Say the word “insect” and what comes to mind? I asked that question to nonentomology, first-year undergraduate students on the first day of class. The typical response: “Insects suck.”
I suspect they were thinking of the suffering inflicted by mosquitoes and other blood-feeding insects, crop damage caused by their vegetarian counterparts, and the invasion of human dwellings by cockroaches and termites. Indeed, “pest insects” tend to overshadow the pivotal role that pollinators and other beneficial insects play. It has been estimated that one-third of our total diet is dependent directly or indirectly upon insect-pollinated crops (1). The value of the increased yield and quality to US agriculture achieved through honey bee pollination alone exceeds $14 billion (2). Honey bee workers forage for nectar and pollen to feed their colonies, but, in the process, they pollinate our crops and provide us with honey and other byproducts. Today, however, this mutually beneficial partnership is in jeopardy. For reasons yet to be fully understood, increasingly and unreasonably large proportions of honey bee workers are failing to return home. They are abandoning the hive, leaving behind the queen, immature workers, and food stores in a condition called colony collapse disorder (CCD). “Insects suck?” When I look back at that first day of class, I expected the answer to be “insects are cool” so that I could start discussing unique aspects of their physiology. In PNAS, Mao et al. (3) substantiate my view, unravel unique aspects of the physiology of the honey bee, demonstrate how honey plays a crucial role in bringing bees back home, and suggest that current beekeeping practices may be responsible, at least in part, for CCD.
Mao et al. (3) combine conventional chemical ecology/natural product chemistry approaches with next-generation sequencing to address a fundamental question in bee biology. They design their research to identify what constituents of honey are responsible for up-regulation of genes involved in detoxification of foreign compounds (xenobiotics), and they investigate the complete repertoire of genes recruited by the honey’s factor(s). This research bolsters their previous studies on how honey up-regulates transcription of genes for cytochrome P450 enzymes, which are involved in detoxification of xenobiotics (4) and play versatile roles in insect physiology, ranging from biosynthesis of hormones (5) to degradation of pheromones (6).
First, they pursued a bioassay-guided isolation of the constituents of honey. They incorporated each of the four main peaks isolated by liquid chromatography from honey extracts, in a substitute for honey, the so-called “bee candy.” After feeding bees with candies spiked with honey constituents from each fraction, they quantified by qPCR the levels of transcripts for one gene known to be up-regulated by honey. This approach led them to the most active fractions. Next, they analyzed these fractions by mass spectrometry and identified their active constituents as p-coumaric acid [International Union of Pure and Applied Chemistry (IUPAC) name: (E)-3-(4-hydroxyphenyl)-2-propenoic acid] and pinocembrin [IUPAC name: (2S)-5,7-dihydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one]). p-Coumaric acid is a derivative of cinnamic acid found in a wide variety of plants, including almonds, whereas pinocembrin is a natural antioxidant of the family of flavonoids. Additional bioassays with authentic compounds demonstrated that p-coumaric acid is by far the most active constituent of honey to induce transcription of the gene for a detoxifying enzyme.
Next, Mao et al. (3) elegantly cast a net for the complete repertoire of genes up-regulated in the honey bee when fed with p-coumaric acid by taking advantage of next-generation sequencing technology. They fed control bees with “bee candies” while a treatment group was fed with “bee candies” spiked with the honey-derived compound. Although their quantitative PCR approach was essential to guide the isolation of the active compounds, sequencing RNA (RNASeq) with Illumina technology and subsequent bioinformatics analysis allowed them to identify all genes that were up-regulated by the p-coumaric acid treatment. The results were revealing. As many as 12 xenobiotic-metabolizing genes, including genes encoding enzymes known to metabolize pesticides, were up-regulated by the “honey factor.” Additionally, p-coumaric acid treatment induced up-regulation of genes encoding for antimicrobial peptides–the pillars of the immune system of bees. These findings stress the healing power of honey and question, in part, current beekeeping practices that may be involved in what causes CCD.
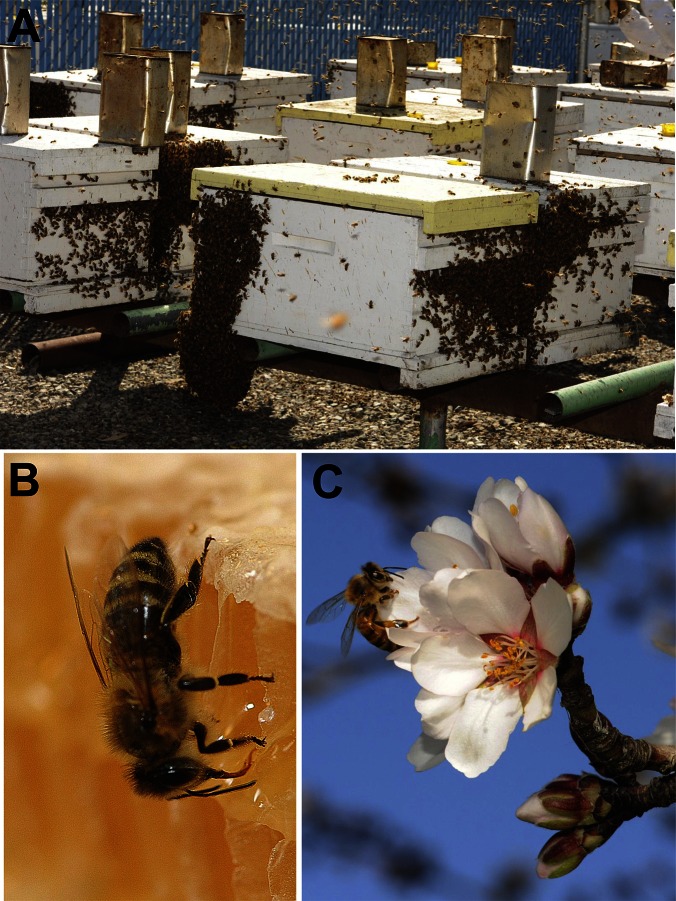
One of the unique aspects of the honey bee physiology is regulation of the P450s, enzymes that detoxify xenobiotics. Contrary to other insects (7), in the honey bee these enzymes may not be induced by a substrate itself. Apparently, the honey bee relies heavily on constituent(s) of honey to prime its detoxifying machinery so that they have “ammunition” to fend off xenobiotics they later encounter in life. Hygiene and exposure to xenobiotics have been problematic in bee hives following the 1980s introduction of the varroa mite, Varroa destructor, into the United States. The problem is twofold: varroa carries new loads of pathogens, and, in attempts to control mites, beekeepers treat the hives with pesticides (acaricides). The paper by Mao et al. (3) suggests that feeding honey bees with honey substitutes (Fig. 1 A and B) might be the last element of a lethal trinity.
Fig. 1.
Snapshots of honey bee in agriculture. (A) Bee hives with artificial bee feeders on top, (B) honey bee feeding on honey, and (C) honey bee pollinating an almond blossom (photos courtesy of Kathy Keatley Garvey).
As highlighted by the example of California’s multibillion dollar almond industry, hives are in high demand. This spring, beekeepers from throughout the country brought in some 1.6 million hives to the Golden State just to pollinate the 800,000 acres of almonds (Fig. 1C). California can supply only a third of the 1.6 million hives needed to pollinate the almonds. With this high demand, beekeepers are compelled to use honey substitutes like a 50:50 blend of sucrose and high fructose corn syrup, as well as other sugar sources, to maintain their colonies. An implication from the paper by Mao et al. (3) is that these sugar sources devoid of the “honey’s factors” do not induce the bee’s biochemical machinery to fight against xenobiotics and microbial agents.
The findings question beekeeping practices that may be involved in what causes CCD.
The paper by Mao et al. (3) is not a panacea for beekeepers whose bees are ravaged by CCD, but an invitation to them to reconsider the use of honey substitutes. Thus, we should investigate whether spiking sugar sources with p-coumaric acid would benefit the bees, resulting in the healing power of honey. More importantly, this paper opens new research avenues aimed at a better understanding of the honey bee immune system and how possibly it can be boosted to improve bee and sustainable agriculture health.
Footnotes
The author declares no conflict of interest.
See companion article on page 8842.
References
- 1.McGregor SE. 2007. Insect pollination of cultivated crop plants. Available at http://web.utk.edu/~jbaile29/publications/pollination/McGregor.pdf. Accessed April 8, 2013.
- 2.Morse RA, Calderone NW. The value of honey bees as pollinators of U.S. crops in 2000. Bee Culture. 2000;128:1–15. [Google Scholar]
- 3.Mao W, Schuler MA, Berenbaum MR. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc Natl Acad Sci USA. 2013;110:8842–8846. doi: 10.1073/pnas.1303884110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RM, et al. Ecologically appropriate xenobiotics induce cytochrome P450s in Apis mellifera. PLoS ONE. 2012;7(2):e31051. doi: 10.1371/journal.pone.0031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daimon T, Shinoda T. Function, diversity, and application of insect juvenile hormone epoxidases (CYP15) Biotechnol Appl Biochem. 2013;60(1):82–91. doi: 10.1002/bab.1058. [DOI] [PubMed] [Google Scholar]
- 6.Maïbèche-Coisne M, Nikonov AA, Ishida Y, Jacquin-Joly E, Leal WS. Pheromone anosmia in a scarab beetle induced by in vivo inhibition of a pheromone-degrading enzyme. Proc Natl Acad Sci USA. 2004;101(31):11459–11464. doi: 10.1073/pnas.0403537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]