Abstract
The ability to rapidly respond to changes in temperature is a critical adaptation for insects and other ectotherms living in thermally variable environments. In a process called rapid cold hardening (RCH), insects significantly enhance cold tolerance following brief (i.e., minutes to hours) exposure to nonlethal chilling. Although the ecological relevance of RCH is well-established, the underlying physiological mechanisms that trigger RCH are poorly understood. RCH can be elicited in isolated tissues ex vivo, suggesting cold-sensing and downstream hardening pathways are governed by brain-independent signaling mechanisms. We previously provided preliminary evidence that calcium is involved in RCH, and here we firmly establish that calcium signaling mediates cold sensing in insect tissues. In tracheal cells of the freeze-tolerant goldenrod gall fly, Eurosta solidaginis, chilling to 0 °C evoked a 40% increase in intracellular calcium concentration as determined by live-cell confocal imaging. Downstream of calcium entry, RCH conditions significantly increased the activity of calcium/calmodulin-dependent protein kinase II (CaMKII) while reducing phosphorylation of the inhibitory Thr306 residue. Pharmacological inhibitors of calcium entry, calmodulin activation, and CaMKII activity all prevented ex vivo RCH in midgut and salivary gland tissues, indicating that calcium signaling is required for RCH to occur. Similar results were obtained for a freeze-intolerant species, adults of the flesh fly, Sarcophaga bullata, suggesting that calcium-mediated cold sensing is a general feature of insects. Our results imply that insect tissues use calcium signaling to instantly detect decreases in temperature and trigger downstream cold-hardening mechanisms.
Keywords: calcium imaging, cold acclimation, environmental stress, overwintering, physiological ecology
Low temperature is one of the primary constraints for insects and other ectotherms living in temperate and polar regions (1). Although seasonal adaptations to cold stress, including environmentally programmed periods of dormancy called diapause, have been well-studied (2–5), physiological responses to sudden changes in temperature have received less attention. In a process termed rapid cold hardening (RCH), insects dramatically enhance their cold tolerance in a matter of minutes to hours (6). For example, in the flesh fly, Sarcophaga crassipalpis, the first species in which RCH was described, exposure to 0 °C for as little as 30 min significantly enhances cold tolerance at −10 °C (6). RCH has since been described in dozens of insect species (7), including both freeze-intolerant (insects in which internal ice formation is lethal) and freeze-tolerant species (insects that tolerate internal ice formation) (8, 9). Naturally occurring thermoperiods can elicit RCH (10), and RCH preserves essential functions such as courtship and mating (11, 12), supporting the relevance of this process to natural populations.
Although the ecological relevance of RCH has been established, the physiological mechanisms are poorly understood. RCH results in a slight increase in the cryoprotectant glycerol (6, 13), changes in cell membrane composition and fluidity (14–16), and up-regulation of a single heat shock protein in the brain (17). However, gene expression does not appear to be a major driver of RCH; in the flesh fly, Sarcophaga bullata, no transcripts (out of ∼15,000 tested) were differentially expressed following 2 h of RCH (18) whereas, in Drosophila melanogaster, RCH failed to increase the expression of five genes previously linked to cold tolerance (19). Thus, we hypothesize that RCH is primarily mediated by second messenger systems that do not require the synthesis of new gene products. Indeed, signaling pathways such as MAP kinase (20, 21) and apoptosis signaling (22, 23) have been linked to RCH although the upstream cold-sensing mechanisms that trigger these pathways are unknown.
One of the most remarkable features of RCH is that isolated tissues retain the capacity for RCH ex vivo (24, 25), indicating that cells directly respond to low temperature in the absence of stimulation from the brain and hormones. However, the mechanism by which tissues detect changes in temperature and trigger downstream cold-hardening pathways is unknown. Chilling causes a rapid loss of ion homeostasis in insect tissues (26–33), but whether these ion movements play a role in tissue-level cold sensing has not been assessed. Cold acclimation in plants, which requires days to weeks for induction (34), is regulated by cold-induced calcium influx (35–37). In addition, fruit flies (D. melanogaster) with a mutant copy of the membrane protein dystroglycan have elevated levels of intracellular calcium, which correlates with improved performance and survival at low temperatures (38). We recently provided pharmacological evidence that calcium and calmodulin are required for RCH in the Antarctic midge, Belgica antarctica (25), but cold-induced calcium signaling has not been examined in detail. Here, we provide evidence that chilling rapidly elevates intracellular calcium, and that calcium- and calmodulin-dependent signaling events are required for cold sensing and RCH in insect tissues. Experiments were conducted in two well-studied models of insect cold tolerance, the freeze-tolerant goldenrod gall fly, Eurosta solidaginis, and the freeze-intolerant flesh fly, S. bullata. RCH is the fastest cold-hardening process that has been described, and we provide clear evidence of the underlying cold-sensing mechanisms.
Results and Discussion
Ex Vivo RCH in E. solidaginis.
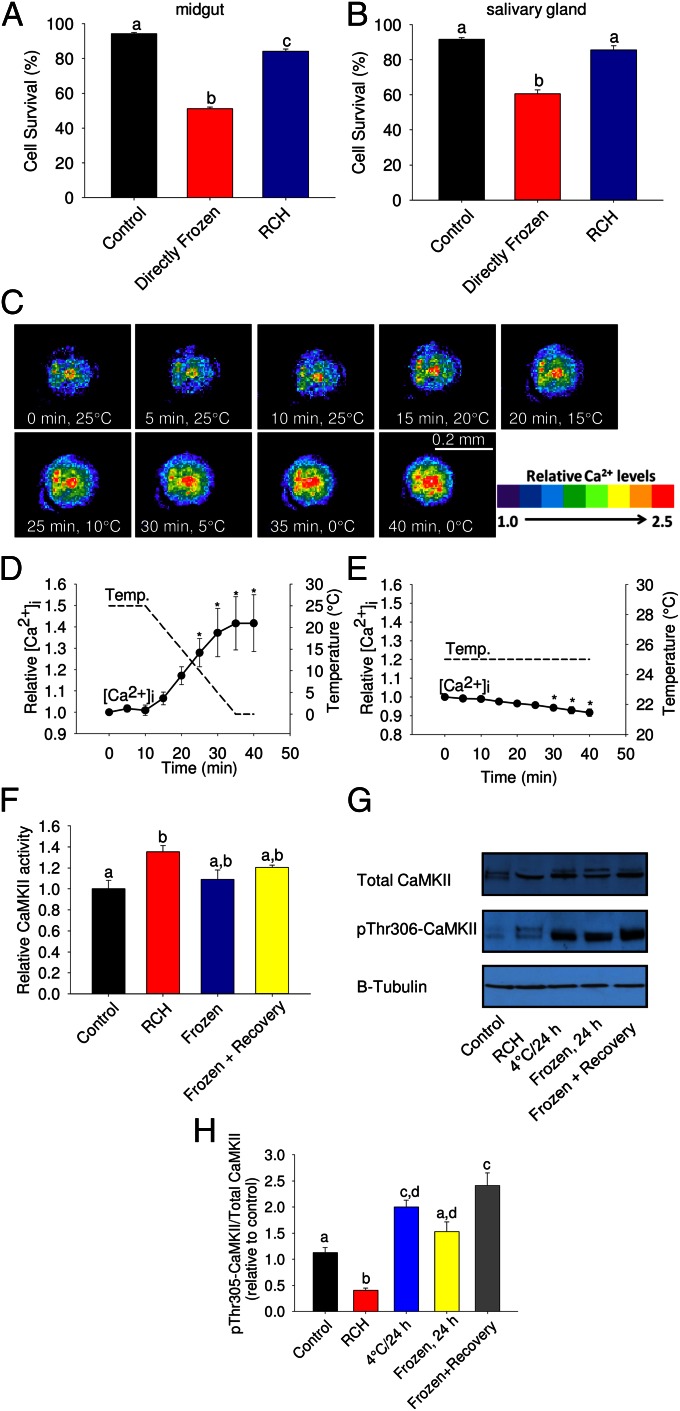
The goldenrod gall fly, E. solidaginis, is one of the most cold-hardy temperate species known and has long been a model for studying freeze tolerance (39). Larvae of E. solidaginis are capable of RCH (40) although the capacity for RCH in isolated tissues has not been addressed in this species. When midgut and salivary gland tissue from winter-acclimatized third instar larvae were removed and directly frozen at −20 °C, high mortality resulted. Cell survival of midgut tissue dropped to ∼50% (Fig. 1A) whereas that of the salivary gland was ∼60% (Fig. 1B), in contrast to untreated controls held at 4 °C, which had >90% survival (Fig. 1 A and B). However, when tissues were first given a 1-h RCH period before freezing, in which temperature was gradually lowered from 4 °C to −20 °C, survival increased by 25–30% in each tissue (Fig. 1 A and B). This cold-hardening response occurred ex vivo in the absence of stimulation from nerves or hormones, indicating that RCH in tissues of E. solidaginis is independent of the nervous system.
Fig. 1.
Rapid cold hardening reduces freezing injury in tissues of the goldenrod gall fly, E. solidaginis and activates calcium-signaling pathways. (A and B) Effect of ex vivo RCH (slow ramping from 4 °C to −20 °C over 1 h) on cell viability of (A) midgut and (B) salivary gland following freezing at −20 °C. (C–E) Intracellular calcium as a function of temperature in tracheal cells of E. solidaginis. (F) Activity of calcium/calmodulin-dependent protein kinase II (CaMKII) following RCH, freezing for 24 h, and freezing with 2 h recovery. (G) Levels of both total CaMKII and phospho-Thr306 CaMKII in response to low temperature. (H) The ratio of phospho-Thr306 CaMKII to total CaMKII in response to low temperature. In A and B, “Control” tissues were held at 4 °C for 3 h, “Directly Frozen” tissues were directly transferred from 18 °C to −20 °C for 2 h whereas “RCH” tissues were gradually chilled from 4 °C to −20 °C over 1 h, then held at −20 °C for 2 h. In C, relative calcium concentration of a representative tracheal end cell is indicated by the provided color scale. In D and E, an asterisk indicates a significant difference between a particular time point and the initial calcium concentration (repeated measures ANOVA, post hoc Bonferroni, P < 0.05). In F–H, CaMKII activity and phosphorylation were measured in whole-body protein extracts. All values are mean ± SEM, n ≥ 4, with different letters indicating significant differences (ANOVA, Tukey, P < 0.05).
Chilling Increases Intracellular Calcium in Tissues of E. solidaginis.
Using live-cell confocal imaging, we observed a 40% increase in intracellular calcium in tracheal cells of E. solidaginis when temperature was lowered from 25 °C to 0 °C at 1 °C/min (Fig. 1 C and D). Lowering temperature from 25 °C to 20 °C raised intracellular calcium by 7%, and calcium rose continuously as temperature decreased. Maintaining temperature at 25 °C for the duration of the 40-min experiment failed to elicit a significant increase in cytosolic calcium (Fig. 1E). Cold-induced increases in cytosolic calcium have also been demonstrated in plants (35) although cold exposure in plants causes a single, sharp spike in intracellular calcium, suggesting that a different mechanism is at play. Modest elevation of intracellular calcium at low temperature is also known in fish (41) and mammals (42, 43) although not in the context of cold hardening. In insects, disruption of ion gradients during chilling is responsible for the loss of neuromuscular function at low temperature (26–33), but whether these ion movements are used to trigger cold hardening has not been addressed.
RCH Activates Calcium-Dependent Signaling Pathways.
Downstream of calcium entry into the cell, chilling activated calcium-dependent signaling pathways in E. solidaginis. Specifically, we monitored the activity and phosphorylation state of the calcium-dependent signaling enzyme calcium/calmodulin dependent protein kinase II (CaMKII) in response to low temperature. CaMKII is a multifunctional signaling enzyme that regulates numerous metabolic and gene expression processes (44), and whereas this enzyme has been linked to cellular stress in mammalian systems (e.g., ref. 45), it has not been associated with environmental stress. In larvae of E. solidaginis, RCH conditions induced a significant increase (from 0.65 to 0.88 pmol⋅min−1⋅μg protein−1) in the activity of CaMKII (Fig. 1F). Although this protein was activated during RCH, after prolonged freezing at −15 °C for 24 h, with and without 2 h recovery at 18 °C, CaMKII activity decreased to levels that were indistinguishable from both control and RCH-treated larvae (Fig. 1F). Thus, activation of this protein occurred specifically during the RCH period. Additionally, RCH decreased phosphorylation at the inhibitory Thr306 residue by 60% (Fig. 1 G and H), which is consistent with enzyme activation (44). In contrast, chilling at 4 °C for 24 h and 2 h recovery from freezing significantly increased the phosphorylation ratio (Fig. 1 G and H), suggesting deactivation of this enzyme in response to prolonged chilling and recovery from freezing. We cloned and sequenced full-length calmodulin (GenBank accession no. KC810053) and CaMKII (GenBank accession no. KC810054) transcripts from E. solidaginis and measured their tissue-specific expression. Predicted amino acid sequences for both proteins indicated that this signaling axis is highly conserved across insects and mammals (Figs. S1A and S2A), and transcripts were expressed in all six larval tissues tested (Figs. S1B and S2B), suggesting that these genes could mediate cold sensing throughout the body.
Functional Significance of Calcium Signaling During RCH.
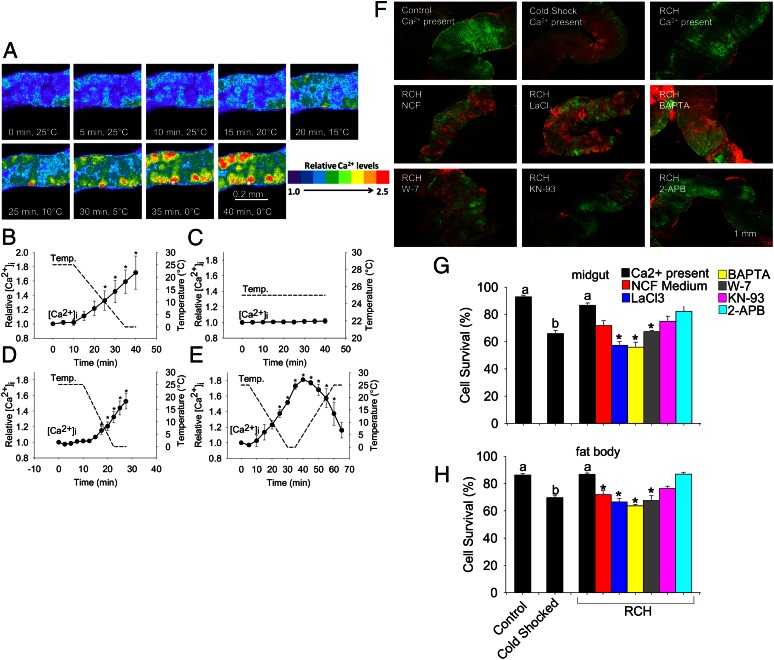
To determine the functional significance of calcium signaling during cold sensing, we used pharmacological inhibitors to block various components of calcium-signaling pathways during RCH. In midgut and salivary gland tissues of larval E. solidaginis, removing calcium from the medium, blocking calcium channels with LaCl3, and chelating intracellular calcium with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, acetoxymethyl ester (BAPTA-AM) all inhibited ex vivo RCH, reducing survival 17–47% relative to tissues incubated with calcium (Fig. 2). In addition, blocking calmodulin with the drug N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7) and inhibiting CaMKII with 2-[N-(2-hydroxyethyl)-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine (KN-93) similarly reduced cell survival following RCH (Fig. 2). However, inhibition of inositol triphosphate (IP3)-mediated calcium signaling had no effect on survival, suggesting that IP3-mediated calcium release is not involved in cold sensing. These drugs were not toxic to tissues held at 4 °C and had little effect on the survival of tissues directly frozen at −20 °C (Fig. S3 A and B). Furthermore, at milder temperatures, inhibition of calcium signaling reduced baseline freezing tolerance. After direct freezing at −17.5 °C, cell survival was significantly higher than at −20 °C (71.1% vs. 51.1% for midgut, 85.9% vs. 60.5% for salivary gland; Fig. S3 C and D), but inhibition of calcium signaling reduced survival at −17.5 °C (Fig. S3 E and F). These pharmacological data demonstrate that calcium entry is not simply a symptom of low temperature but is an essential cold-sensing mechanism mediating rapid responses to cold.
Fig. 2.
Pharmacological inhibition of calcium signaling blocks rapid cold hardening in tissues of E. solidaginis. (A) Fluorescent cell viability images of midgut tissue incubated with the indicated drug at the following temperature conditions: Control, 4 °C/3 h; Directly Frozen, direct transfer to −20 °C for 2 h; RCH, slow ramp from 4 °C to −20 °C over 1 h, then held at −20 °C for 2 h. Green nuclei represent live cells, and red nuclei represent dead cells. (B and C) Quantified viability results for (B) midgut and (C) salivary gland tissue. All values are mean ± SEM, n = 4, and different letters indicate significant differences among the three temperature treatments where an asterisk indicates a significant reduction in survival in the RCH group relative to tissues incubated with calcium present (ANOVA, Tukey, P < 0.05). NCF, nominally calcium free medium. LaCl3 is a general calcium channel blocker, BAPTA is an intracellular calcium chelator, W-7 is an antagonist of the calcium binding protein calmodulin, and KN-93 inhibits activation of CaMKII whereas 2-APB is an inhibitor of IP3-mediated calcium signaling.
Calcium Signaling also Mediates RCH in a Freeze-Intolerant Insect.
The above results clearly demonstrate a role for calcium signaling during RCH in larvae of E. solidaginis, a freeze-tolerant organism. Subsequent experiments indicated that calcium signaling also governs cold sensing and RCH in a freeze-intolerant species, adults of the flesh fly S. bullata. Salivary gland tissues from this species were more amenable to calcium imaging, allowing a more in-depth analysis of the dynamics of cold-induced calcium movements. Chilling to 0 °C elicited a 70% increase in intracellular calcium in adult salivary gland tissue (Fig. 3 A and B) whereas maintaining tissues at 25 °C for the duration of the experiment failed to evoke a calcium response (Fig. 3C). When the cooling rate was increased to 2 °C/min, calcium concentrations showed a similar increase although the concentration at a given temperature was consistently lower than when tissues were cooled at 1 °C/min (Fig. 3D). Warming back to 25 °C from 0 °C caused an immediate reversal in calcium flux (Fig. 3E), which may in part explain the rapid attenuation of RCH upon warming (46).
Fig. 3.
Calcium signaling also mediates cold sensing and rapid cold hardening in a freeze-intolerant fly, S. bullata. Intracellular calcium concentration in salivary glands in response to (A and B) chilling at 1 °C/min, (C) control conditions of 25 °C, (D) chilling at 2 °C/min, and (E) chilling at 1 °C/min followed by warming at 1 °C/min. (F) Fluorescent cell viability images of midgut tissue incubated with the indicated drug at the following temperature conditions: Control: 25 °C/4 h; Cold Shock: −14 °C/2 h; RCH: 0 °C/2 h, −14 °C/2 h. Green nuclei represent live cells and red nuclei represent dead cells. (G and H) Quantified viability results for (G) midgut and (H) fat body tissue. Values are mean ± SEM, n = 3 for (B–D), n = 2 for (E), n = 4 for (G and H). In A, relative calcium concentration of a representative segment of salivary gland tissue is indicated by the provided color scale. In B–E, an asterisk indicates a significant difference between a particular time point and the initial calcium concentration (repeated measures ANOVA, post hoc Bonferroni, P < 0.05). In G and H, an asterisk indicates a significant reduction in survival in the RCH group relative to tissues incubated with calcium present (ANOVA, Tukey, P < 0.05). NCF, nominally calcium free medium. LaCl3 is a general calcium channel blocker, BAPTA is an intracellular calcium chelator, W-7 is an antagonist of the calcium binding protein calmodulin, and KN-93 inhibits activation of CaMKII whereas 2-APB is an inhibitor of IP3-mediated calcium signaling.
Like E. solidaginis, RCH in isolated tissues of S. bullata was dependent on calcium-signaling pathways. For these experiments, RCH was generated by directly transferring tissues to 0 °C for 2 h, before transferring tissues to the discriminating temperature (−14 °C) for 2 h. This stepwise protocol is the typical approach for demonstrating RCH in the laboratory (7). In both midgut and fat body tissue from adults of S. bullata, blocking calcium entry and calmodulin activation prevented RCH (Fig. 3 F–H) whereas these drugs had no effect on control tissues and tissues directly cold shocked at −14 °C (Fig. S3 G and H). In this species, calmodulin (GenBank accession no. KC810055) and CaMKII (GenBank accession no. KC810056) were expressed in all postembryonic developmental stages and in nearly every tissue (Figs. S4 and S5), suggesting that this signaling axis could mediate cold sensing throughout the body in every developmental stage. Thus, although the capacity for freeze tolerance has evolved numerous times in the insect lineage (47), cold-induced calcium signaling appears to be a general response to chilling in insects.
Conceptual Model for Calcium-Mediated Cold Sensing.
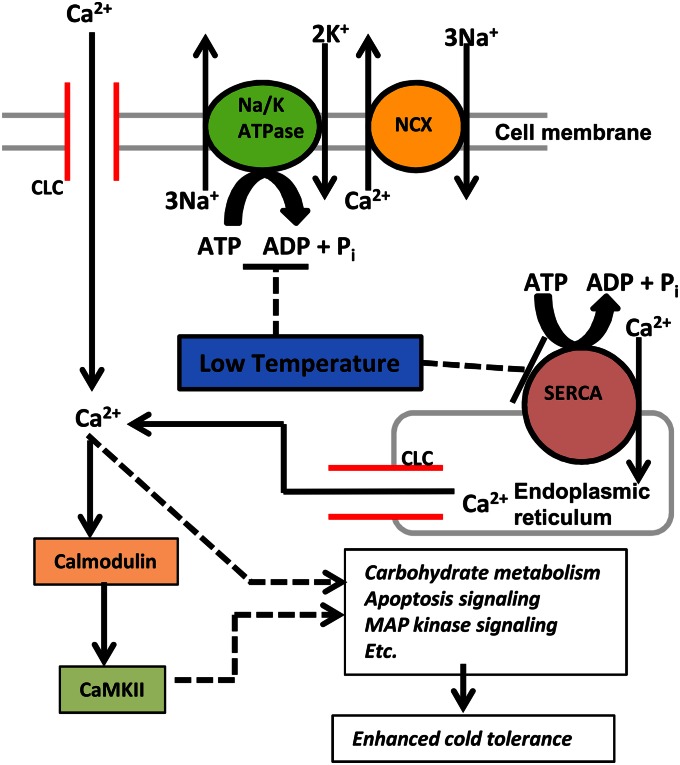
The results presented here represent a significant advance in our understanding of the cell physiology of insect low temperature response. A hypothetical model for the mode of calcium entry and the downstream targets of calcium signaling in response to low temperature is presented in Fig. 4. Low temperature directly inhibits ATP-dependent calcium-exporting functions, such as the Na+/Ca2+-exchanger coupled to Na+/K+-ATPase, causing a gradual leak of calcium ions into the cell (33). This mechanism of cold-induced calcium influx is different from cold sensing in the nervous system, which involves direct activation of excitable, cold-sensitive transient receptor potential channels in sensory neurons (48) that carry the signal to specific regions of the brain (49). Although we cannot rule out that RCH is simply responding to cold injury, with calcium influx as the token symptom, the observation that calcium levels closely track environmental temperature (Fig. 1 C and D and 3 B–E) suggests a general cold-sensing role for calcium. Although the downstream targets of calcium signaling during RCH have not been established, calcium signaling interacts with a number of processes known to be important for cold hardening, including apoptosis signaling (22, 23) and carbohydrate mobilization (50). In the cold hardy atsugari mutant of D. melanogaster, increased intracellular calcium improves cold tolerance by boosting mitochondrial metabolism at low temperature (38), and subsequent experiments will test whether metabolic changes associated with RCH (e.g., 13, 18, 51) are indeed calcium-regulated. Also, although the present study addressed only the calcium/calmodulin/CaMKII signaling axis, several other calcium-dependent signaling pathways are known to mediate stress responses. For example, the calcium-dependent enzyme apoptosis signal-regulating kinase I regulates p38 MAP kinase signaling (52), thus providing a potential link between calcium and p38 signaling (20) during RCH.
Fig. 4.
Working model for the role of calcium signaling during cold sensing and rapid cold hardening. Low temperature causes an increase in intracellular calcium concentration, and we hypothesize that this calcium influx occurs by low temperature inhibition of ATP-dependent calcium export mechanisms coupled with calcium entry through calcium leak channels (CLC). In this model, low temperature inhibits the activity of both sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA) and sodium/potassium ATPase (Na/K ATPase) coupled to the sodium calcium exchanger (NCX). Inside the cell, calcium, via calcium/calmodulin-dependent protein kinase II (CaMKII) and other unknown mechanisms, triggers pathways involved in rapid cold hardening, thereby enhancing the cell’s cold tolerance. Dashed lines and arrows indicate speculative relationships that were not experimentally determined in the present study.
Calcium signaling provides a direct, intuitive means by which isolated insect tissues detect and respond to low temperature. Although the sensing mechanisms governing behavioral responses to low temperature in the nervous system are well-established (48), cold-sensing mechanisms in nonnervous tissues have been hitherto unexplored. Understanding the mechanisms by which insects tolerate low temperature is essential for predicting their response to a changing climate (53). Furthermore, mechanisms of cold hardening can be exploited to manipulate populations of insect pests (54). Our group has recently explored the possibility of disrupting overwintering diapause to control pest populations (55), and we envision disruption of acute cold hardening to be an equally effective strategy.
Methods
Animals.
Galls containing third instar larvae of E. solidaginis, located on the stems of senesced Solidago canidensis plants, were collected from various goldenrod fields in central and southwest Ohio from September to January 2007–2012. Galls were stored at 18 °C, and larvae were removed just before experimentation. Flesh flies, S. bullata, were laboratory-reared according to Denlinger et al. (56) under nondiapausing conditions (25 °C, 16:8 light:dark). Adults were fed sugar and liver ad libitum, and males were used for experiments 4–8 d after eclosion.
Calcium Imaging.
Tissues from E. solidaginis and S. bullata were dissected in Coast’s solution (57) containing (in mM) 100 NaCl, 8.6 KCl, 4.0 NaHCO3, 4.0 NaH2PO4-H2O, 1.5 CaCl2-2H2O, 8.5 MgCl2-6H2O, 24 glucose, 25 Hepes, and 56 sucrose. For experiments with E. solidaginis, the Coast’s solution was supplemented with 250 mM glycerol. After dissection, tissues were loaded with 10 µM fluo-3 AM (Life Technologies) for 1 h at room temperature. The loading solution contained 0.2% pluronic F-127 (Life Technologies) to disperse the dye. After loading, cells were rinsed twice in Coast’s solution, kept in the dark at room temperature, and imaged within the next 3 h. Preliminary experiments established that tracheal cells from E. solidaginis and salivary gland cells from S. bullata were most amenable to calcium imaging. Other tissues were difficult to maintain in the proper focal plane or failed to take up the calcium imaging dye. Tissues were imaged with a Visitech Infinity3 Hawk 2D Array live-cell confocal microscope at the Ohio State Campus Microscopy and Imaging Facility. Cells were excited at 488 nm, and fluorescence at 520 nM was measured every 10 s for the duration of the experiment. Temperature was controlled with an Instec TSA02i inverted microscope thermal stage (Instec). Although images were acquired every 10 s, low temperature caused a slight drift in the focal plane in most samples, necessitating periodic refocusing. As a result, samples were refocused every 2.5–5 min, so results are presented only in 2.5- to 5-min increments. Fluorescence was recorded in a region of interest that consisted of a single tracheal end cell in E. solidaginis and four to five salivary gland epithelial cells in S. bullata. Intracellular calcium concentration was calculated according the following equation: [Ca2+]i = Kd(F/Fo)/(Kd/[Ca2+]i-rest + 1 − F/Fo), where F is the fluorescence, Fo is the initial fluorescence, and [Ca2+]i-rest is the resting calcium concentration, which was estimated to be 100 nm, based on measurements of blow fly salivary glands conducted by Zimmermann and Walz (58). The Kd for fluo-3 was corrected for changes in temperature according to Woodruff et al. (59).
CaMKII Assays.
CaMKII activity was measured with the Signatect CaMKII Assay Kit (Promega). In this assay, CaMKII transfers phosphate from [γ-32P]ATP to a synthetic substrate for CaMKII, which is immobilized on a biotinylated membrane and measured with a scintillation counter. Protein was extracted from whole larvae of E. solidaginis with radioimmunoprecipitation assay buffer containing the Halt Protease and Phosphatase Inhibitor mixture (ThermoFisher Scientific), and 25 µg of protein was loaded into each reaction. For Western blotting, protein samples were generated in the same manner, and 35 µg of protein from each sample was loaded into a 4–15% gradient SDS/PAGE gel. Western blotting was conducted according to Yi et al. (23). Rabbit anti-CaMKII (total) was obtained from Santa Cruz Biotechnology and rabbit anti-CAMKII (pThr305) was obtained from Millipore whereas mouse anti-β-tubulin was obtained from the Developmental Studies Hybridoma Bank. Densitometry was conducted with AlphaView SA software (ProteinSimple), with each sample normalized to β-tubulin. Each Western blot was repeated with six independent biological replicates. To verify whether mammalian-based assays and antibodies would work, we cloned and characterized full-length calmodulin and CaMKII cDNA sequences using the Clontech SMARTer RACE kit (Clontech Laboratories). Additionally, in E. solidaginis, we measured tissue specific expression in larval brains, midguts, fat body, salivary glands, Malpighian tubules, and epidermis using RT-PCR (Figs. S1 and S2). In S. bullata, we measured both developmental and tissue-specific expression profiles (Figs. S4 and S5). Primer sequences for RACE and PCR detection of transcripts are provided in Table S1.
Cell Viability Assays.
Tissues were dissected in Coast’s solution at room temperature. Tissues from E. solidaginis were exposed to the following temperature conditions: control (4 °C, 3 h), directly frozen (directly transferred from room temperature to −17.5 or −20 °C, and held there for 2 h), and RCH (slowly ramped from 4 °C to test temperature over 1 h, then held at test temperature for 2 h). For S. bullata, tissues were exposed to control (25 °C, 4 h), cold shock (−14 °C, 2 h), and RCH (0 °C 2h, −14 °C 2h) conditions; all tissues remained supercooled at −14 °C. Cell viability was assessed with the LIVE/DEAD sperm viability assay (Life Technologies) (24), which is a membrane integrity assay consisting of SYBR green and propidium iodide. In this assay, living cells with intact membranes fluoresce green whereas dead cells with damaged membranes fluoresce red. Viability is expressed as the percentage survival based on the counts of 300 cells per sample. To test whether calcium signaling is essential for RCH, tissues were exposed to the following solutions and drugs to manipulate calcium signaling: nominally calcium free (NCF) medium, Coast’s solution prepared without calcium; 250 µM LaCl3, a general calcium channel blocker; 100 µM BAPTA-AM, an intracellular calcium chelator; 50 µM W-7, an inhibitor of calmodulin; 100 µM KN-93, an inhibitor of CaMKII, and 10 µM 2-aminoethoxydiphenyl borate (2-APB), an inhibitor of IP3-mediated calcium signaling. Tissues were loaded with drugs for 30–60 min before conducting the same temperature experiments described above.
Statistics.
All data are expressed as mean ± SE. Data from calcium-imaging experiments were analyzed with repeated measures ANOVA and a post hoc Bonferroni test. All other means were compared with ANOVA and Tukey’s post hoc multiple comparisons procedure in JMP9 (SAS Institute). For calcium-imaging experiments, n = 2–5 for each experiment whereas, for CaMKII assays, n = 5–6 for each treatment. Cell viability assays were conducted with four biological replicates per treatment, and data were arcsin square-root transformed before analysis.
Supplementary Material
Acknowledgments
We thank Sarah Cole, Brian Kemmenoe, Richard Montione, and other members of the Ohio State Campus Microscopy and Imaging Facility for assistance with these experiments. We also thank Dr. Michael Ibba for providing laboratory space for experiments requiring radiolabeled substrates, Josh Stapleton for help with densitometry, and members of the Laboratory for Ecophysiological Cryobiology at Miami University for assistance with gall collecting. Peter Piermarini and Larry Phelan, Ohio State University, kindly provided comments on a draft of this manuscript. We acknowledge Brent Sinclair, University of Western Ontario, and John Duman, University of Notre Dame, for critically reviewing the paper. This work was supported by National Science Foundation Grant IOS-0840772.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KC810053–KC810056).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306705110/-/DCSupplemental.
References
- 1. Denlinger DL, Lee RE (1998) Physiology of cold sensitivity. Temperature Sensitivity in Insects and Application in Integrated Pest Management, eds Hallman GJ, Denlinger DL (Westview, Boulder, CO), pp 55–95.
- 2.Denlinger DL, Yocum GD, Rinehart JP. 2005. Hormonal control of diapause. Comprehensive Insect Molecular Science, eds Gilbert LI, Iatrou K, Gill S (Elsevier, Amsterdam), pp 615–650.
- 3.Denlinger DL. 1991. Relationship between cold hardiness and diapause. Insects at Low Temperature, eds Lee RE, Denlinger DL (Chapman and Hall, New York), pp 174–198.
- 4.Hahn DA, Denlinger DL. Energetics of insect diapause. Annu Rev Entomol. 2011;56:103–121. doi: 10.1146/annurev-ento-112408-085436. [DOI] [PubMed] [Google Scholar]
- 5.Kostál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52(2):113–127. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Lee RE, Jr, Chen CP, Denlinger DL. A rapid cold-hardening process in insects. Science. 1987;238(4832):1415–1417. doi: 10.1126/science.238.4832.1415. [DOI] [PubMed] [Google Scholar]
- 7.Lee RE, Denlinger DL. 2010. Rapid cold-hardening: Ecological significance and underpinning mechanisms. Low Temperature Biology of Insects, eds Denlinger DL, Lee RE (Cambridge Univ Press, Cambridge, UK), pp 35–58.
- 8.Lee RE, Jr, et al. Rapid cold-hardening increases the freezing tolerance of the Antarctic midge Belgica antarctica. J Exp Biol. 2006;209(3):399–406. doi: 10.1242/jeb.02001. [DOI] [PubMed] [Google Scholar]
- 9.Everatt MJ, Worland MR, Bale JS, Convey P, Hayward SAL. Pre-adapted to the maritime Antarctic?—Rapid cold hardening of the midge, Eretmoptera murphyi. J Insect Physiol. 2012;58(8):1104–1111. doi: 10.1016/j.jinsphys.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Kelty J. Rapid cold-hardening of Drosophila melanogaster in a field setting. Physiol Entomol. 2007;32(4):343–350. [Google Scholar]
- 11.Shreve SM, Kelty JD, Lee RE., Jr Preservation of reproductive behaviors during modest cooling: Rapid cold-hardening fine-tunes organismal response. J Exp Biol. 2004;207(11):1797–1802. doi: 10.1242/jeb.00951. [DOI] [PubMed] [Google Scholar]
- 12.Rinehart JP, Yocum GD, Denlinger DL. Thermotolerance and rapid cold hardening ameliorate the negative effects of brief exposures to high or low temperatures on fecundity in the flesh fly, Sarcophaga crassipalpis. Physiol Entomol. 2000;25(4):330–336. [Google Scholar]
- 13.Michaud MR, Denlinger DL. Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): A metabolomic comparison. J Comp Physiol B. 2007;177(7):753–763. doi: 10.1007/s00360-007-0172-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee RE, Jr, Damodaran K, Yi S-X, Lorigan GA. Rapid cold-hardening increases membrane fluidity and cold tolerance of insect cells. Cryobiology. 2006;52(3):459–463. doi: 10.1016/j.cryobiol.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Michaud MR, Denlinger DL. Oleic acid is elevated in cell membranes during rapid cold-hardening and pupal diapause in the flesh fly, Sarcophaga crassipalpis. J Insect Physiol. 2006;52(10):1073–1082. doi: 10.1016/j.jinsphys.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Overgaard J, Sørensen JG, Petersen SO, Loeschcke V, Holmstrup M. Changes in membrane lipid composition following rapid cold hardening in Drosophila melanogaster. J Insect Physiol. 2005;51(11):1173–1182. doi: 10.1016/j.jinsphys.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Li A, Denlinger DL. Rapid cold hardening elicits changes in brain protein profiles of the flesh fly, Sarcophaga crassipalpis. Insect Mol Biol. 2008;17(5):565–572. doi: 10.1111/j.1365-2583.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 18.Teets NM, et al. Combined transcriptomic and metabolomic approach uncovers molecular mechanisms of cold tolerance in a temperate flesh fly. Physiol Genomics. 2012;44(15):764–777. doi: 10.1152/physiolgenomics.00042.2012. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Mol Biol. 2007;16(4):435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara Y, Denlinger DL. p38 MAPK is a likely component of the signal transduction pathway triggering rapid cold hardening in the flesh fly Sarcophaga crassipalpis. J Exp Biol. 2007;210(18):3295–3300. doi: 10.1242/jeb.006536. [DOI] [PubMed] [Google Scholar]
- 21.Li F-F, Xia J, Li J-M, Liu S-S, Wang X-W. p38 MAPK is a component of the signal transduction pathway triggering cold stress response in the MED cryptic species of Bemisia tabaci. J Integr Agric. 2012;11(2):303–311. [Google Scholar]
- 22.Yi S-X, Lee RE., Jr Rapid cold-hardening blocks cold-induced apoptosis by inhibiting the activation of pro-caspases in the flesh fly Sarcophaga crassipalpis. Apoptosis. 2011;16(3):249–255. doi: 10.1007/s10495-010-0570-0. [DOI] [PubMed] [Google Scholar]
- 23.Yi S-X, Moore CW, Lee RE., Jr Rapid cold-hardening protects Drosophila melanogaster from cold-induced apoptosis. Apoptosis. 2007;12(7):1183–1193. doi: 10.1007/s10495-006-0048-2. [DOI] [PubMed] [Google Scholar]
- 24.Yi S-X, Lee RE., Jr In vivo and in vitro rapid cold-hardening protects cells from cold-shock injury in the flesh fly. J Comp Physiol B. 2004;174(8):611–615. doi: 10.1007/s00360-004-0450-4. [DOI] [PubMed] [Google Scholar]
- 25.Teets NM, et al. Rapid cold-hardening in larvae of the Antarctic midge Belgica antarctica: Cellular cold-sensing and a role for calcium. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1938–R1946. doi: 10.1152/ajpregu.00459.2007. [DOI] [PubMed] [Google Scholar]
- 26.Macmillan HA, Sinclair BJ. Mechanisms underlying insect chill-coma. J Insect Physiol. 2011;57(1):12–20. doi: 10.1016/j.jinsphys.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 27.MacMillan HA, Sinclair BJ. The role of the gut in insect chilling injury: Cold-induced disruption of osmoregulation in the fall field cricket, Gryllus pennsylvanicus. J Exp Biol. 2011;214(5):726–734. doi: 10.1242/jeb.051540. [DOI] [PubMed] [Google Scholar]
- 28.MacMillan HA, Williams CM, Staples JF, Sinclair BJ. Reestablishment of ion homeostasis during chill-coma recovery in the cricket Gryllus pennsylvanicus. Proc Natl Acad Sci USA. 2012;109(50):20750–20755. doi: 10.1073/pnas.1212788109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kostál V, Renault D, Mehrabianová A, Bastl J. Insect cold tolerance and repair of chill-injury at fluctuating thermal regimes: Role of ion homeostasis. Comp Biochem Physiol A Mol Integr Physiol. 2007;147(1):231–238. doi: 10.1016/j.cbpa.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Kostál V, Vambera J, Bastl J. On the nature of pre-freeze mortality in insects: Water balance, ion homeostasis and energy charge in the adults of Pyrrhocoris apterus. J Exp Biol. 2004;207(9):1509–1521. doi: 10.1242/jeb.00923. [DOI] [PubMed] [Google Scholar]
- 31.Kostál V, Yanagimoto M, Bastl J. Chilling-injury and disturbance of ion homeostasis in the coxal muscle of the tropical cockroach (Nauphoeta cinerea) Comp Biochem Physiol B Biochem Mol Biol. 2006;143(2):171–179. doi: 10.1016/j.cbpb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Kristiansen E, Zachariassen KE. Effect of freezing on the transmembrane distribution of ions in freeze-tolerant larvae of the wood fly Xylophagus cinctus (Diptera, Xylophagidae) J Insect Physiol. 2001;47(6):585–592. doi: 10.1016/s0022-1910(00)00157-8. [DOI] [PubMed] [Google Scholar]
- 33.Zachariassen KE, Kristiansen E, Pedersen SA. Inorganic ions in cold-hardiness. Cryobiology. 2004;48(2):126–133. doi: 10.1016/j.cryobiol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Thomashow MF. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 35.Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8(3):489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monroy AF, Dhindsa RS. Low-temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25 °C. Plant Cell. 1995;7(3):321–331. doi: 10.1105/tpc.7.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monroy AF, Sarhan F, Dhindsa RS. Cold-induced changes in freezing tolerance, protein-phosphorylation, and gene exression - Evidence for a role of calcium. Plant Physiol. 1993;102(4):1227–1235. doi: 10.1104/pp.102.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeuchi K, et al. Changes in temperature preferences and energy homeostasis in dystroglycan mutants. Science. 2009;323(5922):1740–1743. doi: 10.1126/science.1165712. [DOI] [PubMed] [Google Scholar]
- 39.Bennett VA, Lee RE. Modeling seasonal changes in intracellular freeze-tolerance of fat body cells of the gall fly Eurosta solidaginis (Diptera, Tephritidae) J Exp Biol. 1997;200(1):185–192. doi: 10.1242/jeb.200.1.185. [DOI] [PubMed] [Google Scholar]
- 40.Levis NA, Yi SX, Lee RE., Jr Mild desiccation rapidly increases freeze tolerance of the goldenrod gall fly, Eurosta solidaginis: Evidence for drought-induced rapid cold-hardening. J Exp Biol. 2012;215(21):3768–3773. doi: 10.1242/jeb.076885. [DOI] [PubMed] [Google Scholar]
- 41.Shiels HA, Di Maio A, Thompson S, Block BA. 2011. Warm fish with cold hearts: Thermal plasticity of excitation-contraction coupling in bluefin tuna. Proc R Soc B Biol Sci 278(1702):18–27. [DOI] [PMC free article] [PubMed]
- 42.Haddad P, Cabrillac JC, Piche D, Musallam L, Huet PM. Changes in intracellular calcium induced by acute hypothermia in parenchymal, endothelial, and Kupffer cells of the rat liver. Cryobiology. 1999;39(1):69–79. doi: 10.1006/cryo.1999.2186. [DOI] [PubMed] [Google Scholar]
- 43.Wang SQ, Lakatta EG, Cheng H, Zhou ZQ. Adaptive mechanisms of intracellular calcium homeostasis in mammalian hibernators. J Exp Biol. 2002;205(19):2957–2962. doi: 10.1242/jeb.205.19.2957. [DOI] [PubMed] [Google Scholar]
- 44.Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378(1):1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119(10):2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coulson SJ, Bale JS. Characterization and limitations of the rapid cold-hardening response in the housefly Musca domestica (Diptera, Muscidae) J Insect Physiol. 1990;36(3):207–211. [Google Scholar]
- 47.Sinclair BJ, Addo-Bediako A, Chown SL. Climatic variability and the evolution of insect freeze tolerance. Biol Rev Camb Philos Soc. 2003;78(2):181–195. doi: 10.1017/s1464793102006024. [DOI] [PubMed] [Google Scholar]
- 48.Rosenzweig M, Kang KJ, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci USA. 2008;105(38):14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144(4):614–624. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson LN. Glycogen phosphorylase: Control by phosphorylation and allosteric effectors. FASEB J. 1992;6(6):2274–2282. doi: 10.1096/fasebj.6.6.1544539. [DOI] [PubMed] [Google Scholar]
- 51.Overgaard J, et al. Metabolomic profiling of rapid cold hardening and cold shock in Drosophila melanogaster. J Insect Physiol. 2007;53(12):1218–1232. doi: 10.1016/j.jinsphys.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Takeda K, et al. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004;5(2):161–166. doi: 10.1038/sj.embor.7400072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bale JS, Hayward SAL. Insect overwintering in a changing climate. J Exp Biol. 2010;213(6):980–994. doi: 10.1242/jeb.037911. [DOI] [PubMed] [Google Scholar]
- 54.Lee RE, Lee MR, Strong-Gunderson JM. Insect cold-hardiness and ice nucleating active microorganisms including their potential use for biological-control. J Insect Physiol. 1993;39(1):1–12. [Google Scholar]
- 55.Zhang Q, Nachman RJ, Kaczmarek K, Zabrocki J, Denlinger DL. Disruption of insect diapause using agonists and an antagonist of diapause hormone. Proc Natl Acad Sci USA. 2011;108(41):16922–16926. doi: 10.1073/pnas.1113863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denlinger DL. Induction and termination of pupal diapause in Sarcophaga (Diptera - Sarcophagidae) Biol Bull. 1972;142(1):11–24. [Google Scholar]
- 57.Coast GM, Krasnoff SB. Fluid secretion by single isolated Malpighian tubules of the house cricket, Acheta domesticus, and their response to diuretic hormone. Physiol Entomol. 1988;13(4):381–391. [Google Scholar]
- 58.Zimmermann B, Walz B. The mechanism mediating regenerative intercellular Ca2+ waves in the blowfly salivary gland. EMBO J. 1999;18(12):3222–3231. doi: 10.1093/emboj/18.12.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodruff ML, et al. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542(3):843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.