Abstract
Soft corals of the family Xeniidae exhibit a unique, rhythmic pulsation of their tentacles (Movie S1), first noted by Lamarck nearly 200 y ago. However, the adaptive benefit of this perpetual, energetically costly motion is poorly understood. Using in situ underwater particle image velocimetry, we found that the pulsation motions thrust water upward and enhance mixing across the coral–water boundary layer. The induced upward motion effectively prevents refiltration of water by neighboring polyps, while the intensification of mixing, together with the upward flow, greatly enhances the coral’s photosynthesis. A series of controlled laboratory experiments with the common xeniid coral Heteroxenia fuscescens showed that the net photosynthesis rate during pulsation was up to an order of magnitude higher than during the coral’s resting, nonpulsating state. This enhancement diminished when the concentration of oxygen in the ambient water was artificially raised, indicating that the enhancement of photosynthesis was due to a greater efflux of oxygen from the coral tissues. By lowering the internal oxygen concentration, pulsation alleviates the problem of reduced affinity of ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) to CO2 under conditions of high oxygen concentrations. The photosynthesis–respiration ratio of the pulsating H. fuscescens was markedly higher than the ratios reported for nonpulsating soft and stony corals. Although pulsation is commonly used for locomotion and filtration in marine mobile animals, its occurrence in sessile (bottom-attached) species is limited to members of the ancient phylum Cnidaria, where it is used to accelerate water and enhance physiological processes.
Keywords: mass balance, diffusion, mass transfer coefficient, Red Sea
A unique characteristic of soft corals belonging to the family Xeniidae (phylum Cnidaria) is the perpetual, nonsynchronous pulsation of the colony’s polyps (Movie S1). This unique motion, consisting of a rhythmic extension and contraction of the tentacles, was first noted two centuries ago by Lamarck (1). However, neither its biomechanical effects nor its benefits to the coral are understood. To the best of our knowledge, no other sessile organism in the world’s oceans exhibits such a behavior. The closest motion resembling this pulsation is exhibited by medusae that rhythmically contract their bell (exumbrella) to swim (2, 3) or enhance nutrient uptake (4–6).
As pulsation in xeniid corals is vigorous and perpetual, its benefit should be considerable. Because all xeniid corals host symbiotic algae, and because food particles are rarely found in their gastrovascular cavity (7, 8), a positive effect of pulsation on carbon acquisition via photosynthesis of their symbionts or on the uptake of dissolved matter from the surrounding waters can be especially beneficial for these animals.
Here, we measured the metabolic cost of pulsation and tested the hypothesis that the key benefits of pulsation are the enhancement of photosynthesis by the coral’s symbiotic algae and the prevention of water refiltration by neighboring polyps. The proposed effect on photosynthesis is based on the prediction that pulsation intensifies mixing and accelerates the flow over the coral–water interface, which, in turn, increases the efflux of oxygen away from the coral’s tissues (9). The maintenance of low concentration of oxygen inside the coral is expected to enhance CO2 binding by ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) (10), thereby increasing the rate of photosynthesis by the coral’s endosymbiontic algae (9). Our study of the above predictions, including a characterization of the pulsation rhythm and its hydrodynamic effects, was carried out with the xeniid coral Heteroxenia fuscescens (Fig. 1) from the coral reef of Eilat, Red Sea, where this and several other xeniid species are common (10, 11).
Fig. 1.
The Xeniid coral Heteroxenia fuscescens during pulsation (A) and rest (B). Note the different postures of the tentacles among the pulsating polyps, demonstrating the absence of phase synchronization among the polyps within the colony. (C) A schematic illustration of the stem and tentacles of a single polyp.
Results
Pulsation Cycles.
Pulsation activity during day and night was examined in situ with four different H. fuscescens colonies at 5- to 10-m depth, using an underwater infrared (IR)-sensitive video camera cabled to shore. Underwater IR illumination was used during the night (Materials and Methods). The period of one full pulsation cycle was on average (±SD) 1.6 s (±0.18 s) (n = 72), with no phase synchronization among neighboring polyps within the colony (Fig. 1A).
Complete diel (24-h) records obtained for three of the colonies showed that the corals pulsate continuously during most (>95%) of the time. Short (15–30 min) intervals without pulsation (hereafter “rest”; Fig. 1B) were observed once a day, usually in late afternoon (Fig. 2). The entire record (>350 h) showed that resting intervals always occurred when the intensity of solar radiation was <50% of the daily maximum.
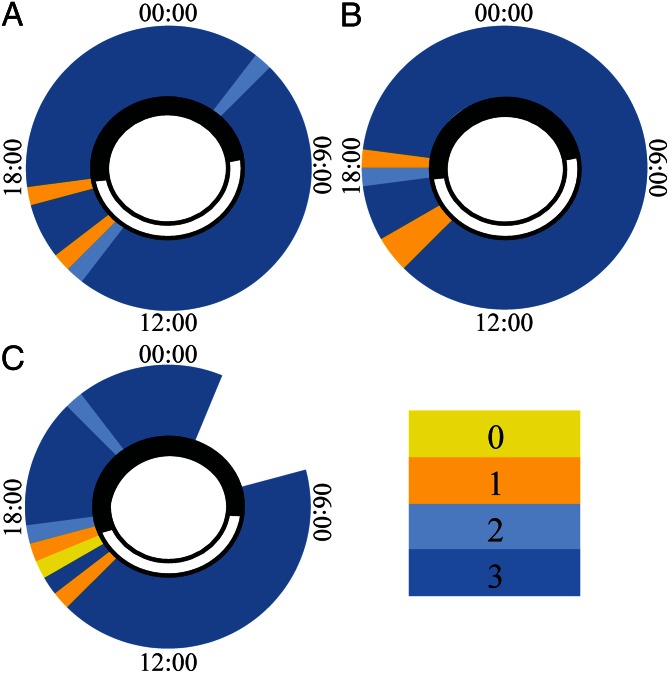
Fig. 2.
Diel patterns of pulsation and rest in three different H. fuscescens colonies recorded in (A) October 2010; (B) December 2010; and (C) December 2012. Color codes indicate four levels of pulsation activity: 0, inactive; 1 and 2, intermediate; 3, fully active; empty sections, missing data. The black and white sections around the inner circle indicate night and day, respectively.
Pulsation, Respiration, and Photosynthesis.
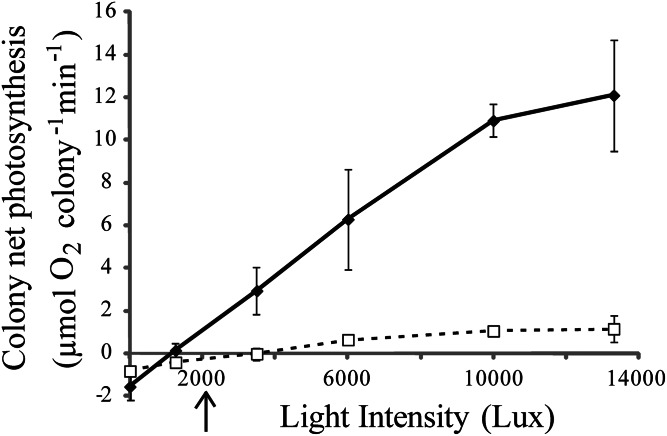
A comparison of respiration and photosynthesis between pulsating and resting H. fuscescens was carried out in the laboratory with freshly collected colonies (Materials and Methods). As shown in Fig. 3, these measurements indicate that the coral’s respiration rate during pulsation was approximately two times higher than during rest periods (mean ± SD = ×2.06 ± 0.28), whereas the gross photosynthesis was sevenfold higher (×7.11 ± 0.96); Fisher’s combined probability test: P < 0.01, n = 3 measurements for each of the three colonies for each of the respiration and photosynthesis measurements. A similar pattern was found in a different experiment where we measured the change in net photosynthesis as function of irradiance intensity (P–I curves). The results (Fig. 4) show that in the pulsating corals the slope of the P–I curve was much steeper, reaching about an order of magnitude higher Pmax than in the resting corals. Here, the enhancement of photosynthesis was already noticeable at dim light (3,500 lx), equivalent to the intensity of light at 10-m depth about 1 h after sunrise or before sunset.
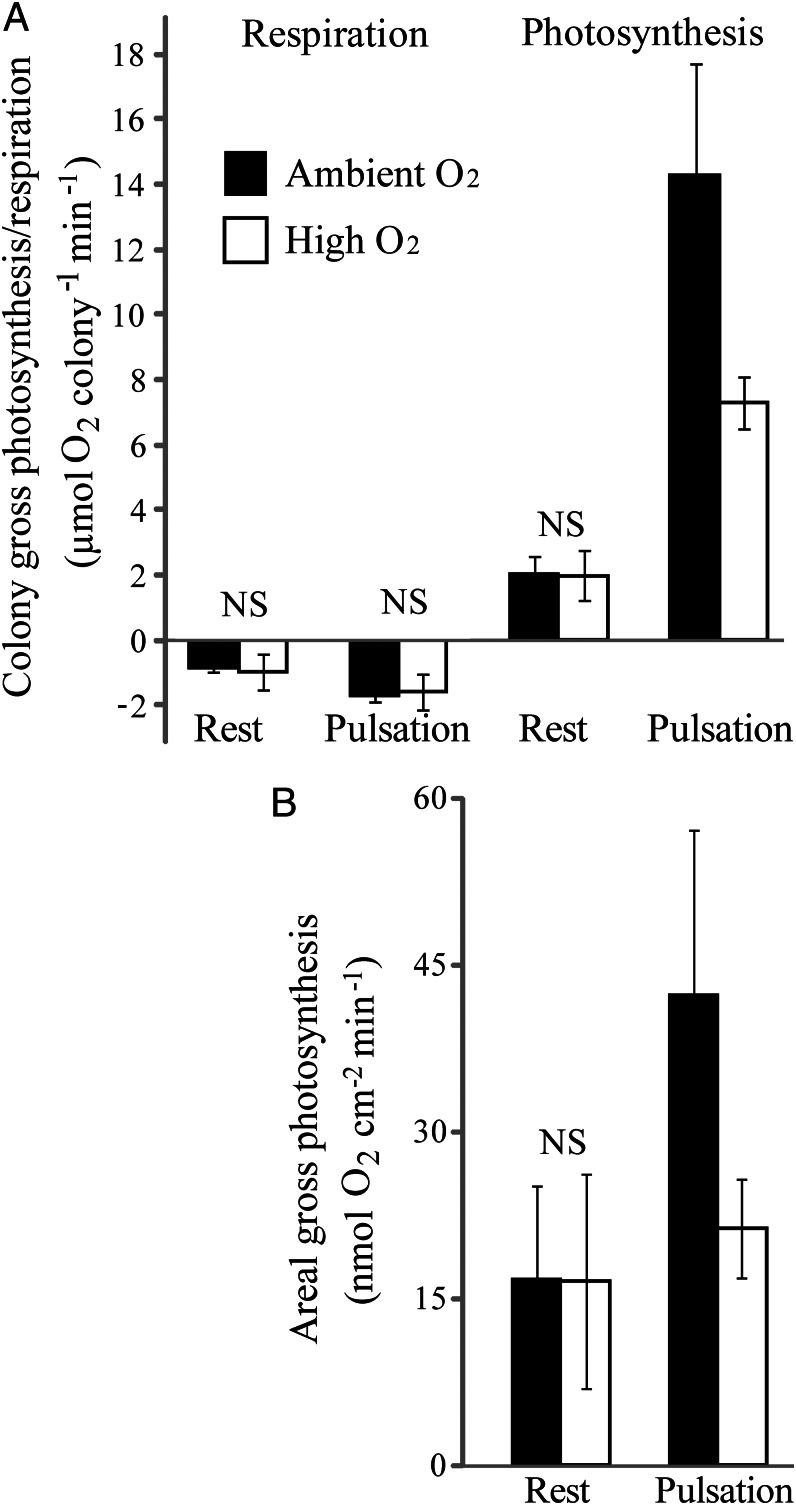
Fig. 3.
Effects of pulsation and ambient oxygen concentration on the rates of gross photosynthesis and respiration in H. fuscescens (n = 3). (A) Average rates (±SD) of gross photosynthesis and dark respiration under conditions of ambient (filled bars) and high (open bars) oxygen concentrations in pulsating and resting states. (B) Average rates (±SD) of gross photosynthesis normalized by the colony’s surface area exposed to downwelling light (see text). Note that the artificially elevated oxygen concentration in the chamber water practically eliminated the effect of pulsation on normalized photosynthesis (Mann–Whitney, P = 0.51, n = 3 colonies, testing for a difference in gross photosynthesis between pulsation under high oxygen and rest under normal oxygen). In both A and B, the difference in gross photosynthesis under pulsating state between ambient and high oxygen conditions was significant (Mann–Whitney, P < 0.05, n = 3).
Fig. 4.
P–I curves of pulsating (full line) and resting (dotted line) H. fuscescens. Data points indicate the average ± SD (n = 3 colonies) of net photosynthesis at different irradiance intensities. Arrow below the horizontal axis indicates the in situ light intensity 1 h before darkness at ∼10 m depth in the corl reef of Eilat during summer.
During pulsation, the stems of the active polyps are always extended and their tentacles rhythmically change their posture from being fully extended in the open state, to being tightly packed in the closed posture (Fig. 1 and Movie S1). Consequently, the surface area exposed to downwelling light during pulsation was on average (±SD) 2.69 (±0.26) times greater than during the resting state (n = 3). When considering this area, the normalized photosynthesis during pulsation was on average (±SD) 2.68 ± 0.57 times higher than during rest (Fig. 3B; Fisher’s combined probability, P < 0.01; N as above). The normalized value reflects the “net” effect (free of surface area changes) of pulsation on photosynthesis, likely due to pulsation-driven changes in the flow velocity and mixing intensity across the coral–water boundary layer (see below).
As stated above, we assume that the effect of pulsation on photosynthesis was an outcome of enhanced oxygen efflux from the coral tissues. A test of this assumption was carried out by repeating the metabolic measurements under conditions of artificially increased oxygen concentrations (300–500 µM) and comparing the results with those obtained under normal concentrations (∼200 µM).
Indeed, the increase in ambient oxygen practically eliminated the effect of pulsation on photosynthesis (Fig. 3B; Mann–Whitney, P = 0.51, n = 3 colonies, testing for a difference in gross photosynthesis between pulsation under high oxygen and rest under normal oxygen). Note that the photosynthesis rate in the nonpulsating state was unaffected by the change in ambient oxygen (Mann–Whitney, P = 0.83, n = 3).
Hydrodynamic Effects of Pulsation.
The flow field generated by pulsation was recorded in situ using our custom-built underwater particle image velocimetry (UPIV) system (Materials and Methods), positioned at the reef so that the measurement domain was located above the center of the coral colony (Fig. S1). Pulsation markedly changed the flow pattern above the coral polyps (Fig. S1). Most noticeable was the vertical (upward) thrust driven by pulsation near the coral–water interface (Fig. S2A): whereas during rest the vertical velocity near that interface was weaker than that measured at 1.8 cm above the coral, under pulsating state the former velocity was about twice the latter.
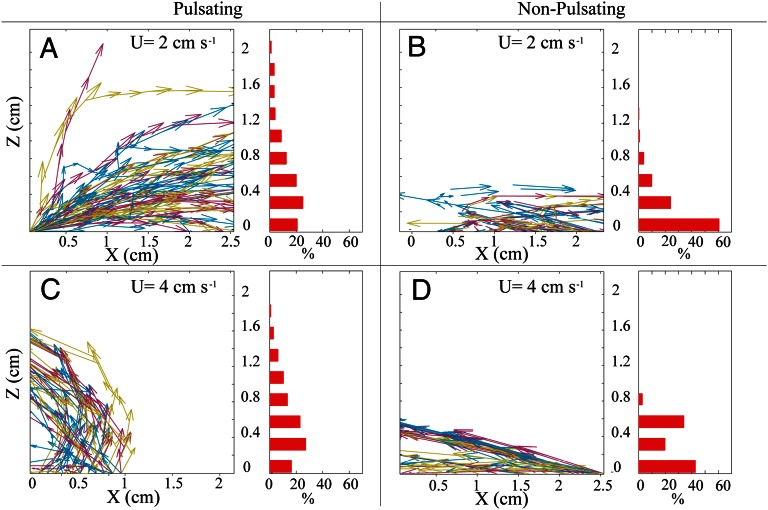
Lagrangian simulations were used to generate particle trajectories to further explore the flow patterns generated by pulsation. To do so, imaginary particles were “released” at the coral–water interface and tracked using our UPIV time series until they exited the measurement domain (Materials and Methods). As each trajectory started at the coral surface, where waterborne commodities such as dissolved gasses and nutrients are taken up by the coral, the simulation allowed us to assess the effect of pulsation on the probability of water refiltration by neighboring polyps. The results show that pulsation substantially reduced that probability; whereas in resting corals >50% of the imaginary particles (of a total of 1,379 trajectories) were refiltered at least once (Fig. 5 B and D), less than 20% of the trajectories (of a total of 4,949) showed refiltration during pulsation (Fig. 5 A and C). Moreover, during pulsation the frequency distribution of heights above the coral surface at which the virtual particles left the measurement domain exhibited a much wider range with greater proportions reaching >5 mm height, compared with the resting state (Kolmogorov–Smirnov, P < 0.01, testing for a difference between the frequency distribution of A vs. B and C vs. D in Fig. 5). Because the horizontal velocity component always increases with height (Fig. S2B), this upward thrust of water by pulsation is an effective means to remove postfiltered water away from the colony.
Fig. 5.
Trajectories of imaginary waterborne particles originating at the coral surface. Left part of each panel: ∼50 representative trajectories. Right part of each panel: histograms showing the percentage of particles (of all trajectories) exiting the measurement domain at different altitudes above the coral. (A) Pulsating H. fuscescens, ambient horizontal velocity (U) = 2 cm/s, n = 4,202 trajectories; (B) nonpulsating stony coral (Favia favus), U = 2 cm/s, n = 1,309; (C) pulsating H. fuscescens, U = 4 cm/s, n = 747; (D) nonpulsating H. fuscescens, U = 4 cm/s, n = 70. Pulsation significantly induced an upward thrust of water parcels for both of the ambient flow speeds (Kolmogorov–Smirnov test, P < 0.007 for horizontal velocity of 2 cm/s; P < 0.003 for 4 cm/s). Note that the Left section of each panel presents the trajectories in a 2.5 × 2 cm region located just above the central section of the coral, as seen in Fig. S1.
Discussion
We found that the net photosynthesis in the soft coral H. fuscescens was an order of magnitude higher during the coral’s pulsation than during its rest (Fig. 3). In terms of absolute gain, the increase in the colony’s photosynthesis rate by pulsation (+12.2 µmol O2⋅min−1) greatly surpasses the added metabolic cost (−0.9 µmol O2⋅min−1). Although part of that benefit (+5.3 µmol O2⋅min−1) is due to the increase in light-exposed surface area during pulsation, the remaining increase, due to motion alone, is remarkable, consisting of 56% of the total benefit. Clearly, a major benefit of pulsation in H. fuscescens is a substantial enhancement of photosynthesis.
How does pulsation enhance photosynthesis? Our findings indicate that this enhancement is due to the pulsation-driven modulation of the flow. That strong flow enhances photosynthesis is well documented in corals, algae, and sea grasses (9, 12–17). A recent study (9) showed that the mechanism involved is a flow-driven increase in oxygen efflux from the organism’s tissues. With no flow, the accumulation of photosynthetically produced oxygen in the tissue reduces the binding capability of CO2 by RuBisCO, thereby reducing photosynthesis and channeling carbon to the wasteful photorespiration pathway (9, 10). The enhancement of photosynthesis by pulsation in H. fuscescens seems to follow suit; our in situ UPIV measurements show that pulsation both intensifies mixing (Fig. 6) and propels water away from the oxygen-rich coral–water interface (Fig. S2). However, the conclusive evidence for the above pathway is our finding that no augmentation of photosynthesis by pulsation occurred when the ambient oxygen concentration was artificially raised (Fig. 3B). Note that the raised oxygen concentration in the ambient water had no effect on photosynthesis during rest (Fig. 3B). Apparently, under the quiescent conditions of the resting state, the local accumulation of oxygen within the coral tissues reached 600–1,000 µM (see oxygen transfer modeling in SI Text), thereby overwhelming the effect of ambient oxygen on CO2 binding capability by RuBisCO. High oxygen concentrations, ranging from 550 to 600 µM, were observed in the tissues of other corals under no-flow conditions (9, 18).
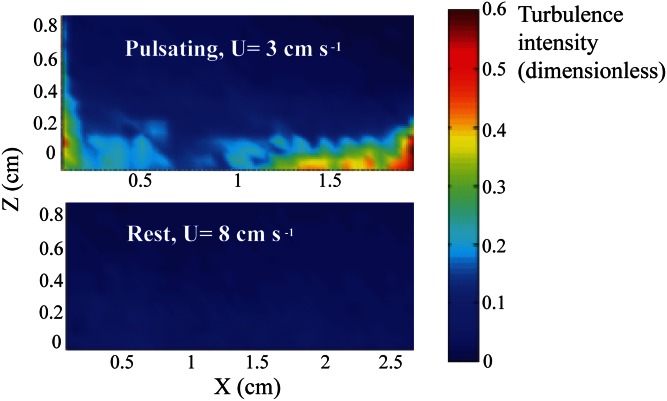
Fig. 6.
In situ turbulence intensity (color bar) across the boundary layer over H. fuscescens. (Upper) A pulsating colony under conditions of weak ambient current (U = 3 cm/s). (Lower) A resting colony under conditions of strong ambient current (U = 8 cm/s). See SI Text for methods.
Reassurance of the mechanism proposed above is provided by the calculation of the mass transfer coefficient (L) for each coral based on our experimental data (SI Text, Table S1, and Fig. S3). On average, L (±SD) was 9.42 (±2.46) times higher during pulsation than during resting states, reflecting the large effect of pulsation.
The findings that pulsation increases photosynthesis were all based on experiments in a closed chamber. How relevant are these findings to in situ conditions, where currents prevail most of the time? To address this question, we used our UPIV records to compare the in situ turbulence intensity (SI Text and Fig. S4) across the coral–water boundary layer between conditions of a pulsating coral in a weak ambient current (3 cm/s) to those of a resting coral in a strong current (8 cm/s; that is almost more than three times the average current velocity at the height of the corals above the bottom in the coral reefs of Eilat; see, for example, figure 4 in ref. 19). The results (Fig. 6) demonstrate the overwhelming effect of pulsation, with the turbulence intensity being at least an order of magnitude stronger under the former conditions.
Why does H. fuscescens pulsate at night (Fig. 2)? As hypoxic conditions commonly develop between coral branches at night (20, 21), the strong mixing induced by pulsation (Fig. 6) may enhance aeration, much like the nocturnal aeration of stony corals by their symbiotic, sleep-swimming fish (21). However, the occurrence of oxygen-limited respiration in H. fuscescens in the dark was not supported by our observation that in the resting state respiration was unaffected by the artificially elevated oxygen concentrations (Fig. 3A). Another benefit of pulsation during the night is the reduced probability of water refiltration by neighboring polyps (Fig. 5). Lower refiltration should improve both the uptake of waterborne commodities and the removal of excreted matter. For colonial organisms such as corals, where all of the polyps are “peas of the same pod” that share commodities (22, 23), the prevention of refiltration can benefit the entire colony. Although the effect of flow on the uptake of dissolved and particulate matter in corals is well documented (12, 24–28), the added value of reduced refiltration seems to be unique for pulsating corals.
Overall, our study shows that the adaptive benefit of pulsation for H. fuscescens consists of an order of magnitude enhancement of photosynthesis during the day and the prevention of refiltration during both day and night.
Although the cause of rest in H. fuscescens is not yet understood, its occurrence in late afternoon—early evening (Fig. 2), when the light intensity was always less than 50% of the corresponding daily maximum, indicates that the break in pulsation occurs when the dependency of photosynthesis rate on oxygen removal from the tissues is lessened.
The similarity between the perpetual pulsation and its frequency in H. fuscescens (0.6 ± 0.1 Hz) and those of the upside-down medusa, Cassiopea spp. (frequency of 0.9 ± 0.1 Hz; ref. 6) is intriguing. Both taxa are sedentary cnidarians, living in the quiescent benthic boundary layer. Both host symbiotic algae and in both photosynthesis is thought to be the main nutrition pathway. These similarities, despite the fact that the corals pulsate by using their tentacles and the medusa pulsate by using their bell, suggest that pulsation in Cassiopea may also augment photosynthesis, not only nutrient uptake (4).
A comparison of the photosynthesis-to-respiration (P:R) ratio in H. fuscescens (P:R = 8.3) with corresponding values reported for nine other, nonpulsating corals (five soft corals and four stony corals; Table S2) indicates that the ratio in H. fuscescens greatly surpasses that of all of the nonpulsating corals (ranging from 1.17 to 5.08). Pulsation appears to allow H. fuscescens to reach an exceedingly high energetic yield. The high P:R ratio may account for the enigmatic scarcity of food in the digestive cavity of the pulsating xeniid corals (8).
Pulsation is a common mechanism in the animal kingdom for thrusting fluids internally, most notably in the propulsion of blood by the heart. The use of pulsation to thrust water externally is found in several free-living animals, such as medusa, salps, and cephalopods, where its main function is locomotion. An added benefit, the enhancement of physiological processes, is found in sessile Cnidaria. Given the recent advents in biomimetics (29, 30), xeniid morphology and pulsation may serve as a model for the design of unique artificial pumps in engineering and medicine where enhancement of both material exchanges with the medium and its propulsion are needed.
Materials and Methods
Heteroxenia fuscescens.
Soft corals (Cnidaria: Octocorallia) belonging to the family Xeniidae are common in the Red Sea and the Indo-Pacific (11, 31, 32). Many, but not all, xeniids pulsate. Their gastrovascular cavity is reduced and its capacity for secretion of enzymes and for the endocytosis of food particles is distinctly diminished (33). Some studies classified the Xeniidae (both Xenia and Heteroxenia) as completely autotrophic, unable to take up particulate food (34, 35), although at least one study (36) reported that some prey items were found in the gastrovascular cavities of Xenia. Two studies (8, 33) suggested that xeniid corals can supplement their feeding by consuming dissolved organic matter.
In Situ Observations.
An IR-sensitive underwater video camera cabled to a VCR on the shore laboratory was used to record the in situ pulsation behavior of H. fuscescens living at 5- to 10-m depth in the coral reef of Eilat, Red Sea (in front of the Heinz Steinitz Marine Biology Laboratory; 29°30′ N, 34°56′ E). The video camera was positioned on a tripod oriented each time onto a single H. fuscescens colony, so that the recorded image included several dozens of polyps. Night records were made using IR illumination (880 nm), generated with eight custom-made underwater light sources, each consisting of 30 light-emitting diodes. No apparent response by H. fuscescens to that IR light was detected. Records were obtained for a total of four colonies in different seasons during 2009–2012, with each record lasting continuously several days, totaling more than 350 h of data. The activity of the corals was semiqualitatively characterized using the following “activity index”: “0,” a resting coral with its tentacles contracted for at least 30 s; “3,” fully active, all polyps in the frame continuously pulsate with their tentacles fully expanded during each pulse; “1” and “2” are intermediate states, where in “1” more than one-half of the polyps in the frame are either at rest or only partially expanding their tentacles; “2,” more than one-half of the polyps in the frame are fully active, but some polyps are at rest or only partially pulsating. This scaling allowed us to examine the occurrence of a diel pattern in the pulsation activity (as shown in Fig. 2).
Laboratory Metabolic Measurements.
H. fuscescens was collected by scuba divers at depths of 5–11 m. Colonies that were attached to small stones were selected and collected together with the stone to avoid injury during separation from the substrate. The stone was thoroughly brushed off from fouling algae and invertebrates to avoid external effects on our measurements and the coral was then placed in the metabolic chamber, a transparent, sealed aquarium, 13 × 15 × 25 cm in size. The colony’s photosynthesis and respiration rates were calculated based on measured changes in oxygen concentration in the chamber during 20-min intervals under light and dark conditions, respectively, with a single colony at a time. Changes in oxygen concentration, reflecting respiration or photosynthesis rates, were nearly constant during the measurement intervals, with the linear regression coefficients typically exceeding 0.95. Oxygen concentration was measured using an optode (mini Fibox 3; Presense) calibrated every 2 wk using the Winkler method. The optode tip was positioned at the upper section of the chamber, ∼15 cm away from the coral surface. A small magnetic stirrer was used to gently mix the water in the chamber, assuring that our measurements represented changes taking place in the entire volume. Water temperature in the chamber was similar to that found at the local coral reef (∼22 °C), maintained by immersing the metabolic chamber in a large (96-L), temperature-controlled water bath. Two metal-halide lamps (230 V, 150 W) were used as a light source with intensity similar to that at the reef during midday at 10-m depth (∼13,000 lx). All measurements were replicated three times with each colony under each of the pulsating and nonpulsating states, each under dark and light conditions, with a total of three colonies.
To induce a resting mode, we used the fact that, when lightly touched, H. fuscescens stops pulsating for a few minutes. Accordingly, a small plastic rod (5 cm long, 2 mm wide) with a small magnet (2 × 4 × 5 cm3) attached at its proximal end, was laid on the floor of the metabolic chamber. For consistency, the rod and magnet were kept in the metabolic chamber also during trials without induction of rest. To induce a resting state, the rod was raised from the floor using an outside magnet and then gently moved with its distal (plastic) part “lightly patting” the upper tips of the coral polyps. Morphologically, the induced and natural rest were indistinguishable. The only observed difference between natural and induced rest was the transition time from active to resting state: ∼5 min compared with a few seconds, respectively. During the first ∼7 min after inducing rest, every minute or so the tentacles started to slightly move, necessitating an additional slight touch to induce reretraction. After that interval, the coral usually stopped pulsating altogether for about 30 min. Therefore, our measurements of respiration or photosynthesis always started >10 min after the first touch and typically lasted 10–20 min, always more than 5 min. Occasionally, when slight tentacle movements were noticed, an additional gentle touch was necessary during the measurements.
Our use of induced, rather than natural rest, where the coral in the metabolic chamber rested with no artificial intervention, allowed us to replicate rest time measurements during single days. A separate set of measurements showed that neither photosynthesis nor respiration rates during induced rest differed significantly from those under natural rest (paired t test, P > 0.59 and P > 0.65 for photosynthesis and respiration, respectively; n = 3 colonies each).
If pulsation affects photosynthesis by enhancing the efflux of oxygen from the coral tissues (9), this effect is expected to diminish when the ambient concentration of oxygen is artificially elevated. To examine this, we measured photosynthesis and respiration before and after elevating the ambient oxygen concentration from ∼200 µM to 350–550 µM by bubbling pure oxygen gas through the water for a few minutes. In this experiment, the chamber was sealed and measurements started a few minutes after all visible bubbles disappeared.
Respiration and photosynthesis rates were calculated based on the change in oxygen concentration in the aquarium. Considering the volume of the metabolic chamber (3.8 L) and the surface area of each coral, we calculated the coral’s specific (per square centimeter) respiration, gross photosynthesis, and net photosynthesis rates during pulsating and rest states. The surface area of each coral was calculated based on its measured radius (obtained from digital photographs), assuming the outer geometry of the colony can be approximated as a half-sphere dome shape (2πr2; see Table S1 for values).
UPIV.
The velocity field above two colonies of H. fuscescens was measured in situ using our recently developed UPIV system (37) (Fig. S1). In general, PIV is a noninvasive optical measurement technique that provides 2D, two-component instantaneous velocity fields through a cross-correlation image analysis procedure applied to pairs of images recorded with an imaging system. Small natural particles are illuminated by two Nd:YAG pulsed lasers (200 mJ/pulse; Quantel; Big Sky) and recorded on image files (1,024 × 1,392) pixels using a double-shutter CCD camera (Pixelfly qe; PCO) equipped with a 200-mm Nikkor lens. The image area size was 2.62 × 1.87 cm2 located just above the center of the coral, covering approximately two polyps. Custom-made underwater housings that were manufactured for the two lasers and the camera systems (Fig. S1) were mounted on a special underwater metal frame, which allowed a fine alignment of their position and orientation (see SI Text for more details). The UPIV measurements were carried out in situ from April 26 to June 1, 2010, at ∼5-m depth in the coral reef of Eilat. The database consisted of “sets,” ranging in length between a few seconds to minutes, each consisting of image pairs recorded at 5 Hz (See Table S3 for information about our UPIV dataset). The records were obtained during the night assuring a dark background, which is ideal for generating high-quality PIV images. Postprocessing of the collected realizations for each set the provided the mean velocity fields and Lagrangian second-order implicit trajectory simulations as described in SI Text. In addition to our focal species, H. fuscescens, we used two colonies of the stony coral Favia verroni of a similar size as a nonpulsating “control.” The round, sphere-like morphology of F. verroni is similar to that of H. fuscescens, its polyps are smaller, lack a “stem,” and have smaller gaps between them.
Statistical Analysis.
A Fisher combined probability test (ref. 38, pp 779–782) was performed to test for the effect of pulsation on photosynthesis and respiration. The combined test consisted of three independent Mann–Whitney tests (38), each performed on the three sets of measurements obtained for a single colony. A Mann–Whitney test was used to test for the effect of high ambient oxygen concentration on the photosynthesis rate of H. fuscescens during pulsating and nonpulsating conditions. Kolmogorov–Smirnov test (38) was used to test for the effect of pulsation on distributions of Lagrangian trajectories leaving the coral surface. The Mann–Whitney and Kolmogorov–Smirnov tests were performed using SYSTAT (version 9.0), whereas Fisher’s test was performed manually.
Supplementary Material
Acknowledgments
We are indebted to E. Foran and I. Kolesnikova for assistance; to C. Reimers, N. Keren, and K. Fabricius for valuable advice; to V. China for photography; to the staff of the Interuniversity Institute for Marine Sciences of Eilat for logistic help; to Y. and Y. Mass from Orion Marine Services, Ltd., for the design and building of the housing for the UPIV; to M. Koehl, R. Holzman, and M. Zarubin for comments on earlier drafts; and to two anonymous reviewers for insightful comments. This study was funded by the Israel Science Foundation Grants 620/07 and 1487/10.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8767.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301826110/-/DCSupplemental.
References
- 1.Lamarck JBPA. 1816. Histoire Naturelle des Animeaux sans Vertèbres [Natural History of Invertebrate Animals] (Verdière, Paris), Vol 2, pp 409–410. French.
- 2.Costello JH, Colin SP. Morphology, fluid motion and predation by the scyphomedusa Aurelia aurita. Mar Biol. 1994;121:327–334. [Google Scholar]
- 3.Katija K, Dabiri JO. A viscosity-enhanced mechanism for biogenic ocean mixing. Nature. 2009;460(7255):624–626. doi: 10.1038/nature08207. [DOI] [PubMed] [Google Scholar]
- 4.Jantzen C, Wild C, Rasheed M, El-Zibdah M, Richter C. Enhanced pore-water nutrient fluxes by the upside-down jellyfish Cassiopea sp. in a Red Sea coral reef. Mar Ecol Prog Ser. 2010;411:117–125. [Google Scholar]
- 5.Hamlet CL, Santhanakrishnan A, Miller LA. A numerical study of the effects of bell pulsation dynamics and oral arms on the exchange currents generated by the upside-down jellyfish Cassiopea xamachana. J Exp Biol. 2011;214(Pt 11):1911–1921. doi: 10.1242/jeb.052506. [DOI] [PubMed] [Google Scholar]
- 6.Santhanakrishnan A, Dollinger M, Hamlet CL, Colin SP, Miller LA. Flow structure and transport characteristics of feeding and exchange currents generated by upside-down Cassiopea jellyfish. J Exp Biol. 2012;215(Pt 14):2369–2381. doi: 10.1242/jeb.053744. [DOI] [PubMed] [Google Scholar]
- 7.Fabricius KE, Alderslade P. Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Townsville, QLD, Australia: Australian Institute of Marine Science; 2001. pp. 138–142. [Google Scholar]
- 8.Fabricius KE, Klumpp DW. Widespread mixotrophy in reef-inhabiting soft corals: The influence of depth, and colony expansion and contraction on photosynthesis. Mar Ecol Prog Ser. 1995;125(1–3):195–204. [Google Scholar]
- 9.Mass T, Genin A, Shavit U, Grinstein M, Tchernov D. Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proc Natl Acad Sci USA. 2010;107(6):2527–2531. doi: 10.1073/pnas.0912348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldsworthy A. Photorespiration. Bot Rev. 1970;36:321–340. [Google Scholar]
- 11.Fishelson L. Scleraetinian anthozoans of shallow waters of the Red Sea (Eilat) Mar Biol. 1970;6:106–116. [Google Scholar]
- 12.Patterson MR, Sebens KP, Olson RR. In situ measurements of flow effects on primary production and dark respiration in reef corals. Limnol Oceanogr. 1991;36:936–948. [Google Scholar]
- 13.Finelli CM, Helmuth BST, Pentcheff ND, Wethey DS. Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs. 2006;25:47–57. [Google Scholar]
- 14.Dennison WC, Barnes DJ. Effect of water motion on coral photosynthesis and calcification. J Exp Mar Biol Ecol. 1988;115:67–77. [Google Scholar]
- 15.Carpenter R, Williams S. Mass transfer limitation of photosynthesis of coral reef algal turfs. Mar Biol. 2007;151:435–450. [Google Scholar]
- 16.Koehl MAR, Alberte RS. Flow, flapping, and photosynthesis of Nereocystis luetkeana: A functional comparison of undulate and flat blade morphologies. Mar Biol. 1988;99:435–444. [Google Scholar]
- 17.Enriquez S, Rodriguez-Roman A. Effect of water flow on the photosynthesis of three marine macrophytes from a fringing-reef lagoon. Mar Ecol Prog Ser. 2006;323:119–132. [Google Scholar]
- 18.Kühl M, Cohen Y, Dalsgaard T, Jørgensen BB, Revsbech NP. The microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar Ecol Prog Ser. 1995;117:159–172. [Google Scholar]
- 19.Genin A, Monismith SG, Reidenbach MA, Yahel G, Koseff JR. Intense benthic grazing of phytoplankton in a coral reef. Limnol Oceanogr. 2009;54(3):938–951. [Google Scholar]
- 20.Shashar N, Cohen Y, Loya Y. Extreme diel fluctuations of oxygen in diffusive boundary layers surrounding stony corals. Biol Bull. 1993;185:455–461. doi: 10.2307/1542485. [DOI] [PubMed] [Google Scholar]
- 21.Goldshmid R, Holzman R, Weihs D, Genin A. Aeration of corals by sleep-swimming fish. Limnol Oceanogr. 2004;49:1832–1839. [Google Scholar]
- 22.Oren U, Rinkevich B, Loya Y. Oriented intra-colonial transport of C14 labeled materials during regeneration in scleractinian corals. Mar Ecol Prog Ser. 1997;161:117–121. [Google Scholar]
- 23.Pearse VB, Muscatine L. Role of symbiotic algae (zooxanthellae) in coral calcification. Biol Bull. 1971;141:350–363. [Google Scholar]
- 24.Sebens KP, Witting J, Helmuth B. Effects of water flow and branch spacing on particle capture by the reef coral Madracis mirabilis (Duchassaing and Michelotti) J Exp Mar Biol Ecol. 1997;211:1–28. [Google Scholar]
- 25.Falter JLM, Atkinson MJ, Merrifield MA. Mass-transfer limitation of nutrient uptake by a wave-dominated reef flat community. Limnol Oceanogr. 2004;49:1820–1831. [Google Scholar]
- 26.Falter JL, Atkinson MJ, Coimbra CFM. Effects of surface roughness and oscillatory flow on the dissolution of plaster forms: Evidence for nutrient mass transfer to coral reef communities. Limnol Oceanogr. 2005;50:246–254. [Google Scholar]
- 27.Hearn CJ, Atkinson MJ, Falter JL. A physical derivation of nutrient-uptake rates in coral reefs: Effects of roughness and waves. Coral Reefs. 2001;20:347–356. [Google Scholar]
- 28.Reidenbach MA, Monismith SG, Koseff JR, Yahel G, Genin A. Boundary layer turbulence and flow structure over a fringing coral reef. Limnol Oceanogr. 2006;51:1956–1968. [Google Scholar]
- 29.Bar-Cohen Y. Biomimetics: Nature Based Innovation. Boca Raton, FL: CRC; 2011. [Google Scholar]
- 30.Nawroth JC, et al. A tissue-engineered jellyfish with biomimetic propulsion. Nat Biotechnol. 2012;30(8):792–797. doi: 10.1038/nbt.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benayahu Y, Loya Y. Competition for space among coral-reef sessile organisms at Eilat, Red Sea. Bull Mar Sci. 1981;31:514–522. [Google Scholar]
- 32.Dinesen ZD. Patterns in the distribution of soft corals across the central Great Barrier Reef. Coral Reefs. 1983;1:229–236. [Google Scholar]
- 33.Schlichter D. Epidermal nutrition of the Alcyonarian Heteroxenia fuscescens (Ehrb)- absorption of dissolved organic material and lost endogenous photosynthates. Oecologia. 1982;53:40–49. doi: 10.1007/BF00377134. [DOI] [PubMed] [Google Scholar]
- 34.Gohar HAF. 1940. Studies on the Xeniidae of the Red Sea: Their ecology, physiology, taxonomy, and phylogeny. Publ Mar Biol St Al Ghardaqa (Red Sea) 2:24–118.
- 35.Schlichter D, Svoboda A, Kremer BP. Functional autotrophy of Heteroxenia Fuscescens (Anthozoa, Alcyonaria)—carbon assimilation and translocation of photosynthates from symbionts to host. Mar Biol. 1983;78(1):29–38. [Google Scholar]
- 36.Lewis JB. Feeding behaviour and feeding ecology of the Octocorallia (Coelenterata: Anthozoa) J Zool. 1982;196:371–384. [Google Scholar]
- 37.Mass T. 2010. Biological and ecological effects of flow on corals. PhD thesis (Hebrew Univ of Jerusalem, Jerusalem), p 86.
- 38.Sokal RR, Rohlf FJ. Biometry. 2nd Ed. San Francisco: Freeman; 1981. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.