Abstract
The X-linked gene cyclin-dependent kinase-like 5 (CDKL5) is mutated in severe neurodevelopmental disorders, including some forms of atypical Rett syndrome, but the function and regulation of CDKL5 protein in neurons remain to be elucidated. Here, we show that CDKL5 binds to the scaffolding protein postsynaptic density (PSD)-95, and that this binding promotes the targeting of CDKL5 to excitatory synapses. Interestingly, this binding is not constitutive, but governed by palmitate cycling on PSD-95. Furthermore, pathogenic mutations that truncate the C-terminal tail of CDKL5 diminish its binding to PSD-95 and synaptic accumulation. Importantly, down-regulation of CDKL5 by RNA interference (RNAi) or interference with the CDKL5–PSD-95 interaction inhibits dendritic spine formation and growth. These results demonstrate a critical role of the palmitoylation-dependent CDKL5–PSD-95 interaction in localizing CDKL5 to synapses for normal spine development and suggest that disruption of this interaction by pathogenic mutations may be implicated in the pathogenesis of CDKL5-related disorders.
Mutations in the X-linked gene cyclin-dependent kinase-like 5 (CDKL5) have been identified in patients with severe neurodevelopmental disorders, including an early-onset variant of Rett syndrome (1, 2). The predicted protein encoded by CDKL5 gene belongs to the CDKL family that comprises five members, CDKL1 to CDKL5 (3). The expression of CDKL5 is enriched in human and rat brain (4, 5). In murine, CDKL5 is expressed at low levels in embryonic stages and its expression is markedly up-regulated during postnatal development (5, 6). Loss-of-function studies using RNA interference (RNAi) revealed that CDKL5 is required for neurite growth and excitatory synapse stability (5, 7). Deficiency of CDKL5 in mice leads to autistic-like phenotypes, indicating a causal role for CDKL5 loss of function in disease (8).
Several interacting partners of CDKL5 have been reported, including the Rett syndrome–related protein methyl-CpG binding protein 2 (MeCP2) (9), the DNA methyltransferase Dnmt1 (10), and netrin-G1 ligand (NGL-1) (7). All of the three proteins are potential substrates for CDKL5, at least as demonstrated in vitro, suggesting that CDKL5 elicits its function by phosphorylating target proteins. Although the kinase activity of CDKL5 is required for its function and is impaired by some mutations identified in patients (7, 11), the regulation of CDKL5 seems to be equally important in the pathogenesis of disease, which is suggested by a number of pathogenic mutations identified within its C-terminal region (12). Therefore, further identification of CDKL5-interacting proteins may uncover the regulatory mechanisms for CDKL5, which is an important step toward the understanding of the causes of CDKL5-related disorders.
The multidomain protein postsynaptic density (PSD)-95 is a major scaffold in the postsynaptic density (PSD) (13), and has been extensively investigated during the past decade. These studies have established the essential role of PSD-95 in synapse development and function (14–16). In excitatory synapses, PSD-95 associates with neurotransmitter receptors, adhesion molecules, signaling enzymes (17–19), and its synaptic clustering precedes any of these associated partners (20), suggesting that it functions as an organizer to initiate synapse maturation.
The N-terminal domain of PSD-95 is posttranslationally modified by palmitoylation, the attachment of a 16-carbon palmitate group to a cysteine residue via a thioester bond (21, 22). Unlike other lipid modification of proteins, palmitoylation is dynamic and reversible (23). The attachment of palmitic acids is catalyzed by palmitoyl acyltransferases (PATs) and the removal of it by palmitoyl thioesterases (PPTs) (24). Many palmitoylated proteins, including PSD-95, undergo consecutive cycles of palmitoylation and depalmitoylation (25). Importantly, this palmitate cycling can be regulated by some physiological stimuli (26). For PSD-95, depalmitoylation is accelerated by glutamate receptor activation, whereas palmitoylation is increased by blocking synaptic activity or by BDNF stimulation (25, 27, 28).
Palmitate cycling on PSD-95 controls its polarized targeting to synapses, which is essential for its synaptic functions (25). In this study, we show that palmitoylated PSD-95, but not its nonpalmitoylated form, binds to CDKL5 and promotes its synaptic targeting. This interaction is critical for dendritic spine development and is impaired by some pathogenic mutations. These findings implicate CDKL5 in PSD-95–dependent synapse development and provide insights into the pathogenesis of CDKL5-related neurological disorders.
Results
PSD-95 Is a CDKL5-Interacting Protein.
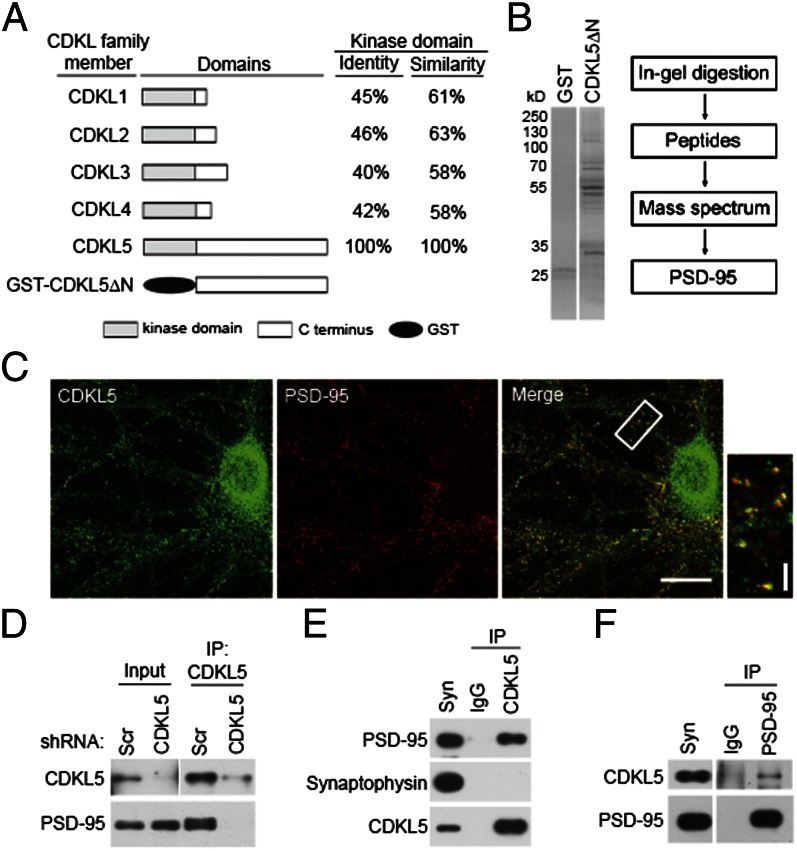
To better understand the mechanisms that underlying the function of CDKL5 in neurons, we searched for its interacting proteins by a glutathione S-transferase (GST) affinity purification method. We previously identified two splicing isoforms named CDKL5a and CDKL5b in the brain (5). Because CDKL5a but not CDKL5b is expressed in neurons, we focused on CDKL5a in this study and refer to CDKL5a as CDKL5 unless otherwise stated. The kinase domains of the CDKL family members show high homology (Fig. 1A); therefore, we chose to use the unique C-terminal region of rat CDKL5 (CDKL5ΔN, amino acids 297–934) as bait (Fig. 1A). Glutathione-Sepharose resins charged with recombinant GST or GST-CDKL5ΔN were incubated with rat brain lysates. Bound proteins were eluted, separated by SDS/PAGE, and subjected to MS analysis (Fig. 1B). This effort yielded a protein known as PSD-95, which showed specific binding to GST-CDKL5ΔN but not to GST control. A direct binding of PSD-95 to CDKL5 was confirmed by a GST pull-down assay using purified proteins (Fig. S1).
Fig. 1.
PSD-95 is a CDKL5-interacting protein. (A) Schematic diagram for domain structures of CDKL family members and GST-CDKL5ΔN recombinant protein. (B) Isolation and identification of CDKL5-interacting proteins. (Left) Coomassie Bright Blue staining of SDS/PAGE gels for the purified proteins. (Right) Flow diagram of mass spectrum analysis. (C) Colocalization of CDKL5 and PSD-95 in hippocampal neurons. Neurons were fixed at 18 DIV and double-stained for CDKL5 and PSD-95. (Scale bar: 20 μm.) The boxed region is magnified to show the colocalization. (Scale bar: 5 μm.) (D) Interaction of CDKL5 and PSD-95 in neurons. Cultured neurons infected with lentivirus expressing scrambled (Scr) shRNA or CDKL5 shRNA were lysed and subjected to immunoprecipitation (IP) using a CDKL5 polyclonal antibody. The immunoprecipitates and the lysates were immunoblotted with CDKL5 or PSD-95 antibody. Interaction of CDKL5 and PSD-95 in the rat brain. Coimmunoprecipitations were performed in rat synaptosome (syn) using CDKL5 (E) or PSD-95 (F) antibody.
We compared the temporal expression profiles of PSD-95 and CDKL5 by immunoblotting of extracts prepared from rat brains at different ages. Both proteins were expressed at low levels in embryonic stages, and their expression increases markedly during early postnatal development (Fig. S2A). Because PSD-95 is a PSD protein (13), we evaluated the distribution of CDKL5 relative to PSD-95 in each fraction in a PSD preparation. In all fractions examined, CDKL5 was copurified with PSD-95 and concentrated in the PSD fractions (Fig. S2B). As with PSD-95, CDKL5 was enriched in the core PSD fraction (PSDIII), which remains after extraction with the strong detergent Sarkosyl (Fig. S2B), suggesting that it was tightly associated with PSD. Double staining for the two proteins showed that CDKL5 and PSD-95 were colocalized in puncta along dendrites in hippocampal neurons (Fig. 1C), indicating a synaptic localization of CDKL5.
We examined whether CDKL5 and PSD-95 interact in vivo using coimmunoprecipitation assays. In lysates of cultured neurons, a CDKL5 polyclonal antibody efficiently coimmunoprecipitated PSD-95, indicating that the two proteins interact in neurons (Fig. 1D). As a negative control, a parallel experiment was carried out using lysates from neurons in which CDKL5 was depleted by a lentivirus-delivered shRNA. The same antibody failed to coimmunoprecipitate PSD-95 in these lysates (Fig. 1D), demonstrating the specificity of this interaction. The two proteins also interacted in intact brain as assayed by coimmunoprecipitation in extracts prepared from a crude synaptosome fraction (P2). In these experiments, PSD-95, but not the presynaptic protein synaptophysin, was coimmunoprecipitated by the CDKL5 antibody (Fig. 1E), and CDKL5 was coimmunoprecipitated with a PSD-95 antibody (Fig. 1F). PSD-95 belongs to the family of membrane-associated guanylate kinase. Because members of this family are structurally similar, we examined whether CDKL5 also interacts with other family members [including PSD-93, synapse-associated protein (SAP) 97, and SAP102]. We found that CDKL5 was efficiently coimmunoprecipitated with PSD-95 (Fig. S2C). Only trace amounts of CDKL5 could be coimmunoprecipitated with PSD-93, and no CDKL5 was precipitated by SAP97 or SAP102. Taken together, these results identify PSD-95 as an interacting protein of CDKL5 and demonstrate that the two proteins interact with each other both in vitro and in vivo.
CDKL5–PSD-95 Interaction Is Controlled by Palmitoylation.
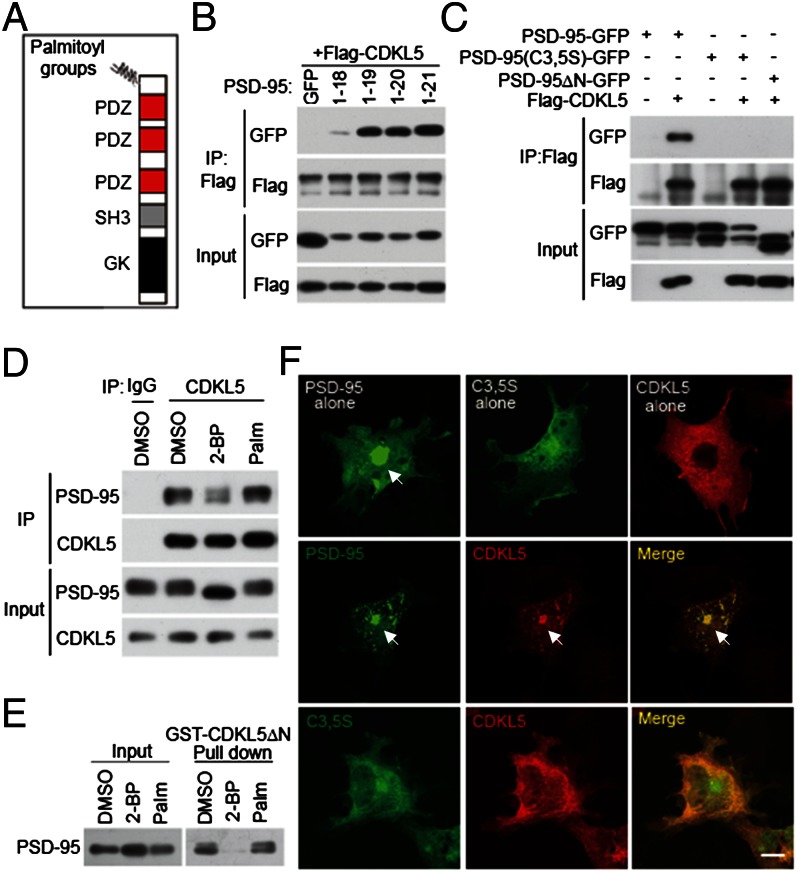
PSD-95 contains multiple domains for protein–protein interaction, including three PSD-95/Discs large/Zona occludens 1 (PDZ) domains, an Src homology 3 (SH3) domain, and a guanylate kinase (GK) domain (Fig. 2A). To determine which domain(s) interacts with CDKL5, we fused GFP to the C terminus of PSD-95 and its truncated derivatives and tested their interaction with Flag-tagged CDKL5 by coimmunoprecipitation. Surprisingly, simultaneous deletion of the three protein–protein interaction modules (PDZ, SH3, and GK) of PSD-95 failed to eliminate its interaction with CDKL5 (Fig. S3 A and B). On the other hand, a PSD-95 mutant (PSD-95ΔN) in which the region of amino acids 1–64 was deleted did not interact with CDKL5 (Fig. S3 A and B). Thus, the N-terminal region of PSD-95 not previously suspected as a module for protein–protein interaction was necessary and sufficient for binding to CDKL5. To identify the minimal binding motif, we generated a series of N-terminal deletion mutants of PSD-95 with GFP fused to their C termini and tested their interaction with CDKL5. We observed that PSD-95 (amino acids 1–21), PSD-95 (amino acids 1–20), and PSD-95 (amino acids 1–19) bound to CDKL5 at comparable levels (Fig. 2B). However, further deletion of amino acid 19 essentially abolished this interaction (Fig. 2B), indicating that the binding motif is localized to amino acids 1–19.
Fig. 2.
The CDKL5–PSD-95 interaction is controlled by palmitoylation. (A) Schematic diagram for domain structures of PSD-95. (B) Determination of the minimal binding region for CDKL5 in PSD-95. GFP or GFP-tagged PSD-95 derivatives (amino acids 1–18, 1–19, 1–20, and 1–21) were cotransfected with Flag-CDKL5 into 293T cells. Coimmunoprecipitation was performed in cell lysates with a Flag antibody. (C) Mutations preventing palmitoylation of PSD-95 abolish its interaction with CDKL5. PSD-95-GFP, PSD-95 (C3,5S)-GFP, and PSD-95ΔN-GFP were transfected individually or together with Flag-CDKL5 into 293T cells. Coimmunoprecipitation was performed in cell lysates with a Flag antibody. (D) Inhibition of palmitoylation decreases the interaction of CDKL5 with PSD-95 in neurons. Cortical neurons were treated with vehicle (DMSO), 20 μM 2-BP, or 20 μM palmitate for 8 h. After treatment, cells were harvested and the lysates were immunoprecipitated with a CDKL5 antibody. (E) GST pull-down experiment showing that the interaction of CDKL5 with PSD-95 is palmitoylation-dependent. Cortical neurons were treated as in D and the lysates were incubated with GST or GST-CDKL5ΔN affinity beads. Total lysates and precipitates were immunoblotted for PSD-95. (F) Palmitoylation-dependent interaction of CDKL5 and PSD-95 in COS-7 cells. COS-7 cells were transfected with PSD-95-GFP, PSD-95 (C3,5S)-GFP, and Flag-CDKL5, individually or together. Cells were fixed and stained for Flag. Arrows indicate the accumulation of proteins at the perinuclear region. (Scale bar: 20 μm.)
We noticed that this binding motif (amino acids 1–19) in PSD-95 overlaps with its palmitoylation motif (amino acids 1–13) (22). Palmitoylation at cysteines 3 and 5 promotes the association of PSD-95 to membranes and is required for its targeting to synapses (22, 25). However, it is unknown whether palmitoylation can influence the direct interaction of PSD-95 with other proteins. We decided to test whether palmitoylation influences the interaction between CDKL5 and PSD-95. Mutation of cysteine to serine at positions 3 and 5 (C3,5S) of PSD-95 prevents palmitoylation (22). Therefore, we surveyed the interaction of CDKL5 with this palmitoylation-deficient mutant. Coimmunoprecipitation assays were performed in 293T cells transfected with GFP-tagged WT PSD-95, PSD-95 (C3,5S), or PSD-95ΔN together with Flag-tagged CDKL5. Similar to deleting the N terminus, mutating cysteines 3 and 5 to serines completely abolished the binding of PSD-95 to CDKL5 (Fig. 2C). Moreover, in a GST pull-down experiment, WT PSD-95 but not PSD-95 (C3,5S) was precipitated by GST-CDKL5ΔN (Fig. S3C), demonstrating a requirement of palmitoylation for the physical interaction of PSD-95 with CDKL5. Deficiency in palmitoylation prevents the association of PSD-95 with membranes (21). Therefore, the failure of PSD-95 (C3,5S) to interact with CDKL5 in living cells could be due to changes in its subcellular location but not to the lack of binding ability. To examine this possibility, we generated a PSD-95 mutant by adding the prenylated motif of paralemmin to the C terminus of PSD-95 (C3,5S). Although not be palmitoylated, this mutant associates with cell membranes and correctly targets to synapses (22). We found that PSD-95(C3,5S)-prenyl did not interact with CDKL5 (Fig. S3D), indicating that the binding of PSD-95 to CDKL5 requires palmitoylation at its N-terminal domain.
To examine whether palmitoylation is important for the interaction of endogenous PSD-95 and CDKL5, we treated cultured neurons with the palmitate analog 2-bromopalmitate (2-BP), a specific palmitoylation inhibitor that effectively blocks palmitoylation (29). Compared with vehicle (DMSO) or palmitate, 2-BP treatment significantly decreased the amount of coimmunoprecipitated PSD-95 by the CDKL5 antibody (Fig. 2D). Similar results were observed by using GST pull-down assays in which 2-BP treatment reduced precipitated PSD-95 to the nearly undetectable level (Fig. 2E). These data obtained using pharmacological tools not only demonstrate the importance of palmitoylation for CDKL5–PSD-95 interaction in neurons, but also argue against the point that cysteine itself, but not palmitoylation, is critical for this interaction. We further demonstrate that the binding of PSD-95 to CDKL5 is not a general phenomenon of palmitoylated proteins. In a GST pull-down assay performed in lysates of neurons, neither of the two well-known palmitoylated proteins, growth associated protein-43 (GAP43) and v-Ha-ras Harvey rat sarcoma viral oncogene homolog (HRas), showed binding to GST-CDKL5ΔN (Fig. S3E). Moreover, when the 13-amino acid palmitoylation motif at the N-terminal region of PSD-95 was replaced by the palmitoylation motif of GAP-43, this chimera protein lost the ability to bind to CDKL5 (Fig. S3D), although it can still be palmitoylated in cells (22).
To visualize where this interaction occurs in intact cells, we transiently transfected COS-7 cells with CDKL5, WT PSD-95, and PSD-95 (C3,5S), individually or together. As previously reported (22), when expressed alone, WT PSD-95 showed a cytoplasmic distribution with accumulation at the perinuclear region, a site for early secretory pathway (Fig. 2F). Unlike WT PSD-95, CDKL5 as well as PSD-95 (C3,5S) appeared in a diffuse pattern throughout the cells (Fig. 2F). However, when CDKL5 was coexpressed with WT PSD-95, it was relocated to the perinuclear region and discrete puncta in the cytoplasm where it colocalized with PSD-95 (Fig. 2F), suggesting that CDKL5 can be recruited to early secretory pathway for trafficking by PSD-95. Consistent with an essential role of palmitoylation in this process, such change in subcellular distribution was not observed in cells coexpressing CDKL5 and PSD-95 (C3,5S) (Fig. 2F). Together, these data demonstrate that CDKL5 binds to PSD-95 in a palmitoylation-dependent fashion, and that this interaction may regulate its subcellular location.
PSD-95 Regulates Synaptic Targeting of CDKL5.
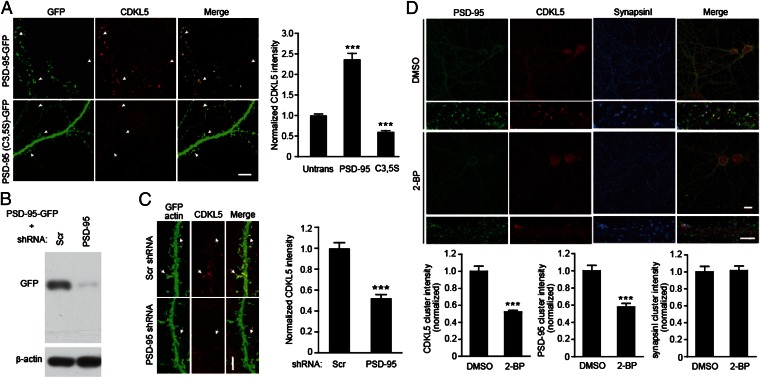
What is the functional significance of this interaction? The redistribution of CDKL5 by PSD-95 (Fig. 2F) gives us a hint that PSD-95 may regulate the location of CDKL5 in cells. A previous report (7) and our data (Fig. 1C) showed that CDKL5 localizes to excitatory synapses; therefore, we asked whether the synaptic targeting of CDKL5 is regulated by PSD-95. We first examined the effect of overexpressing PSD-95 on the subcellular localization of CDKL5. Consistent with previous observation (30), when expressed in hippocampal neurons, GFP-tagged PSD-95 (PSD-95-GFP) targeted faithfully to postsynaptic sites (Fig. 3A). By comparing the immunostaining intensity of endogenous CDKL5 clusters in transfected neurons with that in nearby untransfected neurons, we found that overexpression of PSD-95 significantly increased synaptic CDKL5 levels (Fig. 3A). Overexpression of PSD-95 (C3,5S) did not enhance but did reduce the levels of synaptic CDKL5 (Fig. 3A), suggesting that the promotion effect is dependent on palmitoylation. In addition, PSD-95 was also necessary for synaptic targeting of CDKL5, because the level of synaptic CDKL5 is markedly reduced in PSD-95-knockdown neurons (Fig. 3 B and C).
Fig. 3.
PSD-95 regulates synaptic targeting of CDKL5. (A) Effects of overexpression of WT or palmitoylation-deficient PSD-95 on synaptic localization of CDKL5. Hippocampal neurons were transfected with GFP-tagged WT PSD-95 or PSD-95 (C3,5S). Two days after transfection, cells were fixed and stained for CDKL5. (Scale bar: 5 μm.) Arrows and arrowheads indicate synaptic CDKL5 clusters in transfected and untransfected (Untrans) cells, respectively. Quantified fluorescence intensity data are shown in the bar graph (Right). A total of 20–30 dendritic segments from 10 to 15 neurons were quantified for each condition, ***P < 0.001, compared with untransfected cells. (B) Immunoblots showing the effectiveness of PSD-95 shRNA in down-regulating PSD-95-GFP expression in 293T cells. (C) Down-regulation of PSD-95 reduces synaptic CDKL5 levels. Arrows indicate synaptic CDKL5 clusters. (Scale bar: 5 μm.) n = 12–15 neurons for each condition; ***P < 0.001; t test. (D) Inhibition of palmitoylation reduces synaptic CDKL5 levels. Hippocampal neurons were treated with DMSO or 20 μM 2-BP for 8 h. Cells were fixed and stained for PSD-95, CDKL5, and synapsin I. n =15–20 microscope fields for each condition; ***P < 0.001, compared with DMSO treatment; t test.
We have demonstrated that palmitoylation controls the interaction between CDKL5 and PSD-95. To further demonstrate that palmitoylation of endogenous PSD-95 is important for synaptic targeting of CDKL5, we pharmacologically inhibited palmitoylation in hippocampal neurons. We found that treating neurons with 2-BP but not DMSO resulted in dispersal of synaptic clusters of CDKL5 as well as PSD-95 without causing a general reduction in the cluster intensity of other synaptic proteins such as synapsin I (Fig. 3D). The reduction in CDKL5 clustering in 2-BP–treated neurons is not caused by the decreased expression and/or stabilization of CDKL5, because CDKL5 level was not affected by 2-BP treatment (Fig. 2D).
We performed a series of experiments to examine whether CDKL5 can regulate PSD-95. As assayed by the acyl-biotinyl exchange method, the levels of palmitoylated PSD-95 were not changed by down-regulating CDKL5 (Fig. S4A), suggesting that CDKL5 does not regulate the palmitoylation of PSD-95. We also found that manipulating the CDKL5 level did not change the levels of total and phosphorylated PSD-95 (Fig. S4 B and C). Thus, our results do not support any regulatory role of CDKL5 on PSD-95. Collectively, these results suggest that a major function of the CDKL5–PSD-95 interaction is to regulate the synaptic targeting of CDKL5 and that this process is controlled by palmitoylation.
Effects of Pathogenic Mutations on the CDKL5–PSD-95 Interaction and Synaptic Localization of CDKL5.
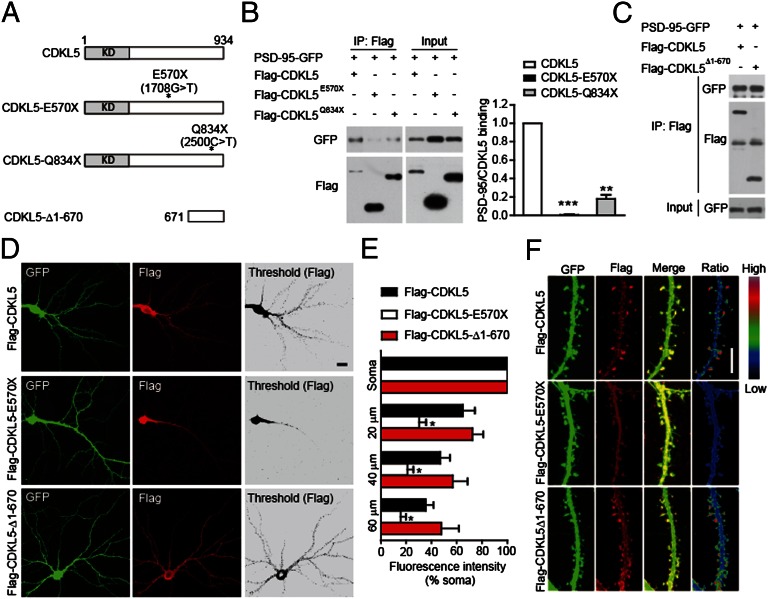
Because mutations in the CDKL5 gene cause severe neurodevelopmental disorders, we examined the impact of human mutations on the CDKL5–PSD-95 interaction. We generated Flag-tagged CDKL5 mutants that bear some of these disease-associated point mutations and examined their interaction with PSD-95. It has been shown that mutations C152F and R175S reduce the catalytic activity of CDKL5 (31). However, we found that CDKL5-C152F and CDKL5-R175S bound to PSD-95 at levels comparable to WT CDKL5 (Fig. S5). Moreover, the kinase-dead mutation (K42R) that completely abolishes kinase activity did not affect the binding of CDKL5 to PSD-95 (Fig. S5), indicating that kinase activity is not required for CDKL5 to bind to PSD-95. On the other hand, the C-terminal truncation mutations either completely abolished (E570×) or markedly reduced (Q834×) the interaction with PSD-95 (Fig. 4 A and B). Furthermore, CDKL5-Δ1-670 containing amino acids 671–934 preserved the interaction with PSD-95 (Fig. 4 A and C). These results indicate that the C-terminal region of CDKL5 mediates the interaction with PSD-95 and that pathogenic mutations affecting this region impair this interaction.
Fig. 4.
Disease-associated mutations impair the interaction of CDKL5 with PSD-95 and its synaptic localization. (A) Schematic diagram showing the structures of CDKL5 and its mutants. KD, kinase domain. (B) Mutations E570× and Q834× impair the interaction of CDKL5 with PSD-95. The interaction was assayed by coimmunoprecipitation in 293T cells coexpressing GFP-tagged PSD-95 and Flag-tagged CDKL5 or its mutants. n = 3; **P < 0.01, ***P < 0.001. (C) Interaction of CDKL5 C-terminal region (CDKL5-Δ1-670) with PSD-95 assayed by coimmunoprecipitation. Subcellular localization of Flag-tagged CDKL5 and its mutants. (D) Representative images of neurons transfected with Flag-tagged CDKL5 or its mutants together with GFP, and stained for GFP and Flag. (Scale bar: 20 μm.) (E) Quantitation of average fluorescence in cell soma and in dendritic segments at the indicated distances from the soma in neurons expressing Flag-tagged proteins. n = 5–6 neurons for each condition; *P < 0.05 compared with Flag-CDKL5. (F) Representative images of dendrites showing the localization of Flag-tagged proteins in dendritic segments of transfected neurons. Ratio images indicate their enrichment in dendritic spines. (Scale bar: 5 μm.)
We have shown that the interaction of CDKL5 with PSD-95 is critical for its synaptic targeting. Therefore, we examined the subcellular localization of CDKL5 and its mutants. We found that the majority of CDKL5-E570× was confined to cell body and nearby dendritic shafts, whereas WT CDKL5 and CDKL5Δ1-670 had apparent synaptic localization (Fig. 4 D and E). In distal dendrites, wild-type CDKL5 and CDKL5-Δ1-670 were enriched in dendritic spines, whereas CDKL5-E570× diffused throughout the dendrites (Fig. 4F). Taken together, these results indicate that pathogenic mutations may impair the binding of CDKL5 to PSD-95 and its synaptic localization.
CDKL5–PSD-95 Interaction Is Critical for Dendritic Spine Development.
Both CDKL5 and PSD-95 play key roles in excitatory synapse development (7, 32–34). Therefore, we investigated the impact of CDKL5–PSD-95 interaction on the development of dendritic spines at which most of the excitatory synapses in the brain are formed. We first examined the effect of loss of CDKL5 on dendritic spine development and found that down-regulation of CDKL5 by RNAi significantly decreased spine density and size (Figs. S6 and S7 A–D), indicating that CDKL5 is required for spine formation and growth. Consistent with a reduction in the number of functional excitatory synapse, the amplitude and frequency of miniature excitatory postsynaptic current, and the surface expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors were decreased in CDKL5-knockdown neurons (Fig. S7 E–H). These results, together with a recent study (7), demonstrate that CDKL5 is essential for spine development. We noticed that the effect of CDKL5 knockdown on spine density is opposite to what was reported previously (7). This difference may be explained by the different experimental procedures: the neurons were transfected and imaged at different ages in the two studies [days in vitro (DIV) 14/18 vs. DIV 7/14].
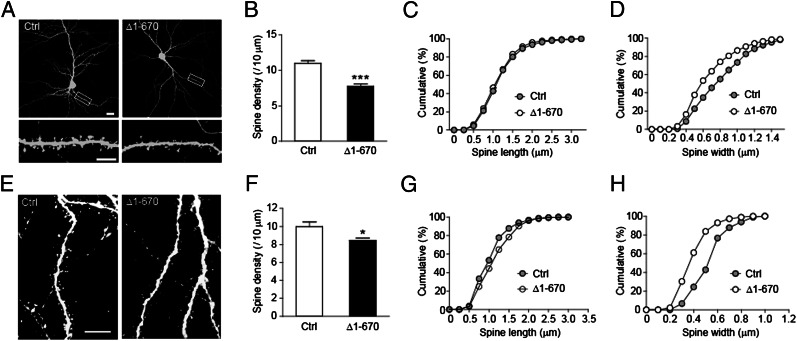
To investigate the involvement of CDKL5–PSD-95 interaction in spine development, we sought to disrupt the interaction between CDKL5 and PSD-95 in neurons. The strong binding to PSD-95 at synapses (Fig. 4 C and F) and the lack of kinase activity suggest that CDKL5-Δ1-670 may exhibit dominant negative effects. Moreover, overexpression of CDKL5-Δ1-670 significantly diminished the binding of full-length CDKL5 to PSD-95 in 293T cells and reduced the clustering of endogenous CDKL5 in dendritic spines (Fig. S8). Therefore, we examined the effect of overexpressing CDKL5-Δ1-670 on spine development. Similar to down-regulation of CDKL5, expression of CDKL5-Δ1-670 in hippocampal neurons resulted in reductions in spine density and size (Fig. 5 A–D), indicating that normal spine formation and maturation are impaired. Similar results were observed in vivo by introducing CDKL5-Δ1-670 into neurons using in utero electroporation (Fig. 5 E–H). Together, these data suggest that the CDKL5–PSD-95 interaction that ensures the targeting of CDKL5 to synapses is essential for spine development.
Fig. 5.
Disruption of CDKL5–PSD-95 interaction inhibits dendritic spine growth. (A) Representative images of hippocampal neurons transfected at DIV 8 with GFP together with empty vector or Flag-CDKL5-Δ1-670. Cells were fixed at DIV 15 and stained for GFP. [Scale bar: 20 μm (Top) and 5 μm (Bottom).] (B) Quantification of spine density in neurons transfected as indicated. n = 20–22 neurons for each; ***P < 0.001; t test. (C) Cumulative distribution of spine length plotted for the indicated conditions. P = 0.2586. Kolmogorov–Smirnov (K–S) test. (D) Cumulative distribution of spine width plotted for the indicated conditions. P < 0.001; K–S test. (E) Representative images of layer II–III pyramidal neurons in postnatal day (P) 14 rat brains transfected with GFP or GFP-CDKL5-Δ1-670 by in utero electroporation. Cells were stained with saturated GFP antibody to circumvent uneven distribution of GFP or GFP-tagged proteins in spines. (Scale bar: 10 μm.) (F) Quantification of spine density in neurons transfected as indicated. n = 5–6 neurons; *P < 0.05; t test. (G) Cumulative distribution of spine length plotted for the indicated conditions. P = 0.0414; K–S test. (H) Cumulative distribution of spine width plotted for the indicated conditions. P < 0.001; K–S test.
Discussion
In this study, we find that CDKL5, a disease-associated protein, is critical for dendritic spine development and that it targets to synapse via its binding to palmitoylated PSD-95. The role of PSD-95 in synapse development has been well demonstrated (32–35). However, the mechanisms underlying its function are not clear. Our data suggest that CDKL5 may mediate, at least in part, the function of PSD-95 in synapse development. First, loss of CDKL5 in neurons causes an inhibition of spine formation and growth, which indicates a key role of CDKL5 in synapse development. Second, PSD-95 seems to be a critical regulator of synaptic targeting of CDKL5. Thus, the impaired synapse development observed in PSD-95–deficient neurons may result directly from reduced accumulation of CDKL5 in synapses. We have previously shown that CDKL5 is critical for dendritic arborization (5). Together, these results underscore its pleiotropic involvement in both spinogenesis and dendritogenesis.
It is not surprising that as a postsynaptic scaffolding protein, PSD-95 can regulate synaptic trafficking of its interacting proteins. For instance, the synaptic content of AMPA receptors is regulated by PSD-95 via its interaction with stargazin, a member of the transmembrane AMPA receptor regulatory protein family (36, 37). What makes the CDKL5–PSD-95 interaction unique is that it is dependent on palmitoylation of PSD-95. This palmitoylation-dependent interaction is well supported by the finding that CDKL5 binds to the N-terminal domain of PSD-95 containing the palmitoylation sites. Because palmitoylation of PSD-95 occurs mainly at two sites—the Golgi apparatus that localizes at the perinuclear region and dendrites (27)—we propose that there are two possible ways by which palmitoylated PSD-95 regulates synaptic targeting of CDKL5. First, CDKL5 may bind to palmitoylated PSD-95 at the Golgi apparatus and moves together with PSD-95 to the postsynaptic site (Fig. S9). This is consistent with the finding of colocalization of CDKL5 and PSD-95 to perinuclear region in COS-7 cells coexpressing the two proteins (Fig. 2F). Second, free CDKL5 is captured by newly palmitoylated PSD-95 at the dendrites, trafficked to synapses, and becomes enriched at the subsynaptic site. The latter way is likely to be important in neurons, because the palmitoylation of PSD-95 at synapses is regulated by neuronal activity (25). This activity-dependent palmitoylation of PSD-95 may provide a regulatory mechanism for synaptic targeting of CDKL5, thereby contributing to activity- or experience-dependent synapse development.
Another interesting finding is that a class of patient-derived mutations impairs the interaction of CDKL5 with PSD-95 and its synaptic localization. Considering that disruption of CDKL5–PSD-95 interaction inhibits spine development, these discoveries link mislocalization of CDKL5 to abnormal development of dendritic spines, which may provide a mechanistic insight into the pathogenesis of a subpopulation of CDKL5-related disorders. A recent study shows that CDKL5 regulates synapse development via phosphorylating NGL-1 (7). Thus, CDKL5, PSD-95, and NGL-1 may define a protein complex that functions coordinately to regulate synapse development.
Finally, we noted that our findings may represent a unique protein trafficking mechanism by which a nonpalmitoylated protein can be delivered to a specific cellular compartment via interaction with a palmitoylated protein. Palmitoylation has emerged as a sorting mechanism for polarized protein trafficking (24, 38, 39). Because palmitoylation requires specific amino acid sequences, relatively few proteins can be palmitoylated considering the number of proteins that are expressed. Thus, using palmitoylated proteins as carriers for polarized delivery is an economic strategy to achieve targeted trafficking. It will be interesting to know whether palmitoylated PSD-95 regulates the trafficking of other proteins and whether it represents a general regulatory mechanism in polarized trafficking of protein complexes.
Materials and Methods
For further details, see SI Materials and Methods.
Cell Cultures and Transfection.
Cultures of cortical neurons were prepared from E18 Sprague–Dawley (SD) rat as previously described (5). Cultures of hippocampal neurons were prepared from P0 SD rat. Procedures involving animals and their care were performed in accordance with the Animal Care and Use Committee of the Institute of Neuroscience. Neurons were transfected using Lipofectamine 2000 following the manufacturer’s instruction (Invitrogen).
GST Pull-Down and Coimmunoprecipitation.
For GST pull-down assay, cells were lysed and the lysates were cleared by centrifugation at 14,000 × g for 20 min. The supernatant was incubated with GST-fusion protein or GST for 4 h at 4 °C. For coimmunoprecipitation, cells were lysed and cleared by centrifugation. Antibodies and protein A/G or antibody-coupled resins were incubated with the cleared lysates. The bound proteins were eluted by 2 × reducing SDS loading buffer, resolved by SDS/PAGE, and immunoblotted with indicated antibodies.
Supplementary Material
Acknowledgments
We thank Dr. Qian Hu for assistance with confocal imaging and Yang Li for help with the in utero electroporation experiments. This work was supported by 973 Program Grant 2011CBA00400; National Natural Science Foundation of China Grants 30925016, 31021063, and 31123002; and Program of Shanghai Subject Chief Scientist Grant 12XD1405500.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300003110/-/DCSupplemental.
References
- 1.Scala E, et al. CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J Med Genet. 2005;42(2):103–107. doi: 10.1136/jmg.2004.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao J, et al. Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am J Hum Genet. 2004;75(6):1149–1154. doi: 10.1086/426460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malumbres M, et al. Cyclin-dependent kinases: A family portrait. Nat Cell Biol. 2009;11(11):1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montini E, et al. Identification and characterization of a novel serine-threonine kinase gene from the Xp22 region. Genomics. 1998;51(3):427–433. doi: 10.1006/geno.1998.5391. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, et al. CDKL5, a protein associated with rett syndrome, regulates neuronal morphogenesis via Rac1 signaling. J Neurosci. 2010;30(38):12777–12786. doi: 10.1523/JNEUROSCI.1102-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rusconi L, et al. CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail. J Biol Chem. 2008;283(44):30101–30111. doi: 10.1074/jbc.M804613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricciardi S, et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol. 2012;14(9):911–923. doi: 10.1038/ncb2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang IT, et al. Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proc Natl Acad Sci USA. 2012;109(52):21516–21521. doi: 10.1073/pnas.1216988110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mari F, et al. CDKL5 belongs to the same molecular pathway of MeCP2 and it is responsible for the early-onset seizure variant of Rett syndrome. Hum Mol Genet. 2005;14(14):1935–1946. doi: 10.1093/hmg/ddi198. [DOI] [PubMed] [Google Scholar]
- 10.Kameshita I, et al. Cyclin-dependent kinase-like 5 binds and phosphorylates DNA methyltransferase 1. Biochem Biophys Res Commun. 2008;377(4):1162–1167. doi: 10.1016/j.bbrc.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 11.Lin C, Franco B, Rosner MR. CDKL5/Stk9 kinase inactivation is associated with neuronal developmental disorders. Hum Mol Genet. 2005;14(24):3775–3786. doi: 10.1093/hmg/ddi391. [DOI] [PubMed] [Google Scholar]
- 12.Kilstrup-Nielsen C, et al. What we know and would like to know about CDKL5 and its involvement in epileptic encephalopathy. Neural Plast. 2012;2012:728267. doi: 10.1155/2012/728267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20(6):264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- 14.Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17(7):343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Xu W. PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr Opin Neurobiol. 2011;21(2):306–312. doi: 10.1016/j.conb.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 17.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269(5231):1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 18.Brenman JE, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84(5):757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 19.Irie M, et al. Binding of neuroligins to PSD-95. Science. 1997;277(5331):1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 20.Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci. 1998;18(4):1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topinka JR, Bredt DS. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel Kv1.4. Neuron. 1998;20(1):125–134. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 22.El-Husseini AE, et al. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000;148(1):159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mumby SM. Reversible palmitoylation of signaling proteins. Curr Opin Cell Biol. 1997;9(2):148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- 24.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11(3):161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 25.El-Husseini Ael-D, et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108(6):849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 26.Iwanaga T, Tsutsumi R, Noritake J, Fukata Y, Fukata M. Dynamic protein palmitoylation in cellular signaling. Prog Lipid Res. 2009;48(3-4):117–127. doi: 10.1016/j.plipres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Noritake J, et al. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J Cell Biol. 2009;186(1):147–160. doi: 10.1083/jcb.200903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshii A, et al. TrkB and protein kinase Mζ regulate synaptic localization of PSD-95 in developing cortex. J Neurosci. 2011;31(33):11894–11904. doi: 10.1523/JNEUROSCI.2190-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275(1):261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 30.Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22(3):497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 31.Bertani I, et al. Functional consequences of mutations in CDKL5, an X-linked gene involved in infantile spasms and mental retardation. J Biol Chem. 2006;281(42):32048–32056. doi: 10.1074/jbc.M606325200. [DOI] [PubMed] [Google Scholar]
- 32.Nikonenko I, et al. PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol. 2008;183(6):1115–1127. doi: 10.1083/jcb.200805132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA. 2007;104(10):4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- 35.Tsai NP, et al. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell. 2012;151(7):1581–1594. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnell E, et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99(21):13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chetkovich DM, Chen L, Stocker TJ, Nicoll RA, Bredt DS. Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci. 2002;22(14):5791–5796. doi: 10.1523/JNEUROSCI.22-14-05791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linder ME, Deschenes RJ. Palmitoylation: Policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 39.el-Husseini Ael-D, Bredt DS. Protein palmitoylation: A regulator of neuronal development and function. Nat Rev Neurosci. 2002;3(10):791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.