Abstract
The differentiated state of mature cells of adult organisms is achieved and maintained through the epigenetic regulation of gene expression, which consists of several mechanisms including DNA methylation. The advent of induced pluripotent stem cell technology enabled the conversion of adult cells into any other cell type passing through a stable pluripotency state. However, indefinite pluripotency is unphysiological, inherently labile, and makes cells prone to culture-induced alterations. The direct conversion of one cell type to another without an intermediate pluripotent stage is also possible but, at present, requires the viral transfection of appropriate transcription factors, limiting its therapeutic potential. The aim of this study was to investigate whether it is possible to achieve the direct conversion of an adult cell by exposing it to a demethylating agent immediately followed by differentiating culture conditions. Adult human skin fibroblasts were exposed for 18 h to the DNA methyltransferase inhibitor 5-azacytidine, followed by a three-step protocol for the induction of endocrine pancreatic differentiation that lasted 36 d. At the end of this treatment, 35 ± 8.9% fibroblasts became pancreatic converted cells that acquired an epithelial morphology, produced insulin, and then released the hormone in response to a physiological glucose challenge in vitro. Furthermore, pancreatic converted cells were able to protect recipient mice against streptozotocin-induced diabetes, restoring a physiological response to glucose tolerance tests. This work shows that it is possible to convert adult fibroblasts into insulin-secreting cells, avoiding both a stable pluripotent stage and any transgenic modification.
Keywords: pancreatic beta cell, cell plasticity
Regenerative medicine requires new cells that can be delivered to patients for repairing and renovating degenerated or damaged tissues (1). When such cells are not readily available, two main strategies have been developed to obtain them: directed differentiation, by which pluripotent cells, exposed to specific cell culture conditions designed to mimic natural events, assume a specific cell fate, and transdifferentiation, also referred to as reprogramming, which enables a fully differentiated cell type to be converted into another without going through an undifferentiated pluripotent stage (2).
Induced pluripotent stem cell (iPSC) technology showed that the stability of a mature phenotype can be overcome when transforming a somatic cell of any patient in an unlimited source of autologous pluripotent cells. The elimination of the immune rejection risk provided by iPSCs immediately boosted the clinical potential of directed differentiation (3). However, the requirement of permanent integration of viral vectors into the host genome to generate iPSCs poses a severe limit to their current therapeutic use (1). This has stimulated the development of several protocols for a virus-free iPSC derivation, but at present, these approaches are generally more technically demanding and less efficient, and therefore have not gained a widespread adoption (2). Similar limitations apply to recent examples of successful transdifferentiation, as the direct conversion of one mature cell type into another has been obtained only with virus-based transfection protocols. As for iPSC derivation, virus-free transdifferentiation protocols would be highly desirable and are being actively pursued (4, 5).
The differentiated state of mature cells of adult organisms is acquired through a gradual loss of differentiative potency (6), leading to a progressive restriction in their options (7), and is physiologically very stable. Differentiation is achieved and maintained through the epigenetic regulation of gene expression, which consists of different mechanisms including DNA methylation, histone modification, nucleosome packaging and rearrangements, higher-order chromatin structures, and the interplay between chromatin and nuclear lamina (8). For this reason, the complete reversal of this process requires extensive reprogramming that makes it inefficient and prone to errors (9).
We reasoned that part of the problem may derive from considering the achievement of a stable pluripotent state as an indispensable step, even if this does not occur during embryonic development, where pluripotency is limited to a short window of time.
Among the different mechanisms involved in lineage specification and cellular reprogramming, DNA demethylation plays a major role both during early embryonic development and during somatic cell reprogramming (10). Therefore, we tested whether a short exposure to a demethylating agent is sufficient to allow the direct conversion of an adult mature cell into another differentiated cell type. To this purpose, we selected the cytidine analog 5-azacytidine (5-aza-CR), a well-characterized DNA methyltransferase inhibitor known to activate the expression of silent genes (11) and to alter the differentiation state of embryonic (12) and mesenchymal cell lines (13).
This approach would be useful for therapeutic applications only if it can be applied to easily accessible primary cells; therefore, we worked on adult dermal fibroblasts that can be simply propagated in vitro with a stable phenotype and that are little prone to genetic instability. 5-aza-CR-treated skin fibroblasts have been exposed to an endoderm differentiation protocol, and we observed the efficient formation of pancreatic beta cell-like cells. We monitored cell morphology and gene expression pattern (Table S1) during the process and confirmed that fibroblasts were transiting along a brief dedifferentiation state and were readdressed to the new cell lineage. Pancreatic converted cells (PCCs) were shown to express early and mature pancreatic lineage specific markers. A functional beta-cell phenotype was validated by their intracellular production and storage of insulin. Furthermore, exposure to glucose triggered a dynamic response, inducing active insulin release in cell supernatants. On transplantation into SCID, mice PCCs were able to protect them against streptozotocin (STZ)-induced hyperglycemia.
Results
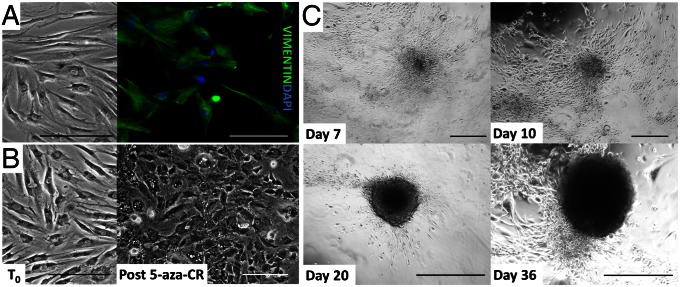
Fibroblasts obtained from skin biopsies formed a monolayer (Fig. 1A), displaying the vigorous growth in culture typical of this cell population (doubling time, 18–24 h; Fig. S1A) and a standard elongated morphology. Uniform immunostaining with the fibroblast-specific marker vimentin (Fig. 1A) and total absence of pluripotency or endoderm/pancreatic markers expression allowed us to determine that we worked with homogeneous cell populations (Fig. 2 and Figs. S2–S4).
Fig. 1.
Morphological changes in adult human skin fibroblasts exposed to 5-aza-CR and subjected to endocrine pancreatic induction. (A) Immunolocalization of vimentin, the typical fibroblast intermediate filament protein. Uniform positivity indicated a homogenous cell population at the onset of the experiments. (Scale bar, 200 µm.) (B) Untreated cells (T0) underwent marked morphological changes in response to an 18-h exposure to 5-aza-CR (post 5-aza-CR). Fibroblasts changed their typical elongated shape into a round epithelioid aspect. Cell size was smaller, and nuclei became larger and more granular. (Scale bar, 200 µm.) (C) Representative pictures of the morphological changes taking place during endocrine pancreatic induction. Cells exposed to activin A gradually organized in clusters (day 7). In response to the addition of retinoic acid, they rearranged in a reticular pattern and clustered in distinguishable aggregates (day 10). These formations progressed with time and were further stimulated by B27/bFGF/ITS, which led to the recruitment of a growing number of cells, aggregating in large 3D colonies (day 20). Finally, colonies became spherical structures that tended to detach and float freely in the culture medium, reminiscent of typical pancreatic islets in vitro (day 36). (Scale bar, 400 µm.)
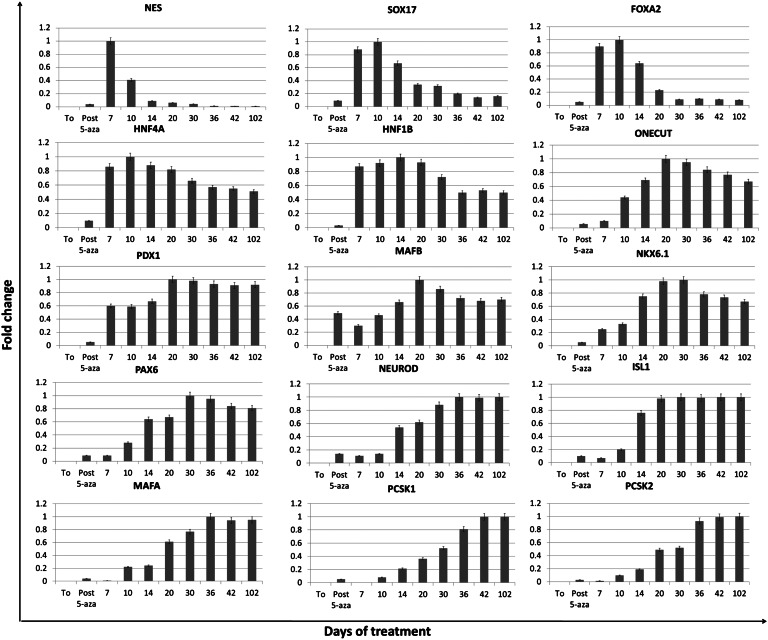
Fig. 2.
Gene expression changes in adult human skin fibroblasts exposed to 5-aza-CR and subjected to endocrine pancreatic induction. Expression pattern of markers of early (NES, SOX17, FOXA2, HNF4A, HNF1B, ONECUT, PDX1, and MAFB) and mature pancreatic precursors (NKX6.1, PAX6, NEUROD, ISL1, MAFA, PCSK1, and PCSK2) in untreated fibroblasts (T0), in fibroblast exposed to 5-aza-CR (post 5-aza-CR), and at different days of pancreatic induction (days 7–102).
After a series of preliminary experiments, we established that 1 µM 5-aza-CR for 18 h represented the optimal combination for dermal fibroblasts. At the end of this treatment, cells were allowed to recover for 3 h in embryonic stem cell (ESC) medium (14). An extensive change of cell phenotype became visible (Fig. 1B), with the typical elongated morphology of untreated fibroblasts [at time 0 (T0)] being replaced by a round or oval shape and with cell size becoming considerably smaller (post 5-aza-CR). Cytoplasm became granular, flattened, and more adherent to the culture surface. Nuclei appeared larger and vacuolated, which is a morphological aspect correlated to a relaxed chromatin structure (15).
Morphological changes were accompanied by the down-regulation of vimentin (VIM); the onset of pluripotency gene expression [POU class 5 homeobox 1 (OCT4), sex determining region Y-box 2 (SOX2), Nanog homeobox (NANOG), and ZFP42 zinc finger protein (REX1)], which were undetectable in untreated fibroblasts (Figs. S2 and S4); and a decrease of global DNA methylation, as indicated by 5-methylcytidine immunostaining (Fig. S4).
Cell proliferation rapidly decreased after exposure to 5-aza-CR (Fig. S1A), and during the following induction period, cell number remained stable as a result of a low apoptotic rate balanced by a similar proliferation rate (Fig. S1B).
Immediately after the 3-h recovery period, we exposed the cells to a three-step protocol originally developed for the pancreatic induction of mouse ESC (16) that was adapted to 5-aza-CR treated human fibroblasts. During the first step, cells were exposed for 7 d to activin A for promoting endoderm commitment. In this interval, cells further flattened and gradually began to organize in clusters (day 7; Fig. 1C). At the same time, pluripotency genes and vimentin were down-regulated (Figs. S2 and S4), whereas we detected an up-regulation of nestin (NES), a gene transiently involved in multilineage progenitor cell differentiation (17), as well as of genes involved in the induction of definitive endoderm [sex determining region Y-box 17 (SOX17), forkhead box A2, (FOXA2)] and primitive gut tube [hepatocyte nuclear factor 4, alpha, (HNF4A), HNF1 homeobox B (HNF1B)] intermediate states (18) (Figs. 2 and 3 and Fig. S4).
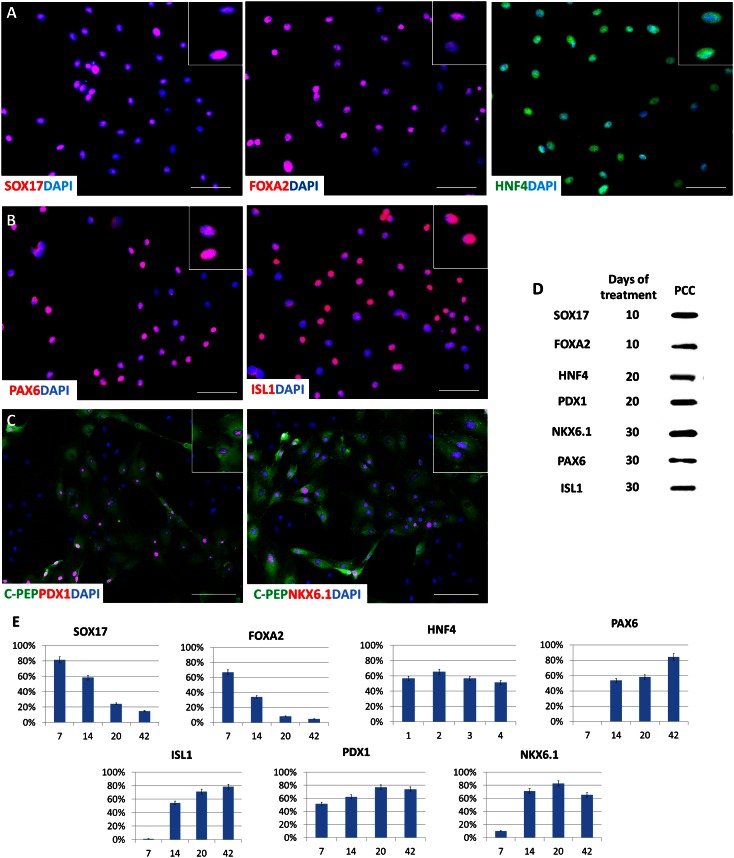
Fig. 3.
Immunocytochemical localization of endoderm and pancreatic markers during human skin fibroblasts’ conversion to endocrine pancreatic cells. (A) Immunolocalization of endoderm (SOX17, FOXA2) and primitive gut tube (HNF4) markers in fibroblasts subjected to pancreatic induction for 10 d. (B) Immunolocalization of PAX6 and ISL1, markers of more advanced pancreatic differentiation, on day 30 after exposure to 5-aza-CR. (C) Colocalization of PDX1 and NKX6.1 with C-peptide was also observed on day 30 after exposure to 5-aza-CR. (D) Western blot immunodetection of SOX17, FOXA2, HNF4, PDX1, NKX6.1, PAX6, and ISL1 in PCCs on different days of treatment. (E) Proportion of positive cells for the different molecules during the differentiation process in vitro. (Scale bars, 200 µm.)
In response to the addition of retinoic acid, used in the second step to further promote pancreatic lineage differentiation in combination with activin A, cells rearranged in a reticular pattern and grew in clearly distinguishable cell aggregates (day 10; Fig. 1C). In parallel, as cells proceeded along the differentiation process, expression of early markers of pancreas progenitor cells [one cut homeobox 1 (ONECUT), pancreatic and duodenal homeobox 1 (PDX1)] were induced and persisted, whereas NES, SOX17 and FOXA2 began to gradually decrease (Figs. 2 and 3E).
The clustering formation process increased with time and was further stimulated by the third step, which consisted of exposure to B27/basic fibroblast growth factor (bFGF)/insulin-transferrin-selenium (ITS) and led to the recruitment of a growing number of cells that aggregated in large 3D colonies (day 20; Fig. 1C).
Around day 36, these colonies appeared as distinct round structures reminiscent of typical pancreatic islets in vitro (19) (day 36; Fig. 1C). Expression of v-maf musculoaponeurotic fibrosarcoma oncogene homolog B (MAFB), NK6 homeobox 1 (NKX6.1), paired box 6 (PAX6), neuronal differentiation 1 (NEUROD), ISL LIM homeobox 1 (ISL1), v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MAFA), proprotein convertase subtilisin/kexin type 1 (PCSK1) and proprotein convertase subtilisin/kexin type 2 (PCSK2) paralleled cell differentiation progression (Figs. 2 and 3). The contemporary increase of the pancreatic precursor marker PDX1 (Figs. 2 and 3) suggests an early appearance of endodermal precursors within the induced population. PAX6, NEUROD, ISL1, MAFA, PCSK1, and PCSK2 displayed a low transcription level until day 10 of induction but exhibited a steadily increasing expression thereafter (Fig. 2), consistent with their key role in cell commitment toward the pancreatic lineage (20, 21). ISL1 is considered a transcriptional activator for insulin, and its interaction with NEUROD is essential and required for transcriptional activity of the insulin gene in pancreatic β-cells (22). In agreement with these data, in our experiments, the simultaneous and coordinated expression of these genes appeared to induce an increase in insulin expression, which was originally undetectable in untreated fibroblasts (T0) and shortly after exposure to 5-aza-CR (post 5-aza-CR), but was present from day 14 onward (Fig. 4 and Fig. S5). The colocalization of C-peptide with both PDX1 and NKX6.1 further confirmed the bona fide nature of PCCs as insulin-producing cells (Fig. 3C).
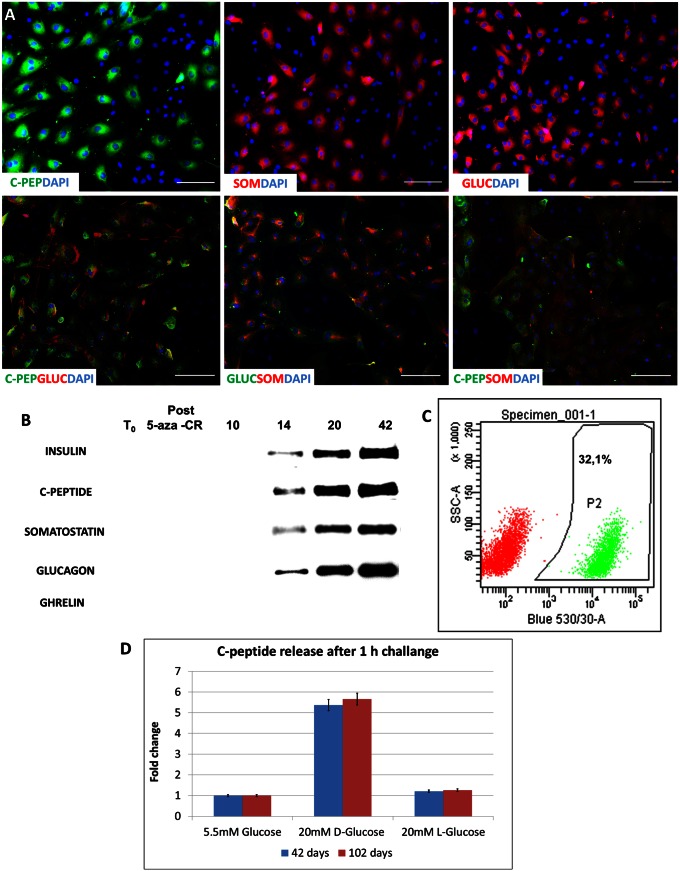
Fig. 4.
Morphologic characterization of human PCCs. (A) Immunostaining of PCCs after 36 d of culture reveals a clear signal of C-peptide, somatostatin, and glucagon in positive cells (Upper). Although some cells expressing only C-peptide can be seen, most cells are positive for more than one hormone (Lower). (Scale bar, 200 µm.) (B) Representative Western blot analysis of constitutive proteins collected at different times of culture. Insulin, C-peptide, somatostatin, and glucagon were detected from day 14 and steadily increased. Consistent with the absence of its mRNA, ghrelin was not detectable. β-Actin was used to check that an equal protein amount was loaded on each lane. (C) Representative output of flow cytometer analysis showing the efficiency toward β-cell differentiation measured counting C-peptide-labeled cells. (D) Quantification of C-peptide release in the culture medium in response to 20 mM d-glucose for 1 h at a different time of culture. Bars represents the mean ± SD of three independent replicates.
Cell differentiation peaked at day 36 and remained stable up to 102 d, when cultures were arrested, maintaining the distinct spherical structure organization (Fig. 1C) as well as the transcription pattern described earlier (Fig. 2 and Fig. S5). Efficiency toward β-cell differentiation was 35 ± 8.9%, as measured counting C-peptide positive cells with a flow cytometer (Fig. 4C).
Exposure to 5-aza-CR resulted in a decrease of 5-methylcytidine signal intensity, which gradually returned to the levels observed in untreated fibroblasts within 3 d of pancreatic induction (Figs. S4 and S6). At the same time, down-regulation of vimentin was observed, together with a contemporary increase of octamer-binding protein 4 (OCT4) and homeobox protein NANOG (Nanog) immunolabeling, which remained clearly visible until day 4. Thereafter, both transcription factors were down-regulated, whereas SRY-related HMG-box transcription factor SOX17 (Sox17) and hepatocyte nuclear factor 4 (Hnf4) signals became gradually more intense (Fig. S4).
If fibroblasts exposed to 5-aza-CR were returned to standard fibroblast culture medium in the absence of any inductive stimulus, they progressively reverted to the original phenotype (Fig. S7A). Within 4 d, the expression of pluripotency genes was completely down-regulated and the vimentin level was back to that of untreated fibroblasts, indicating that the alteration of their differentiation state is transient, reversible, and does not involve toxic effects on the cells (Fig. S7B). This is further supported by cytogenetic analysis showing that treated cells maintained a normal karyotype throughout the entire length of the experiment (Fig. S7C).
Active transcription of α-cell-related genes such as PAX6, MAFB, and PCSK2 together with MAFA, PCSK1, and glucokinase, typically expressed by β-cells, suggested the formation of polyhormonal structures (Fig. 4A). This was confirmed by the observation of PCC transcription for insulin, glucagon somatostatin, and pancreatic polypeptide (Fig. S5). The active synthesis of these hormones was demonstrated by Western blot analysis from day 14 of pancreatic induction and increased along the process (Fig. 4B) in parallel with the respective mRNA transcription (Fig. S5). However, we were unable to detect ghrelin mRNA (Fig. 4B and Fig. S5).
Because changes in ambient glucose represent the primary and physiological stimulus for insulin secretion, we investigated whether PCCs, at day 42 of pancreatic induction, were able to respond to a 20-mM d-glucose exposure. C-peptide release in cell supernatants after a short (1 h) or prolonged (24 h) exposure demonstrated that glucose triggered a dynamic response, inducing active C-peptide secretion in the culture medium (Fig. 4D and Fig. S8). This ability appeared to be stably maintained in time, as PCCs also were able to respond to glucose stimulation in a comparable way after 102 d of culture. Furthermore, after exposure to an equimolar amount of l-glucose, only baseline amounts of the hormone were released, indicating the specific nature of the cellular response.
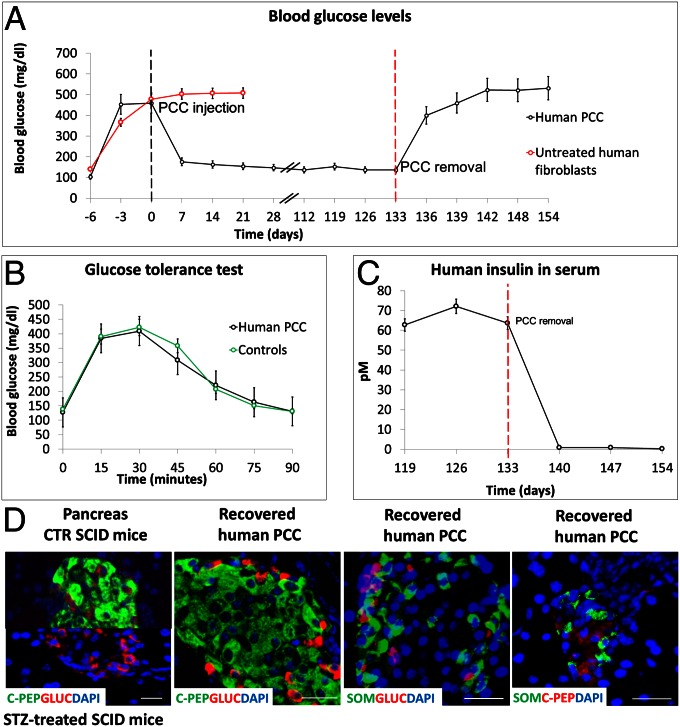
To further test the functional efficiency of pancreatic conversion, 5 × 106 PCCs, cultured for 42 d, were injected into the dorsal s.c. region of 5 SCID mice whose β-cells had been selectively destroyed with STZ (23). The same number of untreated fibroblasts were injected in 5 control mice. PCCs were able to quickly restore the initial glucose blood level and to maintain its physiological level for 133 d in all animals (Fig. 5A), whereas animals injected with untreated fibroblasts died after 4 wk. PCC removal was followed by a rapid increase of glycemia (Fig. 5A), and human insulin, previously detectable in the serum of engrafted mice, rapidly decreased on graft removal (Fig. 5C). During PCC engraftment, SCID mice were subjected to glucose tolerance tests three times at 1-wk intervals. PCCs were able to restore normal glycemic values after 75 min (Fig. 5B). In agreement with recent results (24), recovered human PCC grafts showed the presence of cells individually positive for C-peptide, glucagon, or somatostatin (Fig. 5D).
Fig. 5.
Functional characterization of human PCCs. (A) S.c. injection of 5 × 106 PCCs in STZ-treated SCID mice promptly decreased their glucose blood levels. Glucose levels remained constant up to 133 d. Injection of the same number of untreated fibroblasts did not elicit any effect, and mice died after 4 wk. Removal of PCCs from STZ-treated mice caused a rapid rise in glycemic values, indicating that PCCs were the functional source of insulin. (B) I.p. injection of 3 g per kilogram body weight induced a rise of blood glucose concentration that returned to basal level within 90 min, both in PCC-engrafted and control mice. The test was repeated 3 times at 1-wk intervals. (C) Levels of human insulin in the serum of STZ-treated mice during PCC engraftment and after its removal. (D) Immunolocalization of C-peptide and glucagon in pancreatic islets of control and STZ SCID mice indicate the selective destruction of beta cells. (Scale bar, 20 µm.) Immunolocalization of C-peptide, glucagon, and somatostatin in human PCCs removed from SCID mice. Merged costaining demonstrates that each cell produces a single hormone. (Scale bar, 50 µm.)
Discussion
The capacity of 5-aza-CR to alter gene expression and to transform cell phenotype has been known for a long time (12, 25–27). These interesting observations were based on spontaneous differentiation of immortalized (28) or tumor-derived cell lines (13, 29) occurring several days after the exposure to the drug, which lead to a conversion process characterized by variable efficiency and stability, as well as being mainly limited within the mesoderm linages.
Our results expand these data and show that if a brief exposure of adult primary cells to 5-aza-CR is immediately followed by a specific induction protocol, it leads to efficient conversion into a mature and functional phenotype that spans two different germ lineages. Treated primary cultures of adult dermal fibroblasts respond to a three-step pancreatic differentiation protocol, leading to their differentiation into insulin-secreting cells that are glucose-responsive in vitro and can discriminate between d- and l-glucose, which indicates the attainment of an advanced state of functional differentiation (30). This compares favorably with previous works in which human endocrine pancreatic cells have been generated from ESC, but in which the in vitro differentiation process reached only the fetal β-cell stage characterized by the release of C-peptide in response to a variety of stimuli, but only minimally to glucose (18). Full differentiation of glucose-responsive endocrine cells was obtained only on transplantation of the ESC-derived equivalent of fetal 6- to 9-wk pancreatic tissue into STZ-treated mice for many weeks (21). Alternatively, glucose responsiveness in vitro could be obtained by treating ESC-derived definitive endoderm with a combination of FGF 10 and indolactam V (31). The same cells maintained their ability to secrete insulin after their transplantation under the kidney capsule of nude mice, but in two of six cases, a tumor formation was observed (31), confirming the limitations associated with the use of ESC cells. This was not the case with PCCs, which always maintained a normal karyotype and never induced tumor formation in transplanted mice. In addition, the somatic origin of PCCs makes them potentially patient-specific, similar to iPS and as opposed to ESC.
At present, the differentiation of human iPS into β-like pancreatic cells is possible but has been characterized only partially. In one case, iPS-derived insulin-secreting cells were described as being responsive to a glucose challenge in vitro (32), and in another work, differentiated cells were characterized by examining the expression of beta cell genes after their spontaneous differentiation in embryoid bodies and teratomas or by the presence of detectable serum levels of human C-peptide on the transplantation of pancreatic endocrine progenitors in SCID mice (33). Alternative methods for generating β-like cells include in vivo transfection with three key transcription factors [PDX1, neurogenin 3 (NGN3) and MAFA] of mouse pancreatic exocrine cell (34) or liver cells (35). Irrespective of the degree of functional maturation and efficiency achieved, all these methods require the use of viral vectors, which inherently limits their clinical application potential (1). This is not the case with PCCs, which do not require any genetic modification.
Exposure to 5-aza-CR for 48 h or more has been recently shown to improve the overall efficiency of the reprogramming process required to obtain iPS cells, even if, to maintain a stable undifferentiated phenotype, viral transfection of exogenous transcription factors is still required (36). This effect of 5-aza-CR has been explained as demethylation lowering the energy gradients that must be covered in a counter-current direction to achieve the desired level of plasticity, thus facilitating the transition of mature cells to a higher plasticity state (6). In our work, we took advantage of the same mechanism, but as we did not aim at achieving a stable pluripotency stage, a shorter exposure to 5-aza-CR was sufficient to trigger responsiveness to a directed differentiation protocol.
We determined that a 18-h exposure is the best compromise between efficiency and toxicity that, with longer exposures, leads to an increasing amount of cell death. A possible explanation of the rapid increase of cell toxicity is that 80–90% of 5-aza-CR is incorporated directly into RNA (37). Despite the limited time of exposure, the differentiation process of fibroblasts into β-like cells was accomplished with a remarkable efficiency of over 30%, whereas previously reported differentiation rates of human ESC ranged from 0.8% to 7% (18, 38). This can be explained by the fact that 5-aza-CR is metabolized to 5-aza-2′-deoxycytidine-triphosphate to become incorporated into DNA, where it substitutes for cytidine.
Methyltransferases normally initiate the methylation reaction by a nucleophilic attack, which results in the establishment of a covalent bond between the carbon-6 atom of the cytosine ring and the enzyme. This bond is normally resolved by beta-elimination through the carbon-5 atom, but the reaction is blocked with 5-aza-CR, where carbon-5 is substituted by nitrogen. Thus, the enzyme remains covalently bound to DNA, and its DNA methyltransferase function is blocked (37). As a consequence, as little as 5% of 5-aza-CR substitution is sufficient to cause the demethylation of 85–90% of the genome (39, 40), and the hemimethylation of a few critical sites that takes place within 2 h of exposure is sufficient to induce vast changes in chromatin condensation (41). Therefore, the activation of specific genes is independent from the effect of 5-aza-CR on the respective specific loci. In addition, 5-aza-CR cellular uptake is mediated by four transporter protein families, which include the ubiquitously expressed equilibrative uniporters SLC29A family, enabling a rapid and widespread transport across the cell membrane (37). At the same time, it is important to note that the alteration of the differentiation state induced by this treatment was transient, reversible, and did not involve any toxic effects on the cells, as indicated by the fact that when fibroblasts were returned to standard fibroblast culture medium, they rapidly reverted to the original phenotype. This is consistent with our observations showing that global DNA demethylation takes place shortly after the exposure to 5-aza-CR and that the initial levels are restored within 3–4 d.
Insulin-producing cells generated with this method responded well to a wide range of challenges both in vitro and in vivo, indicating the achievement of a mature differentiated phenotype. This can be related to the fact that, together with insulin, glucagon and somatostatin-producing cells were generated at the end of the procedure. Previous work on mouse ESC, in fact, demonstrated that these cells were unable to properly differentiate in β-cells until they were layered with other endocrine cells (42), highlighting the role of the surrounding microenvironment physiologically provided by the pancreatic islet of Langerhans. The lack of differentiation into epsilon cells and ghrelin expression was a notable exception. A recent study on human pancreas development showed that epsilon cells have developmental features that are distinct from those of the other endocrine cells (43) and that at any stage of pancreatic development through to adult life, ghrelin peptide production is confined to a distinct cell type (18, 44, 45). These data suggest that epsilon cell ontogeny is somehow separated from the rest of the pancreatic endocrine cells and that the conversion process induced by the protocol used in our current study does not recapitulate this part of the pancreatic development.
In conclusion, we suggest that the combined and sequential action of 5-aza-CR with an induction protocol enables an efficient interlineage conversion, as well as a controlled, homogeneous, and stable cell differentiation. Because it avoids the requirement for a stable pluripotent state, the procedure is much closer to the physiological induction process that takes place during embryonic development. The possibility of efficiently converting easily available fibroblasts into insulin-secreting cells with no need for gene transfection represents a promising therapeutic perspective for diabetes as well as for the generation of cells that are otherwise difficult to obtain.
Experimental Procedures
Culture of Skin Fibroblasts.
Two adult human skin fibroblast primary lines were kindly donated by Gianpaolo Zerbini (Scientific Institute San Raffaele, University of Milan, Milan, Italy) and isolated from two female adults aged 35 and 49 y, as previously described and approved by the Ethical Committee of the Department of Internal Medicine of the University of Genoa. (46). Briefly, a skin specimen of ∼2 mm3 was taken by excision under local anesthesia from an avascular area of the anterior aspect of the forearm. Cells were grown to confluence in 60-mm culture dishes in minimum DMEM supplemented with 20% (vol/vol) FBS. After four passages, fibroblasts were harvested and frozen in liquid nitrogen in several aliquots. Another two lines, derived from neonatal foreskin, were commercially available (Gentaur SC101A-HFF and ATCC PCS-201-010). After thawing, cells were grown in standard culture medium consisting of DMEM with 20% (vol/vol) FBS (Gibco), 2 mM 14 glutamine (Sigma), and antibiotics (Sigma). Cells were passaged twice a week in a 1:3 ratio. All experiments were performed on at least three lines.
Treatment of Skin Fibroblasts with 5-Aza-CR.
Cells were plated in a 0.1% gelatin (Sigma) precoated 4-well multidish (Nunc) and exposed to 1 µM 5-aza-CR (Sigma) for 18 h. Concentration and time of exposure were selected according to data in the literature (47) and based on preliminary experiments in which different doses and different incubation times were tested. At the end of the 18-h exposure, cells were rinsed three times and incubated for 3 h with ES cell culture medium (14).
Pancreatic Induction.
Cells were cultured in N2B27 medium supplemented with 0.1 mM β-mercaptoethanol (Sigma), 2 mM glutamine (Sigma), 1 mM MEM nonessential amino acids (Gibco), and 0.5% BSA (Sigma). During the first 6 d, medium was supplemented with 30 ng/mL activin A (Biosource). On day 7, 10 µM retinoic acid (Sigma) was added. Two days later, medium was refreshed and replaced with medium supplemented with 1% B27 (Invitrogen), 20 ng/mL basic fibroblast growth factor (R&D System), and 1% insulin-transferrin-selenium (Invitrogen) to further encourage differentiation. Medium was refreshed daily. Cells were maintained in vitro for a total of 102 d, at which time cultures were arrested.
Cell Analysis.
Cells were analyzed at the following times: untreated fibroblasts (T0), after 18 h exposure to 5-aza-CR (post 5-aza-CR), and then on days 7, 10, 14, 20, 30, 36, 42, and 102 of pancreatic induction. All procedures are described in detail in the SI Experimental Procedures.
PCC Transplantation into Diabetic SCID Mice.
Experimental diabetes was induced in 8-wk-old male SCID mice (Harlan) by a single i.p. injection of STZ (Sigma, 150 mg/kg of body weight) freshly dissolved in 0.1 M citrate buffer at pH 4.6 (23). Six days after STZ injection, the average blood glucose level was 426.30 ± 38.82 mg/dL. After isoflurane anesthesia, cells were injected s.c. in the shoulder area through a 19-gauge hypodermic needle. Five animals received 5 × 106 PCCs, and 5 received the same number of untreated fibroblasts. Blood glucose levels were measured using Accu-Chek glucometer (Roche) at 1-wk intervals.
Glucose Tolerance Test.
Mice fasted for 20 h. Glucose was administered as an i.p. injection of a 30% (wt/vol) dextrose solution at a dose of 3.0 g per kg body weight. Tail blood glucose levels were measured with an Accu-Chek glucometer (Roche) before and 15, 30, 45, 60, 75, and 90 min after glucose administration. Data were analyzed with an independent-samples t test (two-tailed, type 2), using SPSS 19.0, and all values are presented as means ± SD. Differences of P ≤ 0.05 were considered significant.
ELISA.
Blood samples were collected from mice three times at 1-wk intervals during PCC engraftment and after its removal. ELISAs for human C-peptide were performed on serum samples, as described by the manufacturer (Mercodia Insulin ELISA #10-1113-01).
Removal of Grafted PCC.
To verify the PCCs ability to restore blood glucose homeostasis in STZ-treated mice, PCC engrafts were surgically removed from mice under general anesthesia. Blood glucose levels were then monitored in the animals for 3 wk, using an Accu-Chek glucometer (Roche).
Immunofluorescence Analyses.
Pancreas of nontreated and STZ-treated mice and removed grafts were fixed with 10% (wt/vol) formaldehyde for 24 h at 4°C. Tissues were embedded in paraffin and cut into 5-µm sections. Slides were deparaffinized and rehydrated. Aspecific sites were blocked with a solution of PBS containing 5% (wt/vol) BSA and 10% (vol/vol) nonimmune serum. Samples were incubated overnight at 4°C with antibodies specific for insulin, glucagon, and somatostatin (Table S2). Sections were washed three times with PBS and incubated with suitable secondary antibodies (Alexafluor, Invitrogen) for 45 min. Nuclei were stained with DAPI. Slides were observed under a Nikon Eclipse 600 microscope.
Supplementary Material
Acknowledgments
We thank Gianpaolo Zerbini for kindly providing human dermal fibroblasts, Valentina Castiglioni for help with flow cytometry, Valentina Bollati for making available facilities and reagents for methylation analysis, and Alessandro Addis for help with surgical procedures. The study was funded by the Istituto Nazionale Genetica Molecolare (G.P.), Network Lombardo iPS (NetLiPS) Project ID 30190629, Associazione Italiana per la Ricerca sul Cancro (AIRC) IG 10376, and the Carraresi Foundation. The authors are members of the COST Action FA1201 Epiconcept: Epigenetics and Periconception environment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220637110/-/DCSupplemental.
References
- 1.Lengner CJ. iPS cell technology in regenerative medicine. Ann N Y Acad Sci. 2010;1192:38–44. doi: 10.1111/j.1749-6632.2009.05213.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen DE, Melton D. Turning straw into gold: Directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12(4):243–252. doi: 10.1038/nrg2938. [DOI] [PubMed] [Google Scholar]
- 3.Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest. 2010;120(1):51–59. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci. 2010;31(1):36–45. doi: 10.1016/j.tips.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22(7):833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 6.Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: Digging Waddington’s canal. Nat Rev Mol Cell Biol. 2009;10(8):526–537. doi: 10.1038/nrm2727. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Q, Melton DA. Extreme makeover: Converting one cell into another. Cell Stem Cell. 2008;3(4):382–388. doi: 10.1016/j.stem.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Liu G-H, Izpisua Belmonte JC. Navigating the epigenetic landscape of pluripotent stem cells. Nat Rev Mol Cell Biol. 2012;13(8):524–535. doi: 10.1038/nrm3393. [DOI] [PubMed] [Google Scholar]
- 9.Plath K, Lowry WE. Progress in understanding reprogramming to the induced pluripotent state. Nat Rev Genet. 2011;12(4):253–265. doi: 10.1038/nrg2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu SC, Zhang Y. Active DNA demethylation: Many roads lead to Rome. Nat Rev Mol Cell Biol. 2010;11(9):607–620. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones PA. Effects of 5-azacytidine and its 2′-deoxyderivative on cell differentiation and DNA methylation. Pharmacol Ther. 1985;28(1):17–27. doi: 10.1016/0163-7258(85)90080-4. [DOI] [PubMed] [Google Scholar]
- 12.Constantinides PG, Jones PA, Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- 13.Darmon M, Nicolas JF, Lamblin D. 5-Azacytidine is able to induce the conversion of teratocarcinoma-derived mesenchymal cells into epithelia cells. EMBO J. 1984;3(5):961–967. doi: 10.1002/j.1460-2075.1984.tb01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brevini TA, et al. Cell lines derived from human parthenogenetic embryos can display aberrant centriole distribution and altered expression levels of mitotic spindle check-point transcripts. Stem Cell Rev. 2009;5(4):340–352. doi: 10.1007/s12015-009-9086-9. [DOI] [PubMed] [Google Scholar]
- 15.Niwa H. How is pluripotency determined and maintained? Development. 2007;134(4):635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y, et al. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells. 2005;23(5):656–662. doi: 10.1634/stemcells.2004-0241. [DOI] [PubMed] [Google Scholar]
- 17.Wiese C, et al. Nestin expression—a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61(19-20):2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 19.Teramura Y, Iwata H. Bioartificial pancreas microencapsulation and conformal coating of islet of Langerhans. Adv Drug Deliv Rev. 2010;62(7-8):827–840. doi: 10.1016/j.addr.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385(6613):257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- 21.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, et al. The LIM-homeodomain protein ISL1 activates insulin gene promoter directly through synergy with BETA2. J Mol Biol. 2009;392(3):566–577. doi: 10.1016/j.jmb.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 23.Lumelsky N, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292(5520):1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 24.Xie R, et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12(2):224–237. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd AW, Schrader JW. Derivation of macrophage-like lines from the pre-B lymphoma ABLS 8.1 using 5-azacytidine. Nature. 1982;297(5868):691–693. doi: 10.1038/297691a0. [DOI] [PubMed] [Google Scholar]
- 26.Taylor SM, Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17(4):771–779. doi: 10.1016/0092-8674(79)90317-9. [DOI] [PubMed] [Google Scholar]
- 27.Taylor SM, Jones PA. Changes in phenotypic expression in embryonic and adult cells treated with 5-azacytidine. J Cell Physiol. 1982;111(2):187–194. doi: 10.1002/jcp.1041110210. [DOI] [PubMed] [Google Scholar]
- 28.Chiu CP, Blau HM. 5-Azacytidine permits gene activation in a previously noninducible cell type. Cell. 1985;40(2):417–424. doi: 10.1016/0092-8674(85)90155-2. [DOI] [PubMed] [Google Scholar]
- 29.Enjoji M, Nakashima M, Honda M, Sakai H, Nawata H. Hepatocytic phenotypes induced in sarcomatous cholangiocarcinoma cells treated with 5-azacytidine. Hepatology. 1997;26(2):288–294. doi: 10.1002/hep.510260206. [DOI] [PubMed] [Google Scholar]
- 30.Blum B, et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol. 2012;30(3):261–264. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5(4):258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 32.Thatava T, et al. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther. 2011;18(3):283–293. doi: 10.1038/gt.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9(1):17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banga A, Akinci E, Greder LV, Dutton JR, Slack JMW. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci USA. 2012;109(38):15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikkelsen TS, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454(7200):49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008;123(1):8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]
- 38.Borowiak M, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4(4):348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 40.Creusot F, Acs G, Christman JK. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J Biol Chem. 1982;257(4):2041–2048. [PubMed] [Google Scholar]
- 41.Haaf T. The effects of 5-azacytidine and 5-azadeoxycytidine on chromosome structure and function: Implications for methylation-associated cellular processes. Pharmacol Ther. 1995;65(1):19–46. doi: 10.1016/0163-7258(94)00053-6. [DOI] [PubMed] [Google Scholar]
- 42.Kahan BW, et al. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: An in vitro model to study islet differentiation. Diabetes. 2003;52(8):2016–2024. doi: 10.2337/diabetes.52.8.2016. [DOI] [PubMed] [Google Scholar]
- 43.Andralojc KM, et al. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia. 2009;52(3):486–493. doi: 10.1007/s00125-008-1238-y. [DOI] [PubMed] [Google Scholar]
- 44.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: A novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107(1-3):63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 45.Vignjević S, et al. Similar developmental patterns of ghrelin- and glucagon-expressing cells in the human pancreas. Cells Tissues Organs. 2012;196(4):362–373. doi: 10.1159/000335469. [DOI] [PubMed] [Google Scholar]
- 46.Zerbini G, Podesta F, Meregalli G, Deferrari G, Pontremoli R. Fibroblast Na+-Li+ countertransport rate is elevated in essential hypertension. J Hypertens. 2001;19(7):1263–1269. doi: 10.1097/00004872-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Hattori N, et al. Preference of DNA methyltransferases for CpG islands in mouse embryonic stem cells. Genome Res. 2004;14(9):1733–1740. doi: 10.1101/gr.2431504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.