Abstract
High-frequency firing of neurons depresses transmitter release at many synapses. At the glutamatergic synapse of the Drosophila larval neuromuscular junction, we find that presynaptic depression is modulated by postsynaptic ionotropic glutamate receptor (iGluR) activity. Although basal release at low frequency was insensitive to postsynaptic iGluR activity, recovery from depression elicited by high-frequency presynaptic trains decreased with partial block of native iGluRs. Moreover, recovery from depression increased with optical activation of the light-gated mammalian iGluR6 (LiGluR) expressed postsynaptically. The enhancement of recovery from depression occurred within 2 min of optical activation of LiGluR and persisted for minutes after optical deactivation. This effect depended on cAMP-dependent presynaptic recruitment of vesicles from the reserve pool. Our findings reveal a unique dimension to postsynaptic iGluR activity: fast retrograde signaling that preserves transmission efficacy during high-frequency presynaptic firing.
Keywords: optogenetics
Neurons fire in bursts of high-frequency activity in many settings. They do this to overcome the unreliable nature of isolated spikes (1) and, to name a few diverse cases, in response to sensory input (2) or a hippocampal cell’s “place field” (3), throughout the motor system (4), during consolidation of fear extinction in the prefrontal cortex (5, 6), and in many places in the brain during gamma oscillations (7). Whereas the excitability properties of many neurons support reliable high-frequency firing, this kind of activity challenges the synaptic release machinery, especially at high-probability release sites, which tend to depress. Several forms of short-term plasticity that are self-contained in the presynaptic nerve terminal regulate the degree of depression and thus, the fidelity of throughput along the circuit. Here, we show a unique form of regulation of presynaptic depression that is controlled by activity-sensing feedback from the postsynaptic cell.
Our study was performed at the Drosophila larval neuromuscular junction (NMJ), a glutamatergic synapse that has the molecular features of mammalian central synapses, where presynaptic motor neurons fire in bursts (8) and where the synapse exhibits several forms of activity-dependent changes in transmission strength (9), including a retrograde regulation of basal synaptic strength (10). We find that the activation of postsynaptic ionotropic glutamate receptors (iGluRs) influences synaptic transmission during high-frequency bursts. Partial pharmacological block of native iGluRs reduced recovery from presynaptic depression, whereas optical activation of postsynaptic iGluR activity using light-gated mammalian iGluR6 (LiGluR) (11) had the opposite effect, enhancing recovery. This retrograde regulation had no effect on basal transmission. It occurred within 1–2 min of iGluR activation and lasted for minutes without decay, following termination of this activation. The enhanced recovery from presynaptic depression appears to be mediated by enhanced recruitment from the reserve vesicle pool when high-frequency activity depletes the readily releasable pool.
Results
Partial Block of Native GluRs Reduces Recovery from Synaptic Depression During High-Frequency Presynaptic Firing.
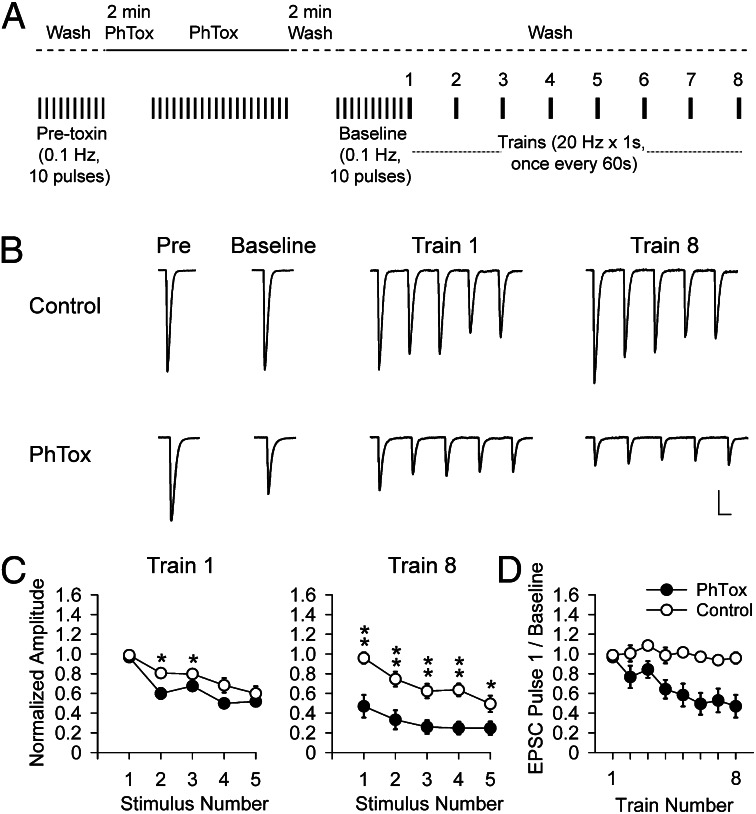
At the Drosophila larval NMJ, high-frequency trains of presynaptic firing elicit a mixture of presynaptic facilitation and depression during the train, with depression dominating at physiological external calcium, where basal quantal content is high, due to the depletion of the readily releasable pool of synaptic vesicles (12, 13). We asked whether the activity of native iGluRs plays a role in regulating this behavior by partially blocking the native iGluRs. Our experiments were performed in the standard larval NMJ preparation (14), where 4 μM of the wasp venom philanothotoxin-433 (PhTox) produced a stable reduction in the amplitude postsynaptic response (Fig. S1), as described earlier (15).
Drosophila motor axons have been observed to fire in bursts of up to 30 Hz (16) lasting for up to 15 s (17) at intervals of 10–60 s (8). We examined the synaptic depression of excitatory postsynaptic currents (EPSCs) induced in the wild-type NMJ by a series of 1-s long 20-Hz trains of presynaptic stimulation, given at 1-min intervals (Fig. 1A). Vehicle-treated NMJs showed a mild depression (Fig. 1 B and C). NMJs treated with 4 μM PhTox, in which basal EPSC amplitude was reduced by 38 ± 0.3% (n = 10) (Fig. 1B), had the same amount of depression during the burst (Fig. 1B and see Fig. S5A), but considerably diminished recovery between bursts (Fig. 1 B–D). An examination of the change in the amplitude of the first EPSC in each train showed that the PhTox effect on recovery from depression becomes evident within 2 min following the start of the high-frequency presynaptic stimulation (Fig. 1D).
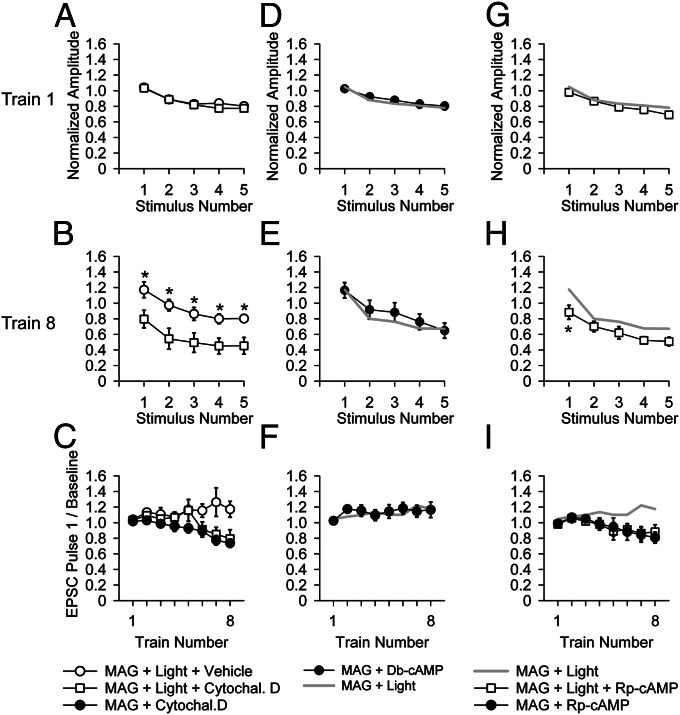
Fig. 1.
Partial block of native GluRs diminishes recovery from synaptic depression during high-frequency trains of presynaptic activity. (A) Schematic of PhTox labeling and washout with high-frequency train stimulation. Ten baseline EPSCs elicited at 0.1 Hz before addition of PhTox, followed by Phtox perfusion (2 min without stimulation, followed by 20 stimuli 0.1 Hz), followed by 2 min of wash (no stimulation), followed by 10 stimuli at 0.1 Hz to generate a baseline EPSC. This was followed by trains at 20 Hz for 1 s, once every 60 s (intertrain interval not shown to scale). (B and C) Traces of average 10 pulses for pretoxin and baseline and first 5 pulses of trains (B) and average amplitudes (± SEM) normalized to average baseline EPSC (C) of first five EPSCs in train 1 (Left) and train 8 (Right) for WT in 0.1% DMSO (control, n = 8) or 4 μM PhTox in 0.1% DMSO (PhTox, n = 10). (C) EPSC amplitudes significantly larger in train 8 in 0.1% DMSO (open symbols) than in 4 μM PhTox in 0.1% DMSO (filled symbols) (Student t test, *P < 0.05, **P < 0.01). (D) Time course of effect on synaptic depression of partial PhTox block seen in plot of first EPSC of the eight trains in PhTox-DMSO (filled symbols) and DMSO alone (open symbols). (Scale bars: B, 25 nA and 20 ms.)
Postsynaptic LiGluR Responds to Presynaptic Glutamate and to Light.
Having seen that a reduction in iGluR activation increased synaptic depression, we set out to determine the effect of an elevation of iGluR activation. Overexpression of single native iGluR subunits does not alter receptor density, apparently because of limited availability of other subunits that are required to form the native heteromeric receptors (18–20). We therefore expressed LiGluR, our light-gated version of a mammalian kainate receptor, which uses a single cysteine substitution in iGluR6 (L439C) to provide an anchoring site for a maleimide-azobenzene-glutamate (MAG) photoswitch to generate the light-gated receptor (11). iGluR6, one of the closest mammalian homologs to the Drosophila iGluR subunits (19), forms homotetrameric channels that share the Drosophila iGluR property of high permeability to calcium (21, 22).
Transgenic flies expressing the upstream activating sequence (UAS)-LiGluR were crossed with yeast GAL4 protein 24B-GAL4 line (23) to restrict LiGluR expression to the muscle. In these muscles, LiGluR was found to distribute throughout the postsynaptic area and to be most concentrated on the peripheral border of synaptic boutons (Fig. S2A), overlapping with the postsynaptic scaffolding protein Discs Large (Dlg), the Drosophila homolog of postsynaptic density protein 95 (24) (Fig. S2B). The Drosophila glutamate receptor subunits GluRIIA and GluRIIB are known to partially overlap with Dlg (25) and we also found LiGluR to partially overlap with GluRIIA, but to lack the punctuate clustering of GluRIIA (Fig. S2C), which is found opposite clusters of the presynaptic active zone protein Bruchpilot (Brp) (26) (Fig. S2D). Importantly, postsynaptic LiGluR expression does not alter native Brp or GluRIIA expression (Fig. S3).
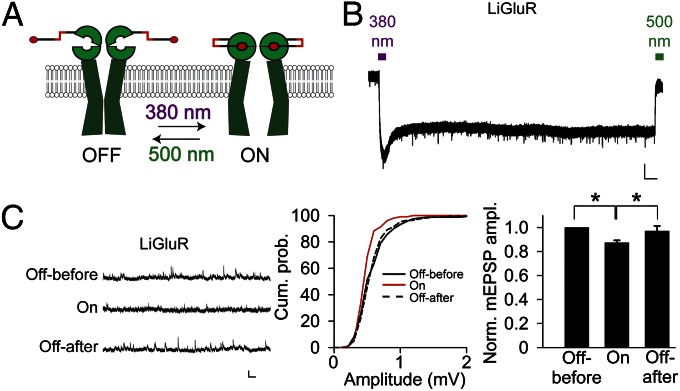
To determine whether LiGluR is functional at the NMJ, we exposed third-instar LiGluR-expressing larva fillets to MAG, performed voltage clamp recording of the larva muscle in thapsigargin to block muscle contraction (27) and Con A (which has no effect on the Drosophila iGluRs) (Fig. S4) to block desensitization of LiGluR (28). A 10-s long pulse of wide-field illumination with 380 nm light to photoisomerize the azobenzene from trans to cis and activate LiGluR (Fig. 2A) generated an inward current that was maintained after the light was turned off and which was then deactivated by a 10-s pulse of illumination with 500 nm light (Fig. 2B). The wavelength dependence of activation and deactivation, as well as the maintained phase of activation in the dark, which is due to the stability of the azobenzene photoswitch in the cis configuration, reflect the previously determined properties of LiGluR (29).
Fig. 2.
Photoactivation of LiGluR reduces mEPSP amplitude. (A) Schematic of LiGluR photoactivation by 380 nm light and photodeactivation by 500 nm light. (B) Photocurrent from LiGluR in voltage clamped muscle activated by 10 s of 380 nm light, remains active in absence of light, and is deactivated by 10 s of 500 nm light. (C) Photoactivation of LiGluR reversibly decreases mEPSP amplitude. Traces (Left) and cumulative amplitude distributions (Center) from representative experiment. Summary bar graphs showing average normalized amplitudes for LiGluR (n = 6), with significant (paired Student t test, P < 0.05) decrease in mEPSP amplitude. (Scale bars: B, 1 nA and 15 s). Error bars in C represent SEM.
Having demonstrated that LiGluR is functional in larval muscle, we asked whether it contributes to the postsynaptic response to glutamate released by the motorneuron. We did this by examining the effect on spontaneous miniature excitatory postsynaptic potential (mEPSP) amplitude of optical activation of the MAG-labeled LiGluR receptors. Our rationale was that optical activation would activate LiGluR with MAG and, therefore, prevent it from responding to synaptically released glutamate. Indeed, we found that in LiGluR-expressing muscle, illumination with 380 nm light reduced mEPSP amplitude and that this reduction was reversed by illumination at 500 nm to deactivate LiGluR (Fig. 2C). This finding indicates that LiGluR localizes to synapses and contributes to the postsynaptic response to glutamate released by presynaptic nerve terminals.
Optical Activation of Postsynaptic LiGluR Selectively Enhances Recovery from Synaptic Depression.
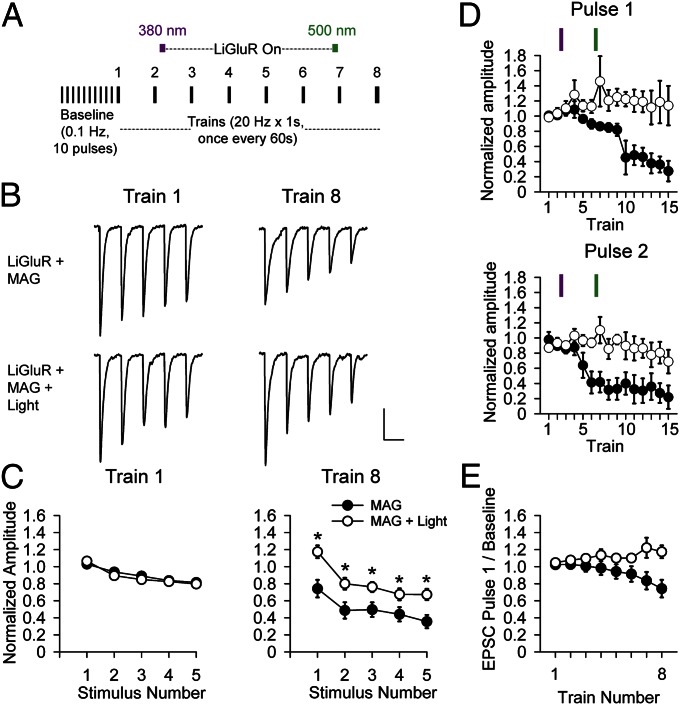
With the ability to photoactivate an iGluR in hand, we next investigated whether postsynaptic LiGluR activation would alter recovery from synaptic depression. As in the PhTox experiments (Fig. 1), presynaptic axons were stimulated with 1-s long 20-Hz trains once every minute (Fig. 3A). LiGluR-expressing NMJs were conjugated with MAG, stimulated with 20-Hz presynaptic trains, and either illuminated to activate LiGluR between trains 2 and 7 or not illuminated to serve as a control. The onset of depression was not affected by expression of LiGluR (Fig. S5B) or by its optical activation (Fig. S5C). Synaptic depression accumulated considerably in absence of light, but NMJs in which LiGluR was photoactivated did not accumulate depression, instead showing a modest enhancement of the first EPSC of the train (Fig. 3 B–E), indicating enhanced recovery from synaptic depression during the interbursts’ interval.
Fig. 3.
Optical activation of LiGluR enhances recovery from synaptic depression during high-frequency presynaptic activity. (A) Schematic of stimulation protocol. Ten baseline EPSCs at 0.1 Hz followed by 8 trains at 20 Hz for 1 s, once every 60 s (intertrain interval not shown to scale). LiGluR was activated midway between trains 2 and 3 by 10-s illumination at 380 nm followed by period of sustained activation in the dark until being turned off by 10-s illumination at 500 nm between trains 6 and 7, for a total of 5 min of activation. (B–D) Traces (B) and average amplitudes (± SEM) (C and D) normalized to average baseline EPSC. (C) First five EPSCs in train 1 (Left) and train 8 (Right) for LiGluR + MAG (no optical activation, filled symbols) and LiGluR + MAG + light (with optical activation, open symbols). No light (n = 12); with light (n = 13). EPSC amplitudes significantly larger in train 8 activated by light (Student t test, P < 0.05). (D) First EPSC (Upper) and second EPSC (Lower) of 15 trains without light (n = 3) or with light (n = 4). (Scale bar: B, 20 nA and 30 ms.) (E) Average of first EPSC of the 8 20-Hz trains in LiGluR + MAG (filled symbols) and LiGluR + MAG + light (open symbols).
The expression of LiGluR driven by 24B-GAL4 in these experiments was strong enough to reduce EPSC amplitude and increase paired pulse ratio (PPR) (Fig. S6 E and F), consistent with a reduction in quantal content. We wondered whether this change in homeostatic set point was responsible for the effect of optical activation of LiGluR on depression. To test this we switched to myocyte enhancer factor-2 GAL4 (Mef2-GAL4) to drive a weaker muscle-specific expression of LiGluR. In these UAS-LiGluR/Mef2-Gal4 NMJs, EPSC amplitude and PPR were unchanged from wild type (Fig. S6 E and F), but optical activation of LiGluR still enhanced recovery from depression (Fig. S6G).
The results with Mef2-Gal4/UAS-LiGluR indicate that the retrograde enhancement of recovery from synaptic depression is triggered by LiGluR activity at the normal homeostatic set point, just as the PhTox block of native iGluRs at the normal homeostatic set point has the opposite effect of suppressing recovery from depression. The LiGluR manipulation affords several advantages over the PhTox manipulation, which led us to focus our analysis on LiGluR. First, LiGluR is confined postsynaptically, whereas native iGluRs are also found in neurons (30). Second, LiGluR activity can be turned off independently of presynaptic activity to determine the persistence of its effect on synaptic transmission. Indeed, we found that the recovery from synaptic depression showed no signs of diminishing 8 min after LiGluR was turned off (Fig. 3D). Third, LiGluR can be activated independently of presynaptic activity, making it possible to compare identical levels of LiGluR signaling on transmission elicited by different presynaptic stimulation patterns. We used this advantage next to ask whether the optical activation of LiGluR alters basal quantal content or is specific to the dynamics of transmission at high presynaptic firing frequencies.
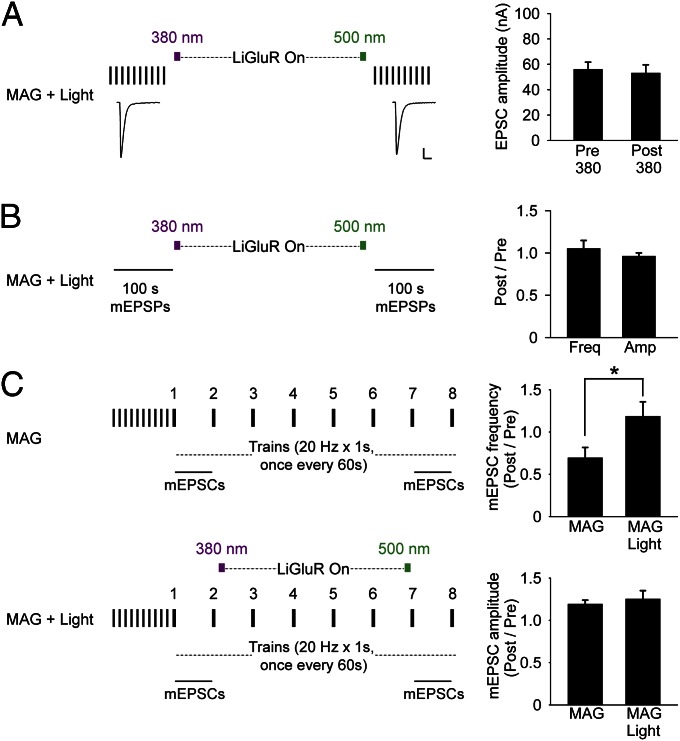
To test whether the optical activation of LiGluR alters basal quantal content, we examined the effect of activation of LiGluR on EPSCs evoked by low-frequency (0.1 Hz) presynaptic stimulation that induces neither facilitation nor depression. We found that optical activation of LiGluR had no effect on the amplitude of EPSCs evoked at 0.1 Hz (Fig. 4A). Moreover, when the same optical activation of LiGluR was given in absence of presynaptic stimulation, there was no effect on the amplitude or frequency of spontaneous mEPSPs before LiGluR activation and after LiGluR deactivation (Fig. 4B). Thus, LiGluR activity does not affect basal transmitter release.
Fig. 4.
Optical activation of LiGluR has no effect on basal EPSCs or basal mEPSPs but prevents reduction of mEPSC frequency following high-frequency presynaptic activity. (A) At low-frequency (0.1 Hz) presynaptic stimulation, there is no rundown of EPSC amplitude and 5 min of photoactivation of LiGluR has no effect on EPSC amplitude. (Left) Representative example of average of 10 EPSCs from one NMJ. (Right) Average EPSC amplitude (± SEM, n = 8) before and after LiGluR photoactivation. (Scale bar: Left, 20 nA and 30 ms.) (B) Frequency and amplitude of mEPSP under basal conditions (no presynaptic stimulation) is not altered by 5 min of photoactivation of LiGluR (n = 6). (C) In absence of photoactivation of LiGluR (Upper Left, n = 8), mEPSC frequency is reduced following 20-Hz trains of presynaptic stimulation, with no change in mEPSC amplitude (Right). Photoactivation of LiGluR (Lower Left, n = 8) prevents reduction in mEPSC frequency following 20-Hz trains (Right, Student t test, P < 0.05).
Synaptic depression at high frequencies of presynaptic firing is generally attributed to a presynaptic process in which the readily releasable pool of vesicles is depleted (31). We found that failure to recover from synaptic depression in NMJs expressing LiGluR in the muscle that was labeled with MAG, but not activated by light, was accompanied by a reduction in the frequency of mEPSCs recorded during the 1-min interval between the seventh and eighth 20-Hz trains (Fig. 4C, Upper). Together with the reduction in EPSC amplitude (Fig. 3C), this suggests that the 20-Hz trains deplete a vesicle pool that subserves both evoked and spontaneous release. When the recovery from synaptic depression was enhanced by optical activation of LiGluR, the reduction in mEPSC frequency was also prevented (Fig. 4C, Lower). In neither case was mEPSC amplitude changed. These results are consistent with a presynaptic locus for the recovery from synaptic depression elicited by the 20-Hz trains. Moreover, the lack of effect of LiGluR on basal transmission and its prevention of both the depression of EPSCs evoked by high-frequency trains and the accompanying decline of mEPSC frequency together suggest that activation of postsynaptic LiGluR triggers a retrograde signal that acts to maintain the level of readily releasable vesicles during elevated rates of release.
LiGluR Effect on Recovery from Synaptic Depression Depends on Actin and cAMP.
One mechanism for maintaining the level of readily releasable vesicles during high rates of presynaptic firing could be by recruiting vesicles from the reserve pool (32). Because vesicle recruitment is actin dependent (12, 13, 32, 33), we tested the effect of the actin depolymerizing agent, cytochalasin D, on the prevention of depression that is triggered by postsynaptic LiGluR. Cytochalasin D (in 0.1% ethanol final concentration) was applied to NMJs from LiGluR-expressing larvae at a concentration of 10 μM for 10 min before presynaptic stimulation at either low or high frequency. At low frequency, cytochalasin D had no effect on transmission (Fig. S7A), consistent with earlier observations in which vesicle cycling in the readily releasable pool was shown to keep up with release (12). However, as seen in wild-type NMJs (12), in NMJs expressing LiGluR in muscle, a long high-frequency train at 10 Hz that yielded no depression under control conditions (0.1% ethanol vehicle alone) produced a substantial depression in the presence of cytochalasin D (Fig. S7B). This is consistent with vesicle recycling being inadequate to maintain release at high frequency, leading to actin-dependent recruitment of vesicles from the reserve pool.
Having seen that cytochalasin D blocks recruitment of vesicles from the reserve pool in LiGluR NMJs, we tested the effect of cytochalasin D on LiGluR-enhanced recovery from depression. We found that cytochalasin D blocked the LiGluR-enhanced recovery from synaptic depression (Fig. 5 A–C). The results suggest postsynaptic LiGluR activity enhances recovery from depression by enhancing recruitment of vesicles from the reserve pool.
Fig. 5.
Role of actin and cAMP in the LiGluR-enhanced recovery from synaptic depression. (A–C) A total of 10 µM cytochalasin D blocks the enhanced recovery from synaptic depression by postsynaptic LiGluR. (A and B) The first five stimuli of train 8 in LiGluR + MAG + light in 0.1% ethanol alone (control) (n = 5, open circles) are similar to those recorded in normal saline (Fig. 3C) and show less depression than seen in LiGluR + MAG + light in 10 µM cytochalasin D and 0.1% ethanol (n = 7, open squares). (C) Greater accumulation of depression in 10 µM cytochalasin D is similar in MAG + light (n = 7, open squares) and MAG without light (n = 5), as tracked in the amplitude of first EPSC of the eight trains. (D–F) A total of 1 mM Db-cAMP, a membrane permeable cAMP analog, applied to LiGluR-expressing NMJ labeled with MAG but not stimulated with light (n = 8, filled circles) mimics the effect of optical stimulation of LiGluR-expressing NMJ labeled with MAG (data from Fig. 3C). (G–I) A total of 100 µM Rp-cAMP, a membrane permeable competitive inhibitor of cAMP-dependent kinase (n = 8, open squares), partially blocks the enhanced recovery from synaptic depression effect by optical stimulation of LiGluR (data from Fig. 3C) (*P < 0.05, Student t test) and is tracked by MAG + Rp-cAMP control (n = 7, filled circles) within the first EPSC of the eight trains.
One presynaptic signal that enhances recruitment of vesicles from the reserve pool at the Drosophila larval NMJ is cAMP (34, 35). We asked whether a membrane-permeable cAMP analog would have an effect similar to the optical stimulation of postsynaptic LiGluR. Indeed, we found that a 1-mM concentration of the membrane-permeable cAMP analog dibutyryl (Db)-cAMP enhanced recovery from synaptic depression elicited by 20-Hz trains of presynaptic stimulation (Fig. 5 D–F), similar to optical activation of postsynaptic LiGluR (Fig. 3). This similarity suggests that LiGluR may signal through cAMP. To test this idea, we examined the effect of the membrane-permeable competitive blocker of cAMP-dependent kinases, R-adenosine, cyclic 3′,5′-(hydrogenphosphorothioate) triethylammonium (Rp)-cAMP. We found that 100 µM Rp-cAMP partially blocked the effect of LiGluR activation on recovery from synaptic depression (Fig. 5 G–I). The degree of depression was not as severe as when LiGluR was not activated at all (compare Figs. 5H and 3C), indicating that the block by Rp-cAMP was incomplete. These results suggest that activation of postsynaptic LiGluR strengthens presynaptic recovery from depression by enhancing recruitment from the reserve pool during high-frequency trains that deplete the readily releasable pool and that this depends, at least in part, on presynaptic cAMP.
Postsynaptic Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) Activity Is Required for LiGluR Recovery from Synaptic Depression.
Our results so far suggest that activation of postsynaptic iGluRs triggers a fast retrograde signal that promotes recovery from presynaptic depression of transmitter release during high-frequency trains. How could iGluR activity translate into generation of such a retrograde signal? Over the developmental time of days, presynaptic compensation to loss of postsynaptic iGluR current in the GluRIIA subunit null mutant was shown earlier to depend on postsynaptic Ca2+/calmodulin-dependent protein kinase II (CaMKII) (36). We asked whether postsynaptic CaMKII also plays a role on the much shorter time scale (<2 min) seen here. To test this, we inhibited postsynaptic CamKII activity by expressing a peptide corresponding to the rat autoinhibitory domain of CaMKII (36, 37). EPSCs during the 20-Hz trains that preceded optical activation of LiGluR were the same in NMJs expressing LiGluR alone and those expressing both LiGluR and the CaMKII inhibitory peptide (Fig. S8A). However, the optical activation of LiGluR fails to promote recovery from synaptic depression in the NMJs that also expressed the CaMKII inhibitory peptide (Fig. S8 B and C). These NMJs depressed similarly to NMJs in which LiGluR was not activated by light (Fig. 3C, Right). This result suggests that postsynaptic CaMKII is involved in the conversion of postsynaptic iGluR activity into retrograde signaling to enhance recovery from synaptic depression.
Discussion
We asked whether iGluR function has a regulatory effect on synaptic transmission at the elevated frequencies of presynaptic firing that typically trigger plasticity changes, and which engage facilitation, potentiation, and depression. We found that at the Drosophila larval NMJ feedback, triggered by postsynaptic iGluR activation, maintains synaptic transmission during repetitive activity at elevated frequencies by regulating recovery of presynaptic depression. Our results suggest a functional importance for this phenomenon in optimizing transmission at synapses with multiple connections of varying strength.
iGluR Activity Enhances Recovery from Synaptic Depression.
We examined the effect of either the reduction or increase of iGluR activity on transmission evoked by high-frequency bursts of presynaptic firing. The activity of native iGluRs was reduced by partial pharmacological block with PhTox. iGluR activity was increased by a unique use of the light-gated mammalian kainate receptor, LiGluR, which we expressed in the postsynaptic muscle, shown to respond to synaptic glutamate as well as to light, and used as an independently controlled mimic for iGluR activation by synaptic glutamate. We find that recovery from depression of synaptic transmission during high-frequency presynaptic firing is reduced when native iGluRs are partially blocked and that it is enhanced when postsynaptic LiGluR is activated by light. These effects occur within 2 min of iGluR activation. The LiGluR effect persists for minutes after LiGluR is turned off and is specific to synaptic depression, with no effect on basal transmission at low-frequency presynaptic firing. The LiGluR effect takes place without a change in unitary mEPSP amplitude, but is tracked by changes in mEPSC frequency, consistent with a presynaptic effect. Taken together, these results suggest that the activity of postsynaptic iGluRs elicits a fast retrograde signal that acts presynaptically to modulate glutamate release dynamics.
How Recovery from Presynaptic Depression Is Enhanced by Retrograde Signaling.
At the Drosophila NMJ, as in mammalian central synapses, there are at least two synaptic vesicle pools, the readily releasable pool and the reserve pool, and high-frequency activity depletes the readily releasable pool and mobilizes vesicles from the reserve pool to replenish the readily releasable pool and sustain release (38). Presynaptic cAMP signaling mobilizes recruitment from the reserve pool to the readily releasable pool (34). We found that the enhancement of recovery from presynaptic depression by activation of LiGluR was blocked by the actin depolymerizing agent cytochalasin D, suggesting that the activity of postsynaptic LiGluR promotes recovery by enhancing recruitment from the reserve vesicle pool. A cAMP analog shown earlier to enhance vesicle recruitment from the reserve pool had the same effect as LiGluR activation, supporting this interpretation, and a cAMP competitor partially blocked the LiGluR effect, suggesting that cAMP is a presynaptic mediator of the retrograde signal by postsynaptic LiGluR.
We asked how recovery from presynaptic depression could be regulated by the activity of postsynaptic iGluRs. Inhibition of postsynaptic CaMKII blocked the LiGluR effect, suggesting that calcium enters through the iGluRs and activates CaMKII. Because the experiments were performed under voltage clamp of the muscle (i.e., in absence of activation of voltage-gated calcium channels) and in thapsigargin (depleting the internal calcium stores before the start of the stimulation), the most likely entry route for calcium into the cytoplasm is the calcium-permeable iGluRs themselves (21).
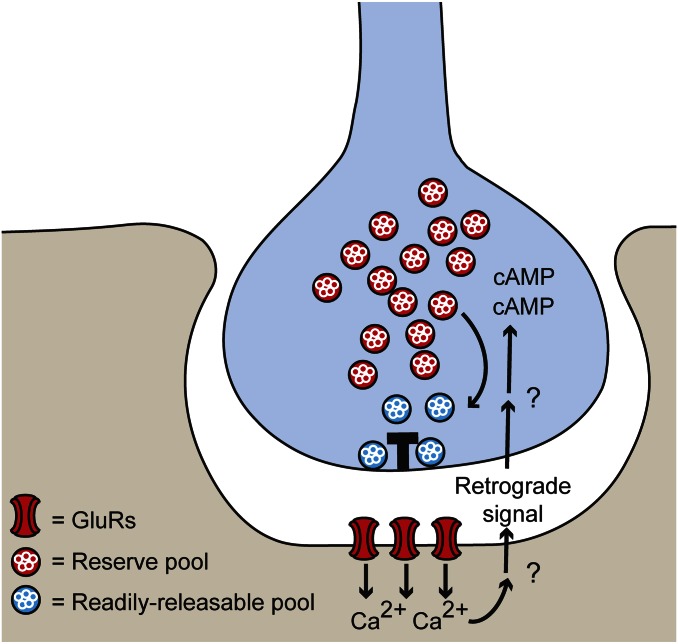
Together, these results lead to a model where glutamate release during high-frequency presynaptic firing triggers postsynaptic calcium influx through iGluRs and a retrograde signal that alleviates the depletion of the readily releasable pool by a cAMP-dependent recruitment of vesicles from the reserve pool (Fig. 6).
Fig. 6.
Postsynaptic receptor activity controls recovery from presynaptic synaptic depression. Schematic illustrates how calcium influx through postsynaptic GluRs that have been activated by anterograde glutamate transmission leads to postsynaptic release of a retrograde signal. This retrograde signal is proposed to increase presynaptic cAMP levels to recruit synaptic vesicles from the reserve pool and replenish the readily releasable pool, thereby promoting recovery from synaptic depression during high-frequency stimulation.
The rapid (minute time scale) enhancement in recovery from presynaptic depression by postsynaptic iGluR activation is reminiscent of the fast compensation pathway that elevates basal transmitter release following block of iGluRs in a semiintact preparation where the connections to the central nervous system are retained. However, we observed this effect on depression in the standard, stretched NMJ preparation, where compensation had earlier been shown not to occur (15). Indeed, our experiments show that optical activation of LiGluR does not activate homeostatic compensation because it does not alter quantal content during basal transmission (Fig. 4 A and B).
Our findings may explain earlier observations on the Drosophila embryonic NMJ, where high-frequency presynaptic trains in low calcium (facilitation dominating over depression) and with iGluRs not blocked, elicited a sustained elevation of mEPSP frequency that depended on postsynaptic calcium-activation of synaptotagmin 4, suggesting mediation by a calcium-dependent release of a retrograde transmitter (39, 40). Our results suggest that this increase in mEPSP frequency may have reflected an enhanced recruitment of vesicles from the reserve pool.
Why Involve Feedback from the Postsynaptic Cell in Recovery from Presynaptic Depression?
Under physiological conditions, many synapses depress during high-frequency activity due to depletion of vesicles from the readily releasable pool (41). Recruitment of vesicles from the reserve pool can mitigate this depletion by a self-contained presynaptic mechanism (38, 41). Our results indicate that recruitment from the reserve pool can be enhanced by feedback from the postsynaptic cell.
What advantage can there be in involving the postsynaptic cell in the regulation of presynaptic recruitment of reserve vesicles? Postsynaptic sensitivity to glutamate can vary considerably between multiple synapses on the same cell (42), including at the Drosophila larval NMJ, where mEPSP amplitudes range over severalfold (43) and glutamate receptor composition can differ (44). Glutamate release sites with more of the active zone protein Bruchpilot (Brp) (26) have the highest release probability (20, 45) and innervate postsynaptic densities with larger iGluR clusters (20, 46). As a result, they are expected to generate larger postsynaptic responses and require the fastest resupply of vesicles. Because vesicles in the reserve pool appear to be shared among multiple release sites (47–50), the participation of postsynaptic iGluR activity in the regulation of presynaptic recruitment of reserve vesicles provides an attractive mechanism for directing reserve pool vesicles to the active zones that carry out the bulk of glutamate release, thereby maintaining stable transmission at high frequencies of firing.
Materials and Methods
LiGluR DNA (11) was cloned into the standard UAS plasmid (pUAST) vector (51). UAS-LiGluR transgenic flies were made with standard P-element genetic transformation (BestGene). All fly lines were used in the w1118 background. The following additional fly stocks were used: 24B-GAL4 (51), UAS-CaMKII inhibitory peptide (Ala) (37), and Mef2-GAL4. Electrophysiology, MAG labeling, optical stimulation, and immunohistochemistry are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dirk Trauner for MAG and Pejmun Haghighi for the CaMKII(Ala) line; Gautam Agarwal for assistance in cloning LiGluR; and Einat Peled, Robin Ball, Zachary Newmann, the rest of the E.Y.I. laboratory, and Tara Tracy for helpful discussion. This work was supported by National Science Foundation GRANT FIBR 0623527) and the National Institutes of Health Nanomedicine Development Center for the Optical Control of Biological Function Grant 2PN2EY018241).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221314110/-/DCSupplemental.
References
- 1.Lisman JE. Bursts as a unit of neural information: Making unreliable synapses reliable. Trends Neurosci. 1997;20(1):38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- 2.Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci. 2004;5(1):13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 4.Grillner S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron. 2006;52(5):751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53(6):871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, et al. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73(5):990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Budnik V, Zhong Y, Wu CF (1990) Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci 10(11):3754–3768. [DOI] [PMC free article] [PubMed]
- 9.Griffith LC, Budnik V. Plasticity and second messengers during synapse development. Int Rev Neurobiol. 2006;75:237–265. doi: 10.1016/S0074-7742(06)75011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marqués G, Zhang B. Retrograde signaling that regulates synaptic development and function at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:267–285. doi: 10.1016/S0074-7742(06)75012-7. [DOI] [PubMed] [Google Scholar]
- 11.Volgraf M, et al. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2(1):47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20(5):917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- 13.Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28(3):941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- 14.Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976;262(1):189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52(4):663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper RL, Neckameyer WS. Dopaminergic modulation of motor neuron activity and neuromuscular function in Drosophila melanogaster. Comp Biochem Physiol B Biochem Mol Biol. 1999;122(2):199–210. doi: 10.1016/s0305-0491(98)10160-8. [DOI] [PubMed] [Google Scholar]
- 17.Cattaert D, Birman S. Blockade of the central generator of locomotor rhythm by noncompetitive NMDA receptor antagonists in Drosophila larvae. J Neurobiol. 2001;48(1):58–73. doi: 10.1002/neu.1042. [DOI] [PubMed] [Google Scholar]
- 18. Featherstone DE, et al. (2005) An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J Neurosci 25(12):3199–3208. [DOI] [PMC free article] [PubMed]
- 19. Qin G, et al. (2005) Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J Neurosci 25(12):3209–3218. [DOI] [PMC free article] [PubMed]
- 20. Marrus SB, DiAntonio A (2004) Preferential localization of glutamate receptors opposite sites of high presynaptic release. Curr Biol 14(11):924–931. [DOI] [PubMed]
- 21.Chang H, Ciani S, Kidokoro Y. Ion permeation properties of the glutamate receptor channel in cultured embryonic Drosophila myotubes. J Physiol. 1994;476(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 22.Egebjerg J, Heinemann SF. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc Natl Acad Sci USA. 1993;90(2):755–759. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17(4):641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 24.Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13(4):823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K, Featherstone DE. Discs-large (DLG) is clustered by presynaptic innervation and regulates postsynaptic glutamate receptor subunit composition in Drosophila. BMC Biol. 2005;3:1. doi: 10.1186/1741-7007-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kittel RJ, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312(5776):1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- 27.Guerrero G, et al. Heterogeneity in synaptic transmission along a Drosophila larval motor axon. Nat Neurosci. 2005;8(9):1188–1196. doi: 10.1038/nn1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11(6):1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- 29.Gorostiza P, et al. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci USA. 2007;104(26):10865–10870. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Völkner M, Lenz-Böhme B, Betz H, Schmitt B. Novel CNS glutamate receptor subunit genes of Drosophila melanogaster. J Neurochem. 2000;75(5):1791–1799. doi: 10.1046/j.1471-4159.2000.0751791.x. [DOI] [PubMed] [Google Scholar]
- 31.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18(6):995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 32.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9(5):344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 33.Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 34.Kuromi H, Kidokoro Y. Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron. 2000;27(1):133–143. doi: 10.1016/s0896-6273(00)00015-5. [DOI] [PubMed] [Google Scholar]
- 35.Cheung U, Atwood HL, Zucker RS. Presynaptic effectors contributing to cAMP-induced synaptic potentiation in Drosophila. J Neurobiol. 2006;66(3):273–280. doi: 10.1002/neu.20218. [DOI] [PubMed] [Google Scholar]
- 36.Haghighi AP, et al. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39(2):255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- 37.Griffith LC, et al. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10(3):501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- 38.Kidokoro Y, et al. Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse. Brain Res Brain Res Rev. 2004;47(1-3):18–32. doi: 10.1016/j.brainresrev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310(5749):858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- 40.Barber CF, Jorquera RA, Melom JE, Littleton JT. Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains. J Cell Biol. 2009;187(2):295–310. doi: 10.1083/jcb.200903098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 42.Branco T, Staras K, Darcy KJ, Goda Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron. 2008;59(3):475–485. doi: 10.1016/j.neuron.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong K, Karunanithi S, Atwood HL. Quantal unit populations at the Drosophila larval neuromuscular junction. J Neurophysiol. 1999;82(3):1497–1511. doi: 10.1152/jn.1999.82.3.1497. [DOI] [PubMed] [Google Scholar]
- 44.Schmid A, et al. Activity-dependent site-specific changes of glutamate receptor composition in vivo. Nat Neurosci. 2008;11(6):659–666. doi: 10.1038/nn.2122. [DOI] [PubMed] [Google Scholar]
- 45.Peled ES, Isacoff EY. Optical quantal analysis of synaptic transmission in wild-type and rab3-mutant Drosophila motor axons. Nat Neurosci. 2011;14(4):519–526. doi: 10.1038/nn.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graf ER, Daniels RW, Burgess RW, Schwarz TL, DiAntonio A. Rab3 dynamically controls protein composition at active zones. Neuron. 2009;64(5):663–677. doi: 10.1016/j.neuron.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staras K, et al. A vesicle superpool spans multiple presynaptic terminals in hippocampal neurons. Neuron. 2010;66(1):37–44. doi: 10.1016/j.neuron.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darcy KJ, Staras K, Collinson LM, Goda Y. Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat Neurosci. 2006;9(3):315–321. doi: 10.1038/nn1640. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Alfonso T, Ryan TA. A heterogeneous “resting” pool of synaptic vesicles that is dynamically interchanged across boutons in mammalian CNS synapses. Brain Cell Biol. 2008;36(1-4):87–100. doi: 10.1007/s11068-008-9030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westphal V, et al. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320(5873):246–249. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- 51.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.