FtsZ in bacterial cytokinesis: Cytoskeleton and force generator all in one. Microbiol Mol Biol Rev. 2010;74(4):504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barák I, Muchová K, Wilkinson AJ, O’Toole PJ, Pavlendová N. Lipid spirals in Bacillus subtilis and their role in cell division. Mol Microbiol. 2008;68(5):1315–1327. doi: 10.1111/j.1365-2958.2008.06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenarcic R, et al. Localisation of DivIVA by targeting to negatively curved membranes. EMBO J. 2009;28(15):2272–2282. doi: 10.1038/emboj.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishov I, Woldringh CL. Visualization of membrane domains in Escherichia coli. Mol Microbiol. 1999;32(6):1166–1172. doi: 10.1046/j.1365-2958.1999.01425.x. [DOI] [PubMed] [Google Scholar]
- 22.Adams DW, Errington J. Bacterial cell division: Assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7(9):642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- 23.Margolin W. Themes and variations in prokaryotic cell division. FEMS Microbiol Rev. 2000;24(4):531–548. doi: 10.1111/j.1574-6976.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 24.Mainardi JL, et al. A novel peptidoglycan cross-linking enzyme for a beta-lactam-resistant transpeptidation pathway. J Biol Chem. 2005;280(46):38146–38152. doi: 10.1074/jbc.M507384200. [DOI] [PubMed] [Google Scholar]
- 25.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2009. [Google Scholar]
- 26.R Development Core Team . Vienna: R Found Stat Comput; 2012. R: A Language and Environment for Statistical Computing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.