Abstract
Studies on circadian entrainment have traditionally been performed under controlled laboratory conditions. Although these studies have served the purpose of providing a broad framework for our understanding of regulation of rhythmic behaviors under cyclic conditions, they do not reveal how organisms keep time in nature. Although a few recent studies have attempted to address this, it is not yet clear which environmental factors regulate rhythmic behaviors in nature and how. Here, we report the results of our studies aimed at examining (i) whether and how changes in natural light affect activity/rest rhythm and (ii) what the functional significance of this rhythmic behavior might be. We found that wild-type strains of fruit flies, Drosophila melanogaster, display morning (M), afternoon (A), and evening (E) peaks of activity under seminatural conditions (SN), whereas under constant darkness in otherwise SN, they exhibited M and E peaks, and under constant light in SN, only the E peak occurred. Unlike the A peak, which requires exposure to bright light in the afternoon, light information is dispensable for the M and E peaks. Visual examination of behaviors suggests that the M peak is associated with courtship-related locomotor activity and the A peak is due to an artifact of the experimental protocol and largely circadian clock independent.
Keywords: circadian rhythms, chronoethogram, courtship, period mutants, afternoon peak
The role of circadian clocks in the temporal regulation of behaviors has been studied mostly under controlled laboratory conditions (1). Because simplified laboratory protocols are far removed from the reality of nature, these studies are limited in their ability to reveal the true features of circadian behavior in nature. For instance, laboratory studies mostly use square waves of one zeitgeber (time cue) such as light or temperature, or in rare cases, a combination of the two, quite unlike multiple, simultaneous, stochastic, and gradually changing factors in nature (2–6). Few recent studies on activity/rest and adult emergence rhythms of fruit flies, Drosophila melanogaster, under seminatural (SN) conditions reported significant differences in the patterns of these rhythms from those observed in the laboratory (2–6). For instance, adult emergence rhythm was more robust under SN compared with the laboratory, and even the period null (per0) flies exhibited rhythmicity (3). An additional afternoon (A) peak of activity was reported under SN (4), which had never been observed in any standard laboratory protocol. Several features of the activity/rest rhythm (anticipation to twilight transitions and midday siesta) were absent under SN, and certain features of the rhythm such as crepuscular pattern and dominance of light over temperature were proposed to be artifacts of laboratory studies (4). The temporal profiles of neuronal expression of clock proteins, PERIOD and TIMELESS were also found to differ between laboratory and nature (6).
At present, the available literature is limited to descriptions of rhythms in nature (2–4). Vanin et al. (4) showed that phases of the morning (M) and evening (E) activity peaks are dependent on the mean daily temperature and that the proportion of flies displaying A peak increased with increasing mean daytime temperature. In a laboratory-based study under gradually varying temperature cycles, the M peak was found to coincide with the morning temperature rise and the E peak with the evening temperature fall (7). Although simulated twilight conditions in the laboratory were able to mimic some features of SN (8), it is not clear how natural light governs the temporal pattern of activity/rest rhythm. Moreover, thus far, there has been no attempt to determine which aspects of light information are crucial for timing of circadian behaviors in nature.
We aimed at examining how natural light modulates the M, A, and E peaks of activity in D. melanogaster by modifying light information under otherwise SN in the following ways: (i) decreasing amplitude of natural light profile to test for the effect of light intensity, (ii) blocking light at different times of the day to examine the effect of exposure to different portion(s) of natural light profile, and (iii) providing constant darkness (DD), or constant light (LL) of different intensities to examine the effects of continuous presence or absence of light. Thus, only light information was altered in our study, allowing other environmental factors to vary naturally. We also aimed to study the functional significance of the three activity peaks by making round-the-clock visual observations of flies. We asked whether flies needed to be active at these times of the day to perform certain critical behaviors such as foraging, searching for mates, courting, and copulating. We scored these behaviors in flies housed solitarily or in groups, and plotted their time course in what we call a “chronoethogram.”
Results
Studies were conducted in an experimental enclosure under SN (3). Based on the light profile (dawn, ∼0600 hours; dusk, ∼1800 hours), we designated specific intervals of time as morning (M, 0400–1000 hours), afternoon (A, 1000–1600 hours) and evening (E, 1600–2200 hours). Under SN, activity of wild-type Canton S (CS) flies (n = 27) had three peaks corresponding to M, A, and E (henceforth, M, A, and E peaks; Fig. 1A, Top Left). Unlike reported previously (4), we found that flies appeared to “anticipate” dawn (Table S1).
Fig. 1.
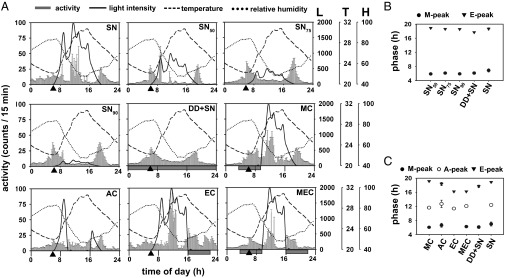
Activity profiles of CS under SN and various light-modified protocols under SN. (A, Top Left) Activity profile of CS under SN averaged across days and flies. Error bars are SEM. Three separate axes on extreme right represent three environmental factors measured: light intensity (L) (in lux), temperature (T) (in degrees Celsius), and relative humidity (H) (in percentage). The black arrowhead on the x axis indicates dawn (>0 lux). (A, Top and Middle) Average activity profiles when naturally varying light intensity was reduced by 50% (SN50), 75% (SN75), and 90% (SN90). Only the amplitude of light waveform was reduced without alteration in qualitative profile of light. (A, Middle) Average activity profile under DD in otherwise SN (DD+SN). (A, Middle and Bottom) Average activity profiles under light-blocking protocols: morning cover (MC), afternoon cover (AC), evening cover (EC), and morning-plus-evening cover (MEC). The shaded horizontal bars below x axis in middle and bottom rows depict durations of light blocking under SN. (B) Phases of the M [F(4,147) = 5.88, P < 0.0002] and E peaks [F(4,143) = 23.03, P < 0.0002] were modulated by light-filtering protocols. The M peak was phase advanced compared with SN (P < 0.05) for the three partial light-filtering protocols, whereas the E-peak phases did not differ from each other or from SN. (C) Light-blocking protocols also modulated phase of the M [F(4,133) = 2.75, P = 0.03], A [F(4,129) = 5.95, P < 0.001], and E peaks [F(5,153) = 147.89, P < 0.0001]. In B and C, error bars are 95% confidence interval (95% CI) to enable visual hypothesis testing.
A Peak Is Light Intensity Dependent.
To determine how natural light might influence activity profiles of flies, we subjected them to SN with altered levels of light while retaining its overall waveform. We used neutral density filters, which reduce the light intensity by 50% (SN50; n = 30), 75% (SN75; n = 26), or 90% (SN90; n = 25) (Fig. 1A). When natural light was cut down by 50% or more, dawn anticipation was enhanced [F(4,149) = 16.76, P < 0.0001; Table S1], the M peak was phase advanced and the A peak was considerably reduced (Fig. 1 A and B). The dependence of the A peak on light is especially evident by its absence under DD+SN (Fig. 1A, middle row). Unlike the M peak, there was no detectable change in phase of the E peak under any light-filtering protocol (Fig. 1 A and B), which suggests that flies are able to track the drop of light intensity from lower levels as well. When light was reduced by 50% or more, activity became distributed mainly in the M and E intervals similar to DD+SN (Fig. S1B). Thus, intensity of natural light modulates phase of the M peak and occurrence of the A peak, whereas the E peak does not depend on light intensity.
A Peak Depends on Afternoon Light, Whereas M Peak Depends on Morning and Evening Light.
Having observed that light intensity is a major determinant of the A peak, we hypothesized that the M, A, and E peaks are regulated by natural light during morning, afternoon, and evening hours, respectively. To test this hypothesis, we blocked natural light from reaching flies at different times of the day by covering the activity monitors during the morning [morning cover (MC); n = 24], afternoon [afternoon cover (AC); n = 26], evening [evening cover (EC); n = 32], or morning-plus-evening [morning-plus-evening cover (MEC); n = 30; Fig. 1A, middle and bottom rows] intervals. Flies deprived of light in the morning (MC) showed greater anticipation than SN [F(5,172) = 9.45, P < 0.0001; Fig. 1A; Table S1]. Blocking natural light in the morning (MC) or morning plus evening (MEC) significantly advanced the M peak making it similar to DD+SN (Fig. 1 A and C). The M peak was diminished under AC as well as EC protocols (Fig. 1A and Fig. S1C). The E peak was not affected by MC or AC (Fig. 1 A and C, and Fig. S1C). However, absence of light in the evening (EC: P < 0.0002; MEC: P < 0.0002; and DD+SN: P < 0.001) advanced the E peak (Fig. 1 A and C). In fact, the E peak was advanced under EC and MEC even more than DD+SN (P < 0.0002; Fig. 1C), suggesting that the E peak coincides with the fall of light intensity to 0 lux, even when this occurred much earlier than civil dusk. Although MC and MEC did not change phase or amplitude of the A peak (Fig. 1 A and C, and Fig. S1C), AC caused drastic reduction of the A-peak amplitude, suggesting that this peak is highly dependent on afternoon light. Flies exposed to natural light in the afternoon (SN, MC, EC, and MEC) were mostly active during the A interval, but when deprived of afternoon light (AC), their activity became distributed nearly equally in all of the three intervals (Fig. 1A and Fig. S1D). Thus, exposure to bright natural light in the afternoon is critical for the A peak.
A Peak Is an Artifact of Experimental Protocol.
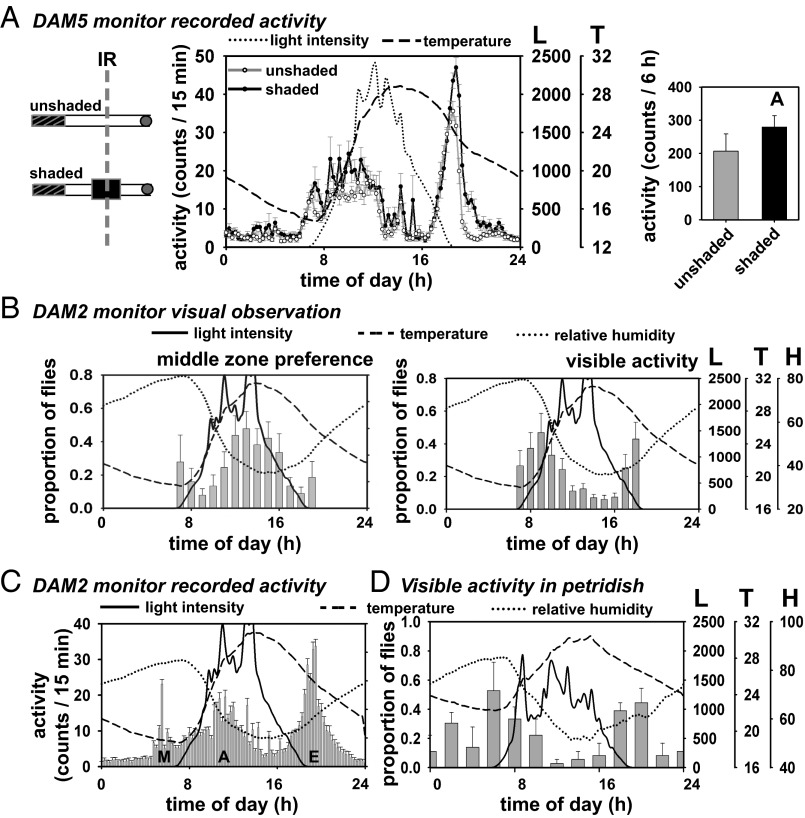
It seemed intuitively less advantageous for flies to be active at a time when they are under higher risk of desiccation (1). We speculated that flies seek the midportion of the activity tube (near the IR beam) during afternoon because this region provides some amount of shade. To test this, we used a flatter version of the recording apparatus—Drosophila activity monitor 5 (DAM5)—and provided additional shade near the middle zone (see schematic; Fig. 2A, Left). Flies with shade near the IR beam showed significantly higher afternoon activity compared with the unshaded controls (Fig. 2A, Right). In a separate experiment, we recorded activity of flies from different regions of the activity tube (near food, middle, and near cotton plug) in the DAM2 monitor and found the A peak to occur under all three cases, albeit with higher amplitude when activity was recorded close to food, followed by the middle zone, followed by the zone near the cotton plug (Fig. S2A). Thus, the A peak is greatly influenced by experimental protocol and location of shade along the activity tube.
Fig. 2.
A peak is an artifact of experimental paradigm. (A, Left) Schematic of experimental setup. (A, Middle) Average activity profiles of flies recorded in flatter version of DAM (DAM5) monitor, with (filled circles) or without shade (unfilled circles) in the middle. Error bars are SEM. Other details are same as Fig. 1A. (A, Right) Activity in the afternoon interval was greater in the shaded compared with unshaded tubes [F(1,29) = 6.02, P < 0.02]. Error bars are 95% CI. (B) Visual observation of flies during daytime in the DAM2 monitor. (B, Left) Flies preferred the middle zone of the tube in the afternoon more than other times of the day [F(11,120) = 19.5, P < 0.001, proportion of flies in the middle zone at 12 and 13 h are significantly greater than at 7–11, 17, and 18 h]. (B, Right) Visual observation of locomotion in the tubes placed in DAM2 monitor showed two peaks of locomotion [F(11,60) = 16.17, P < 0.001]. Error bars are SEM. (C) Average activity recorded in the same DAM2 monitor showed the A peak. Other details are same as Fig. 1A. (D) Proportion of solitary flies in petri dishes exhibiting locomotion as estimated by visual observation. No detectable A peak was observed, but the M and E peaks persisted [F(11,24) = 2.73, P < 0.03].
Visual Observations of Flies Confirm That the A Peak Is Due to Shade Seeking.
To further test our hypothesis that the A peak seen under SN may be an artifact of experimental protocol, during daytime we conducted visual observations of flies placed in the DAM2 monitors (Materials and Methods). Flies showed higher preference for the middle zone of the tubes in the afternoon (Fig. 2B, Left). Locomotion, as determined by visual observations exhibited only M and E peaks, with a trough in the afternoon (Fig. 2B, Right). However, DAM2 recording of the same flies showed a distinct A peak (Fig. 2C), even though flies were observed to be mostly at rest in the afternoon. We propose that the A peak is predominantly due to flies occupying the zone near the IR beam, even though they do not exhibit locomotion. We also conducted another study in which flies were housed in similar tubes, but the tubes were not placed inside DAM monitors, but laid flat on a tray in the same SN enclosure. Tubes were either left unshaded, or shaded near food, in the middle or near the cotton plug (Fig. S2B, schematic). Visual observations revealed that flies in tubes shaded in the middle showed an increased preference for the middle zone in the afternoon, whereas such afternoon preference for middle zone was not seen in the unshaded tubes (Fig. S2B). Overall, flies preferred the shaded region of the tubes with the exception of when shade was provided close to the cotton plug (Fig. S2B). Such preference for shade is consistent with the results when activity was recorded from different zones of the tube (Fig. S2A). We further speculated that, under SN, the A peak is an artifact of recording flies housed in narrow glass tubes. To test this, we conducted visual observations in a larger arena (petri dish) and found that flies display activity corresponding to the M and E peaks obtained in the DAM system but were mostly resting in the afternoon (Fig. 2D).
A Peak Is Largely Clock Independent.
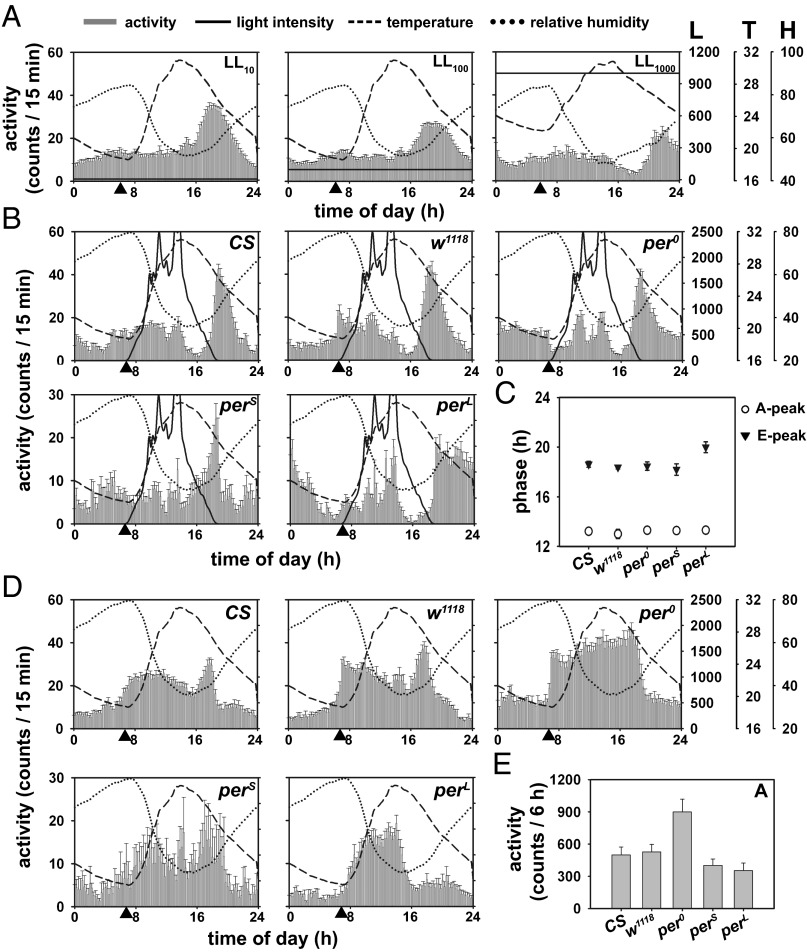
Thus far, our results suggest that the A peak is an artifact of the recording protocol; hence it is unlikely to be circadian clock dependent. However, previous studies (4) had shown that, like the M and E peaks, the A peak is also circadian clock modulated, because it is phase advanced in perS and per0 flies compared with wild-type controls. We examined the role of clock in the regulation of A peak using two separate approaches. First, we recorded activity under LL, which is known to induce behavioral arrhythmicity (9) and disrupt the underlying molecular clock (10). We subjected flies to 10-, 100-, or 1000-lux LL in SN [henceforth, LL10+SN (n = 28); LL100+SN (n = 21); LL1000+SN (n = 29), respectively]. The M peak was abolished in all three LL regimes despite the presence of nonphotic cues (Fig. 3A). Similarly, the A peak was not detectable under any of the LL+SN protocols (Fig. 3A), suggesting that this peak requires natural light in the afternoon.
Fig. 3.
Examining the role of clock in regulating activity peaks under SN. (A, Left to Right) Activity profiles of CS flies under different constant light intensities, 10 (LL10+SN), 100 (LL100+SN), and 1000-lux (LL1000+SN) in otherwise SN conditions assayed in June 2012. (B) Average activity profiles of period mutants (per0, perS, and perL) and their genetic background controls (w1118 for per0, and CS for perS and perL) under SN assayed in February 2013. Other details for A and B are the same as in Fig. 1A. (C) The A-peak phase was not different across genotypes [F(4,87) = 1.32, P = 0.2], but the E peak was delayed in perL compared with its control (P < 0.001). (D) Average activity profiles of per mutants and controls under DD+SN. (E) Afternoon activity level was greater in per0 compared with w1118 (P < 0.001), although perS and perL did not differ from CS. Error bars in C and E represent 95% CI.
Second, we assayed activity of per0 flies (n = 16) under SN and found no difference in phase of the three peaks from their genetic controls (w1118; Fig. S3A). In a separate experiment carried out in February 2013, we assayed the activity of per mutants [perS (n = 8), per0 (n > 23), and perL (n > 20)] under SN and DD+SN (Fig. 3 B–E). Unlike the summer (May to June 2012; Fig. 1A), in this assay (January to February 2013; Fig. 3B) we did not detect a sharp M peak. The A peak in all three per mutants and their genetic controls (n > 15) had similar phase and amplitude under SN (Fig. 3 B and C, and Fig. S3B), which indicates the clock-independent nature of this peak. Nevertheless, the E peak was phase delayed in perL flies compared with CS (Fig. 3 B and C), which suggests that this peak is clock regulated. The amplitude of E peak was lower in perS and perL flies, which can be attributed to the reduced overall activity in these flies (Fig. S3C). Although per0 and perL flies anticipated dawn to a lesser degree, those from other strains anticipated dawn fairly well albeit to variable extents (Table S1). Under DD+SN, we observed higher activity in per0 flies throughout the daytime without any clear peak (Fig. 3D and Fig. S3 D–F) unlike w1118, which displayed relatively clear M and E peaks and a small bout of afternoon activity, probably in response to high temperature (Fig. 3 D and E). This indicates the role of clock in modulating activity levels during the warmest time of day (5); however, this difference is not seen in SN, probably due to masking effects of light. In summary, although the A-peak phase appears to be clock independent, presence of clock helps in the modulation of afternoon activity levels.
Phase of the E Peak Is Light Dependent but in Absence of Light Is Influenced by Temperature and/or Humidity.
The E peak was phase advanced under all LL+SN protocols, with the exception of LL1000+SN (Fig. 3A and Fig. S4A), indicating that flies with no access to natural light use temperature and/or humidity to phase their E peak. Under SN, flies mainly use photic cues to phase their E peak such that it occurs when light falls to 0 lux. The LL10+SN and LL100+SN experiments were conducted separately from the others (about 2 wk later), when high humidity persisted for longer and warm temperature lasted for shorter duration (Fig. 3A, Left and Middle). Under LL1000+SN, the E peak was even more delayed compared with the other two LL+SN protocols (Fig. 3A, Right, and Fig. S4A), probably because during this experiment, the rise in humidity and fall in temperature were more gradual. Under all these protocols, most of the total activity occurred during the evening (or late evening in the case of LL1000+SN; Fig. S4 A–C). These results suggest that, in nature, light information is dispensable for the occurrence of the E peak just as it is for the M peak; presumably, because information regarding changing temperature and/or humidity is sufficient.
Visual Observations Under SN Reveal Rhythmicity in Courtship and Locomotion.
To assess the functional significance of what is recorded as locomotor activity in the DAM monitors, we made visual observations of several easily scorable behaviors, in groups of males and females (three males plus three females), housed together in petri dishes (n = 6 dishes) under SN. Observations were made in parallel with the activity recordings and assays described in Fig. 2 B and C. Flies exhibited rhythmicity in locomotion with clear trough coinciding with temperature maxima (Fig. 4, Top Left), and a trough in rest around dawn (Fig. 4, Top Right). Among the courtship-associated behaviors, males displayed daily rhythm in wing expansion and chasing, both peaking 2–4 h after dawn (Fig. 4, Middle Left and Bottom). Although the highest frequency of copulation occurred around dawn (Fig. 4, Middle Right), this was not statistically significant, probably due to the overall low instances of copulation. Thus, we propose that the M peak is due to courtship-associated activity as corroborated by visual observation of behavior of grouped and solitary flies.
Fig. 4.
Chronoethograms of flies under SN. (Top) Profile of proportion of flies performing locomotion [F(11,48) = 6.96, P < 0.001; Left] or resting [F(11,48) = 9.68, P < 0.001; Right] in petri dishes under group condition of three males and three females. (Bottom) Profile of proportion of flies performing courtship-related activities such as wing expansion [F(11,48) = 4.37, P < 0.001], chasing [F(11,48) = 4.00, P < 0.001], and copulation [F(11,48) = 1.21, P = 0.3]. Courtship-related behaviors peak during the morning hours. Significant effect of time was seen for all behaviors except copulation.
Discussion
A Peak Is an Artifact of Experimental Paradigm.
The A peak was prominent only when sufficiently bright natural light was available during warm afternoons. There was little variation in phase of the A peak among different genotypes and protocols, and it consisted of several subpeaks that mirrored light intensity spikes during midday, indicating a direct and instantaneous response to fluctuations in light intensity (Fig. 1A). Reduction in natural light below 50% resulted in loss of the A peak (Fig. 1A). Similarly, when light was blocked only during afternoon, the A peak was diminished, whereas blocking light at other times of day (morning and/or evening) did not affect the A peak. The A peak can be induced even in the laboratory by subjecting flies to gradually changing, high-amplitude light and temperature cycles (4–7). Furthermore, the effect of blocking morning and/or evening light was not as severe as blocking afternoon light (Fig. S1D), implying that exposure to natural light during midday induces activity.
We speculated that high afternoon activity is due to harsh environmental conditions, inducing flies to seek shade near the IR beam of DAM2 monitors, yielding abnormally high activity counts. Most flies were found to be resting when the DAM2 monitor detected a clear A peak. Consistent with this, flies provided with additional shade in an alternate version of the DAM monitor displayed higher activity in the afternoon (DAM5; Fig. 2A). Similar preference for the shaded portion of the activity tube was apparent when we made observations on flies in tubes with shade provided in different regions (Fig. S2B), and upon automated recording of activity from different zones of the tubes (Fig. S2A). Visual observations of flies whose activity was simultaneously recorded in the DAM2 monitors revealed that majority of them preferred the shaded zone of the activity tube where the IR beam was located (Fig. 2B). The fact that the A peak is an artifact of activity recording protocol was further confirmed in our chronoethogram study where solitary and grouped flies kept in petri dishes did not show the A peak (Figs. 2D and 4).
Is the A Peak Clock Dependent?
Unlike the findings of previous studies (4, 5), which reported clear divergence in phase (up to 3 h), we find that the phase of the A peak in perS, per0, and perL flies did not differ among themselves or from their wild-type controls, suggesting that this peak is clock independent, or at least it does not require PER (Fig. 3 B and C). This inconsistency may be due to differences in experimental protocols and/or in environmental conditions prevailing in tropical and temperate regions; however, this would require further investigation. Clock independence of the A peak in our study is consistent with the notion that, under SN, afternoons are harsh and therefore flies seek shade to avoid stress caused due to bright light and high temperature. Our results clearly suggest that the A peak is an artifact of experimental paradigm and is neither a natural behavior nor under clock control. Nevertheless, weaker dawn anticipation in per0 and perL and delayed E peak in perL indicate some role of circadian clocks in timing of the M and E peaks. It is likely that the three peaks of activity seen under SN are directly driven by environmental factors, and, therefore, we cannot rule out the subtle effects of circadian clocks in the regulation of activity peaks.
Chronoethograms Under SN Reveal Behavioral Correlates of Activity Peaks.
We used the approach of obtaining chronoethograms in which we temporally monitor behaviors such as locomotor activity and rest, courtship-related activities such as chasing, wing expansion, and copulation, which enabled us to assign behavioral correlates to the three activity peaks. Solitary flies in petri dishes under SN showed two distinct peaks in locomotion that corresponded with the activity peaks during dawn and dusk (Fig. 2D). Activity peaks thus obtained were similar to those detected by automated activity recording (Fig. 2 C and D). Previous studies in the laboratory had shown that mating (11) and courtship rhythms (12, 13) are clock controlled with mating frequency being highest around lights-on (zeitgeber time 3–4) (11). Courtship-related activities were found to decline around dusk and remain high during the rest of the day (12, 13). Based on the chronoethograms, we report rhythmicity in courtship-related behaviors under SN. These behaviors mostly comprise chasing, wing expansion, and copulation, which peak around dawn (Fig. 4), closely resembling previous studies in the laboratory (12, 13). We propose that the M peak is due to locomotor activity associated with courtship, whereas the A peak is likely a stress response to harsh afternoon conditions. The E peak corresponded to general locomotion to which no specific behavior could be assigned; hence its significance remains to be established. Although our inferences on the functional significance of activity peaks are based on flies living in groups, we propose that activity related to key behaviors represent innate tendencies that are expressed even in solitary flies.
Light Modulates the M and E Peaks.
The total activity in protocols with reduced light exposure in terms of intensity or duration was lower than in all LL+SN protocols and SN (Figs. S1 B and D, and S4C), indicating that amount of light is crucial in determining activity levels of flies. Flies exposed to less or no light in the morning showed advanced M peak coinciding with temperature troughs and humidity maxima (Fig. 1 A–C). Similarly, in the absence of light the E peak was synchronized with temperature fall and humidity rise, although it otherwise occurred immediately upon light intensity drop (EC, MEC, and SN; Fig. 1 A and C). Neither the M nor E peak seemed to depend on light for their occurrence, although their phases were significantly affected by light. We found that LL abolished the M peak (Fig. 3A), which suggests that changing light is a prerequisite for the M peak. However, under DD+SN, a clear M peak was seen (Fig. 1A). Therefore, LL inhibits M peak, even when other time cues are available. This is consistent with previous findings (14) where discrete temperature cycles induced strong anticipatory morning activity in DD but only a small startle in LL. The M peak also disappeared when flies were deprived of evening light (EC; Fig. 1A), consistent with the notion that evening light affects the M peak (15). In fact, the E peak was the most persistent among the three activity peaks, which suggests that it is least dependent on light information.
In summary, the A peak appears to be an artifact of the experimental paradigm, and largely clock independent, although we find some evidence for clock dependence and light modulation of M and E peaks. Chronoethograms reflected that the M peak is due to courtship-related activities and the A peak is likely to be a stress response to harsh conditions in the afternoon. We speculate that the E peak is associated with foraging-related behavior, although this needs to be verified. Thus, light determines the A peak and modulates morning and evening activity, each of which has distinct functional significance to fly behavior.
Materials and Methods
Detailed methods are provided in SI Text. Most assays were done on virgin male CS flies (unless specified) of age 3–4 d. Mutants of the circadian gene period (per0, perS, and perL) and their genetic controls (w1118 and CS) were also used. The activity recordings and behavioral assays were done in June to July 2012 and January to February 2013 in an outdoor enclosure (3). Locomotor activity was recorded using DAM2 system unless specified. The daily profiles of light, temperature, and humidity were monitored simultaneously using DEnM (Trikinetics). For all light modification protocols, light-tight metal boxes were used. For light-filtering experiments, monitors were covered with neutral density filters (Lee Filters) such that light intensity was reduced by 90% (SN90), 75% (SN75), and 50% (SN50). For light-blocking experiments, activity monitors in SN were covered during morning (MC: 0400–1000 hours), afternoon (AC: 1000–1600 hours), evening (EC: 1600–2200 hours), and morning plus evening (MEC: 0400–1000 and 1600–2200 hours).
Visual Observations of Behaviors Under SN.
Tubes.
Identical to conventional DAM2 recording of single fly activity, except that, additionally, location of fly (near food, middle, or cotton plug) and locomotion were manually recorded every 2 h (in case of the shaded-tube assay) or every 1 h (in parallel to recording in DAM2 monitors).
Petri dishes.
Solitary males or groups of three males and three females were housed in each petri dish with a thin layer of fly food (n = 6 petri dishes for both). Instances of locomotion, rest, wing expansion, chasing, and copulation were recorded manually by visual scanning in 2-h intervals.
Statistical Analyses.
An interval (M, A, or E) was considered to have a peak based on qualitative assessment of the activity profiles (15-min bin) averaged across flies and days of recording. Phases of M, A, and E peaks were estimated by scanning 7-d average activity records of each fly, and identifying that time point corresponding to the highest activity counts observed within that interval. In the afternoon, when there are multiple peaks, the peak closest to the maximum light and temperature was considered, and its phase and amplitude were calculated. The mean phase and amplitude for each peak was obtained for the total number of flies from each genotype and each protocol. The data from each fly was subjected to one-way analysis of variance (ANOVA) to test for the effect of protocol or genotype, for phase, amplitude of activity peaks, and activity levels. Post hoc multiple comparisons were performed using Tukey’s honestly significant difference test. P < 0.05 was considered as level of statistical significance for all analyses. Dawn anticipation index was estimated as the ratio of activity counts for 3-h duration before dawn (the time point when the light intensity value first rose above 0 lux) over activity counts for 6-h duration before dawn (16). To test for the time-of-day effects, two-way ANOVA on activity counts in different intervals under different protocols was followed by post hoc multiple comparisons using Tukey’s test. For chronoethogram assay, proportion of flies showing each behavior at each scan was taken as the basic unit of data. One-way ANOVA was carried out on mating-related movement and on general locomotion to test for time-of-day effects.
Supplementary Material
Acknowledgments
We thank Alok Garg and Manaswini Sarangi for preliminary studies and Reshma Soman for help with experiments. We thank three anonymous reviewers for suggesting some very useful modifications to a previous version of the manuscript. This study was supported by funds from Jawaharlal Nehru Centre for Advanced Scientific Research, Department of Science and Technology Ramanujan Fellowship (to V.S.), and Council of Scientific and Industrial Research Fellowship (to V.V.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220960110/-/DCSupplemental.
References
- 1. Saunders DS (2002) Insect Clocks (Elsevier, Amsterdam)
- 2. Bhutani S (2009) Natural entrainment of the Drosophila melanogaster circadian clock. PhD thesis (University of Leicester, Leicester, UK)
- 3.De J, Varma V, Sharma VK. Adult emergence rhythm of fruit flies Drosophila melanogaster under seminatural conditions. J Biol Rhythms. 2012;27(4):280–286. doi: 10.1177/0748730412448360. [DOI] [PubMed] [Google Scholar]
- 4.Vanin S, et al. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484(7394):371–375. doi: 10.1038/nature10991. [DOI] [PubMed] [Google Scholar]
- 5.Menegazzi P, Yoshii T, Helfrich-Förster C. Laboratory versus nature: The two sides of the Drosophila circadian clock. J Biol Rhythms. 2012;27(6):433–442. doi: 10.1177/0748730412463181. [DOI] [PubMed] [Google Scholar]
- 6.Menegazzi P, et al. Drosophila clock neurons under natural conditions. J Biol Rhythms. 2013;28(1):3–14. doi: 10.1177/0748730412471303. [DOI] [PubMed] [Google Scholar]
- 7.Bywalez W, et al. The dual-oscillator system of Drosophila melanogaster under natural-like temperature cycles. Chronobiol Int. 2012;29(4):395–407. doi: 10.3109/07420528.2012.668505. [DOI] [PubMed] [Google Scholar]
- 8.Yoshii T, Vanin S, Costa R, Helfrich-Förster C. Synergic entrainment of Drosophila’s circadian clock by light and temperature. J Biol Rhythms. 2009;24(6):452–464. doi: 10.1177/0748730409348551. [DOI] [PubMed] [Google Scholar]
- 9.Konopka RJ, Pittendrigh C, Orr D. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet. 1989;6(1):1–10. doi: 10.3109/01677068909107096. [DOI] [PubMed] [Google Scholar]
- 10.Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271(5256):1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 11.Sakai T, Ishida N. Circadian rhythms of female mating activity governed by clock genes in Drosophila. Proc Natl Acad Sci USA. 2001;98(16):9221–9225. doi: 10.1073/pnas.151443298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii S, Krishnan P, Hardin P, Amrein H. Nocturnal male sex drive in Drosophila. Curr Biol. 2007;17(3):244–251. doi: 10.1016/j.cub.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamasaka Y, Suzuki T, Hanai S, Ishida N. Evening circadian oscillator as the primary determinant of rhythmic motivation for Drosophila courtship behavior. Genes Cells. 2010;15(12):1240–1248. doi: 10.1111/j.1365-2443.2010.01456.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto A, Matsumoto N, Harui Y, Sakamoto M, Tomioka K. Light and temperature cooperate to regulate the circadian locomotor rhythm of wild type and period mutants of Drosophila melanogaster. J Insect Physiol. 1998;44(7–8):587–596. doi: 10.1016/s0022-1910(98)00046-8. [DOI] [PubMed] [Google Scholar]
- 15.Bachleitner W, Kempinger L, Wülbeck C, Rieger D, Helfrich-Förster C. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc Natl Acad Sci USA. 2007;104(9):3538–3543. doi: 10.1073/pnas.0606870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrisingh MC, Wu Y, Lnenicka GA, Nitabach MN. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J Neurosci. 2007;27(46):12489–12499. doi: 10.1523/JNEUROSCI.3680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.