Abstract
Trp replacements for conserved Gly–Gly pairs between the N- and C-terminal six-helix bundles on the periplasmic side of lactose permease (LacY) cause complete loss of transport activity with little or no effect on sugar binding. Moreover, the detergent-solubilized mutants exhibit much greater thermal stability than WT LacY. A Cys replacement for Asn245, which is inaccessible/unreactive in WT LacY, alkylates readily in the Gly→Trp mutants, indicating that the periplasmic cavity is patent. Stopped-flow kinetic measurements of sugar binding with the Gly→Trp mutants in detergent reveal linear dependence of binding rates on sugar concentration, as observed with WT or the C154G mutant of LacY, and are compatible with free access to the sugar-binding site in the middle of the molecule. Remarkably, after reconstitution of the Gly→Trp mutants into proteoliposomes, the concentration dependence of sugar-binding rates increases sharply with even faster rates than measured in detergent. Such behavior is strikingly different from that observed for reconstituted WT LacY, in which sugar-binding rates are independent of sugar concentration because opening of the periplasmic cavity is limiting for sugar binding. The observations clearly indicate that Gly→Trp replacements, which introduce bulky residues into tight Gly–Gly interdomain interactions on the periplasmic side of LacY, prevent closure of the periplasmic cavity and, as a result, shift the distribution of LacY toward an outward-open conformation.
Keywords: alternating access, fluorescence, major facilitator superfamily, permease, symport
The lactose permease (LacY) of Escherichia coli, the most intensively studied member of the major facilitator superfamily (1, 2), catalyzes coupled, stoichiometric translocation of a galactopyranoside and an H+ (sugar/H+ symport) across the cytoplasmic membrane (3–5). Because sugar and H+ translocation are obligatorily coupled, LacY transduces the free energy stored in an H+ electrochemical gradient (∆µ̃H+) into a sugar concentration gradient; conversely, downhill transport of galactoside drives uphill transport of H+ with the generation of ∆µ̃H+, the polarity of which is dictated by the direction of the sugar concentration gradient. The highly dynamic nature of LacY is consistent with an alternating access mechanism involving a global conformational change in which sugar and H+ binding sites are alternatively exposed to either side of the membrane (reviewed in ref. 6). Nevertheless, all X-ray structures of LacY obtained so far exhibit an inward-facing conformation, with the sugar-binding site at the apex of a deep hydrophilic cavity open to the cytoplasmic side only (7–10). In this conformation, the periplasmic side is tightly sealed and the sugar-binding site is inaccessible from the periplasm. However, a wealth of biophysical and spectroscopic data, which include site-directed alkylation (11–16), single-molecule fluorescence resonance energy transfer (FRET) (17), double electron-electron resonance (18), site-directed cross-linking (19) and Trp fluorescence studies (20–22), provide converging evidence that the sugar- and H+-binding sites in LacY are alternatively accessible from either side of the membrane (the alternating access model, reviewed in refs. 6 and 23). Moreover, structural modeling of LacY and comparison with the fucose/H+ symporter, which crystallizes in an opposite outward-facing conformation, provide insight into the sequential conformational rearrangements in the transport mechanism of LacY (24–26).
LacY is highly dynamic. Hydrogen/deuterium exchange of virtually all backbone amide protons in the WT occurs at a remarkably rapid rate, with LacY embedded in either a detergent micelle (27, 28) or a phospholipid membrane (29). Moreover, sugar binding by WT LacY is mostly entropic in nature (30), inducing widespread conformational changes (6). Protonation is required for sugar binding, as indicated in the original kinetic model (31–33). Thus, LacY with a pKa of ∼10.5 for sugar binding (34) is protonated under physiological conditions (35). Residues in the H+-binding site are tightly interconnected, define the pKa for sugar binding, and alter galactoside-binding affinity (35, 36). Protonated LacY catalyzes exchange and counterflow of sugar molecules across the membrane by a mechanism that does not include translocation of H+ but involves the global conformational change corresponding to alternating access (31–33, 37–39). The single mutation C154G dramatically decreases conformational flexibility but does not affect sugar or H+ binding, and it practically eliminates all transport reactions (40–44). It is likely that the conformational changes triggered by sugar binding are relatively facile and do not need to overcome high-energy barriers (25).
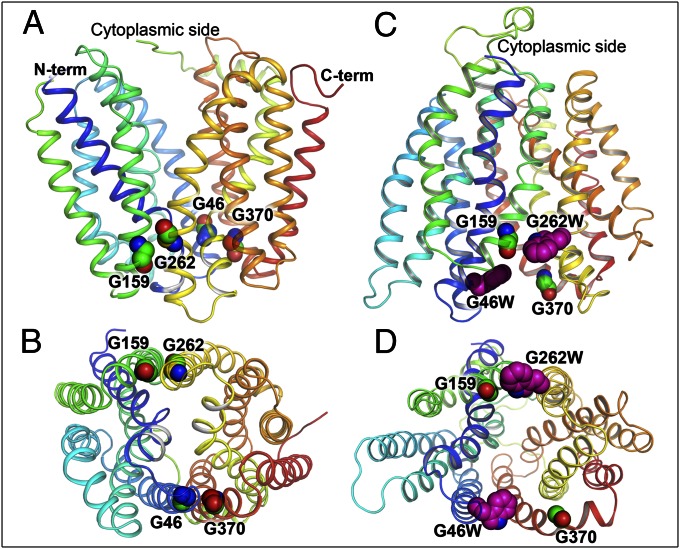
The structural basis for the high flexibility of LacY is provided in part by the irregular shape of the transmembrane helices, with kinks and bends that contain a total of 36 Gly (45) and 12 Pro residues (46), many of which are conserved. Comparison of the LacY sequence with homologous sequences of prokaryotic and eukaryotic major facilitator superfamily transporters and structural threading reveals strong conservation not only of amino acid residues that participate in sugar and H+ binding but also of several important structural elements (47). One such conserved feature is tight interactions between N- and C-terminal six-helix bundles on the periplasmic side of LacY, which is facilitated by two pairs of Gly residues located at the ends of helices 2 and 11 (Gly46 and Gly370, respectively) and helices 5 and 8 (Gly159 and Gly262, respectively) (Fig. 1 and Fig. S1). With no side chain, Gly residues modulate conformational flexibility and also allow close interactions between helices (48–53). Therefore, tight packing of helices on the periplasmic side of LacY, observed in all inward-facing structures, is controlled not only by close proximity of tilted helices 1 and 7 in the middle (19) but also by two direct Gly–Gly contacts between N- and C-terminal six-helix bundles on the periphery of the molecule (Fig. 1B). Notably, these Gly residues are located symmetrically in straight α-helical transmembrane domains (Fig. S2). Cys scanning mutagenesis of LacY (45, 54) shows that individual bulky replacements for Gly46, Gly159, Gly262, and Gly370 practically eliminate transport activity. Thus, each of four single-Cys mutants treated with the thiol reagent N-ethylmaleimide (NEM) retains less than 20% of lactose transport activity (55–58). As a consequence, mutations that severely destabilize interdomain Gly–Gly connections in LacY are expected to produce structures with a less tightly packed periplasmic side. Here we report that site-directed Trp replacements for Gly–Gly pairs on the tightly packed periplasmic side of LacY result in formation of a stable outward-facing conformation of LacY with an open periplasmic cavity.
Fig. 1.
Trp replacements in two pairs of Gly–Gly residues that connect the N- and C-terminal six-helix domains on the periplasmic side of LacY. The 12 transmembrane helices that make up LacY are rainbow colored from blue to red. Gly residues (Gly46, 159, 262, and 370 in helices 2, 5, 8, and 11, respectively) and Trp replacements are shown as spheres. Inward-facing X-ray structure (Protein Data Bank ID code 2CFQ) viewed from the side (A) or from the periplasm (B). Model of the outward-facing conformation of LacY (18, 24) with Gly→Trp replacements (pink spheres) at positions 46 and 262 viewed from the side (C) or the periplasm (D).
Results
Disruption of Gly–Gly Interactions.
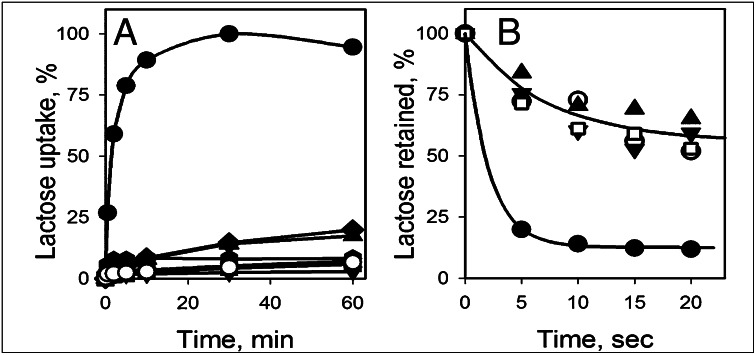
Six mutants were constructed in which Trp residues were introduced into Gly–Gly pairs between the N- and C-terminal domains on the tightly sealed periplasmic aspect of LacY (Fig. 1). Thus, Gly residues at positions 46, 159, 262, or 370 were individually replaced with Trp. In addition, double Trp mutants were constructed by replacing a single Gly residue in each pair (G46W/G262W) or both Gly residues in the same pair (G46W/G370W). All Gly→Trp mutants were expressed in the bacterial membrane at a level comparable to WT LacY. Active lactose accumulation driven by Δμ̃H+ was assayed in E. coli cells expressing WT LacY and the Gly→Trp mutants (Fig. 2A). In marked contrast to WT LacY, none of the mutants catalyzes significant accumulation of lactose. Furthermore, equilibrium exchange of lactose, a reaction that does not involve net H+ translocation, was also tested with the same mutants in right-side-out (RSO) membrane vesicles. As opposed to WT LacY, which exhibits brisk exchange activity, both double Gly→Trp mutants are unable to catalyze exchange of lactose above the levels observed for the negative controls (i.e., RSO vesicles containing no LacY or WT LacY inactivated by NEM) (Fig. 2B).
Fig. 2.
Functional properties of LacY mutants with Gly→Trp replacements. (A) Lactose transport in E. coli cells harboring WT LacY (●), mutants with single or double Gly→Trp replacements: G46W (▲), G159W ( ), G262W (■), G370W (◆), G46W/G262W (▼), G46W/G370W (★), or pT7-5 vector only with no LacY insert (○). Active sugar accumulation was measured at 0.4 mM [14C]-lactose, as described in Materials and Methods. One hundred percent transport corresponds to 160 nmol/mg protein. (B). Equilibrium exchange of lactose by RSO vesicles containing WT LacY (●), NEM-treated WT LacY (□), mutants with double Gly→Trp replacements G46W/G262W (▲), and G46W/G370W (▼), or no permease (○). RSO vesicles equilibrated with 10 mM [14C]lactose were diluted (1:200) into buffer containing 10 mM nonradioactive lactose and, at given times, radioactive lactose retained inside of vesicles was measured by rapid filtration as described in Materials and Methods. Nonspecific exchange of lactose in RSO vesicles containing WT LacY was assayed with an NEM-treated sample (see Materials and Methods for details).
), G262W (■), G370W (◆), G46W/G262W (▼), G46W/G370W (★), or pT7-5 vector only with no LacY insert (○). Active sugar accumulation was measured at 0.4 mM [14C]-lactose, as described in Materials and Methods. One hundred percent transport corresponds to 160 nmol/mg protein. (B). Equilibrium exchange of lactose by RSO vesicles containing WT LacY (●), NEM-treated WT LacY (□), mutants with double Gly→Trp replacements G46W/G262W (▲), and G46W/G370W (▼), or no permease (○). RSO vesicles equilibrated with 10 mM [14C]lactose were diluted (1:200) into buffer containing 10 mM nonradioactive lactose and, at given times, radioactive lactose retained inside of vesicles was measured by rapid filtration as described in Materials and Methods. Nonspecific exchange of lactose in RSO vesicles containing WT LacY was assayed with an NEM-treated sample (see Materials and Methods for details).
Sugar Binding and Thermal Stability.
Galactoside binding to purified mutants with single and double Gly→Trp replacements was measured by FRET (Fig. S3) from Trp151 in sugar-binding site to 4-nitrophenyl-α-d-galactopyranoside (NPG) as an increase in Trp fluorescence after displacement of bound NPG by an excess of β-d-galactosyl-1-thio-β-d-galactopyranoside (TDG) (20). In dodecyl-β-d-maltopyranoside (DDM), all mutants exhibit good sugar binding with 40–60% Trp→NPG FRET (Fig. 3A and Fig. S3C).
Fig. 3.
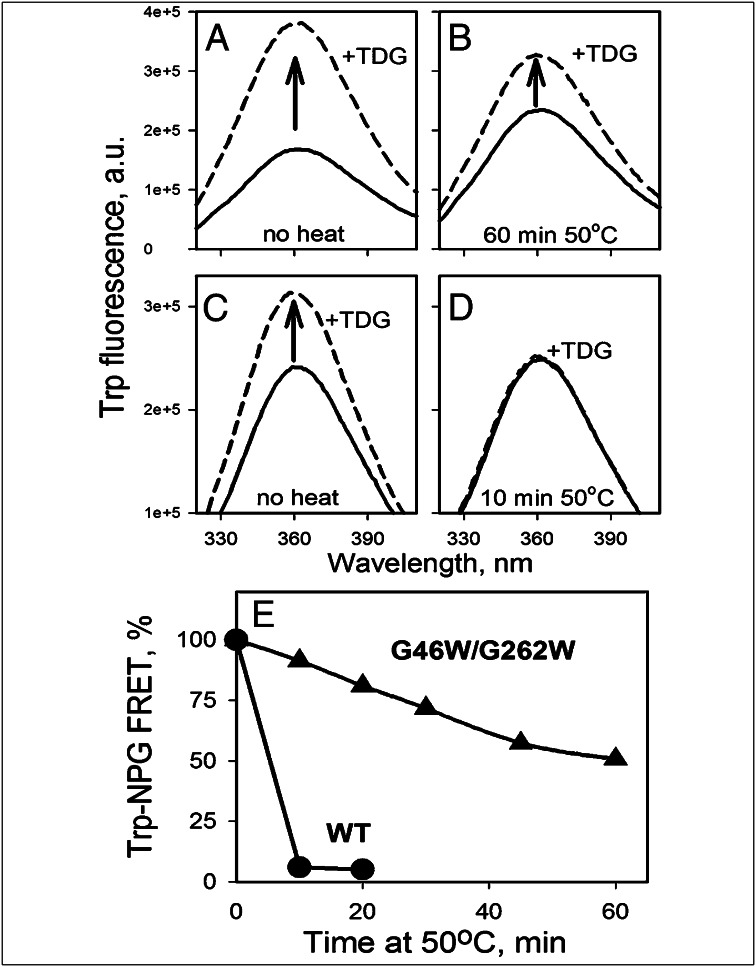
Effect of Gly→Trp replacements on conformational stability of LacY. Purified samples of WT LacY or mutant G46W/G262W were heated in a water bath at 50°C at a protein concentration of 0.3 mg/mL in 50 mM NaPi/0.02% DDM (pH 7.5). At given times, aliquots were cooled on ice, centrifuged for 5 min at 25,000 × g, and assayed for sugar binding at room temperature. Binding of sugar was measured by using Trp→NPG FRET. Trp emission spectra were recorded at excitation wavelength 295 nm with 0.5 μM protein in 50 mM NaPi/0.02% DDM (pH 7.5). The increase in Trp fluorescence after displacement of bound NPG (0.2 mM) with excess TDG (12 mM) is expressed as a percentage of the final fluorescence level after TDG addition. (A and B) Substrate binding to G46W/G262W mutant before heating and after 1 h incubation at 50°C. (C and D) Substrate binding to WT LacY before heating and after 10 min incubation at 50°C. (E) Time courses of thermal inactivation for WT LacY (●) or the G46W/G262W mutant (▲). Binding at zero time (100%) corresponds to 30% FRET with WT LacY and 56% FRET with the mutant. Heavy precipitation was observed with the WT LacY sample after 10 min; only slight aggregation was observed after 1 h with the mutant at 50°C.
The effect of Gly→Trp mutations on conformational stability was tested in DDM by measuring sugar binding to protein samples preincubated at 50°C. In marked contrast to WT LacY, which is completely inactivated after 10 min, mutant G46W/G262W retains substantial sugar binding and exhibits a low propensity to aggregate after 1 h incubation at 50°C (Fig. 3).
Site-Directed Alkylation of a Cys Residue in the Periplasmic Pathway.
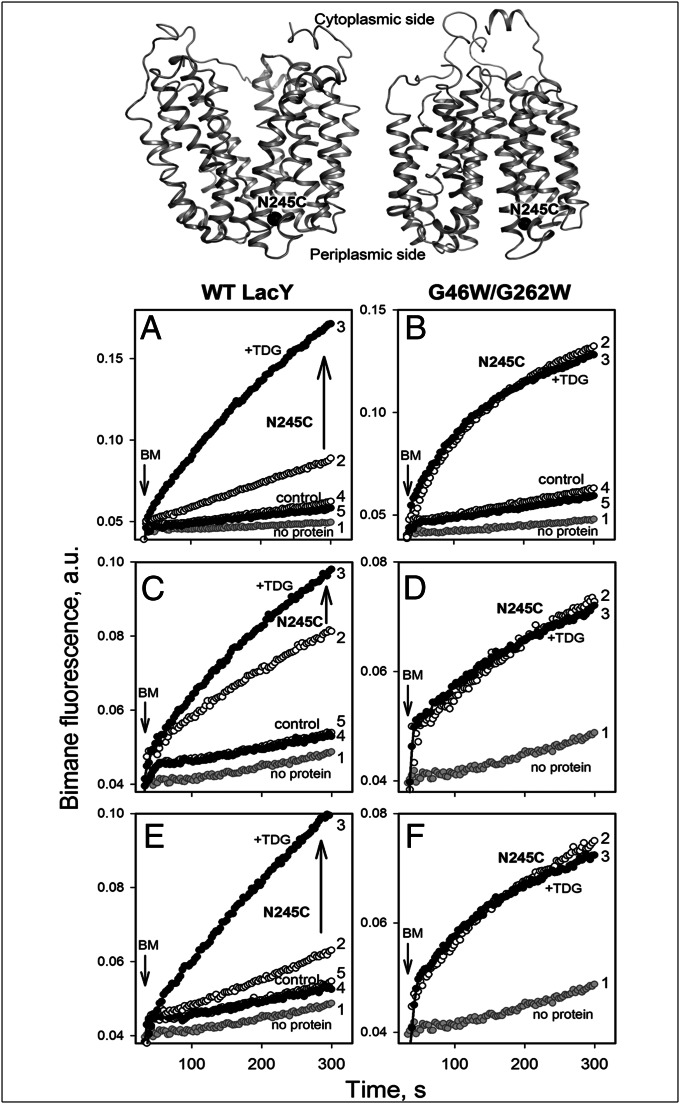
A mutant with Cys in place of Asn245 (N245C) within the tightly packed periplasmic side of LacY (Fig. 4, Top) exhibits a marked increase in reactivity/accessibility to thiol reagents on binding of galactosides, thereby reflecting the opening of the periplasmic cavity (11–13, 15, 19, 59). To examine the effect of Gly→Trp replacements on the conformation of LacY, alkylation of N245C with bimane-C3-maleimide (BM) in WT or G46W/G262W LacY was tested. As opposed to the WT, where N245C labels very slowly in the absence of sugar and TDG causes a dramatic increase in reactivity/accessibility (Fig. 4A, traces 2 and 3), rapid labeling is observed with mutant G46W/G262W in the absence of sugar, and TDG has no significant effect (Fig. 4B, traces 2 and 3). Note that the control samples essentially do not label with BM under the same conditions (Fig. 4 A and B, traces 4 and 5), even though only one of eight native Cys was replaced (Cys148→Met). Rapid labeling is also observed after reconstitution of mutant G46W/G262W/N245C into proteoliposomes with no effect of TDG, whereas the rate of BM labeling of N245C in the WT background is significantly increased by TDG binding (Fig. 4 C and D). Labeling of each protein after dissolving the proteoliposomes in DDM is practically identical to that observed initially (compare Fig. 4, E with A and F with B). Thus, high accessibility/reactivity of Cys245 in mutant G46W/G262W suggests constitutive opening of the periplasmic cavity either in DDM or in proteoliposomes. Moreover, single Gly→Trp replacement in the G370W/N245C mutant was enough to open periplasmic cavity, as evidenced by the high reactivity/accessibility of the introduced Cys245 to BM labeling, although some additional effect of TDG is observed (Fig. S4).
Fig. 4.
Site-directed alkylation of a Cys replacement at position 245 in the periplasmic pathway. The target Cys (mutant N245C) is shown at the top for protein with a closed (Left) or open (Right) periplasmic cavity. Time courses of labeling were recorded at excitation and emission wavelengths of 380 and 465 nm, respectively, in 50 mM NaPi/0.02% DDM (pH 7.5) at 1 μM BM, added to 0.5 μM protein at 30 s, as indicated by an inverted arrow. (Left and Right) BM labeling of WT or mutant G46W/G262W, respectively. The highly reactive/accessible native Cys148 in each mutant was replaced with Met. In each panel, trace 1 was recorded with no protein added and traces 2 and 3 correspond to labeling of Cys245 in the absence of sugar or after addition of 6 mM TDG, respectively. Labeling of control proteins with no Cys replacement at position 245 is shown by trace 4 (no sugar) and trace 5 (6 mM TDG). Labeling of Cys245 with BM was tested with proteins in DDM (A and B), with mutants reconstituted into proteoliposomes (C and D) or with the same proteoliposomes dissolved in DDM (E and F).
Sugar Binding Rates.
Stopped-flow kinetic studies of sugar binding with WT LacY in DDM or after reconstitution into proteoliposomes show that the sugar-binding site is not readily accessible after LacY is reconstituted into proteoliposomes (20, 22). Thus, rates of NPG binding to LacY measured directly by Trp→NPG FRET in DDM increase linearly with NPG concentration, indicating that NPG has free access to the sugar-binding site. However, LacY reconstituted into proteoliposomes binds NPG at a slow rate that is independent of sugar concentration and comparable with the rate of opening of the periplasmic pathway. Therefore, access to the sugar-binding site is limited by the rate of opening of the periplasmic cavity (22).
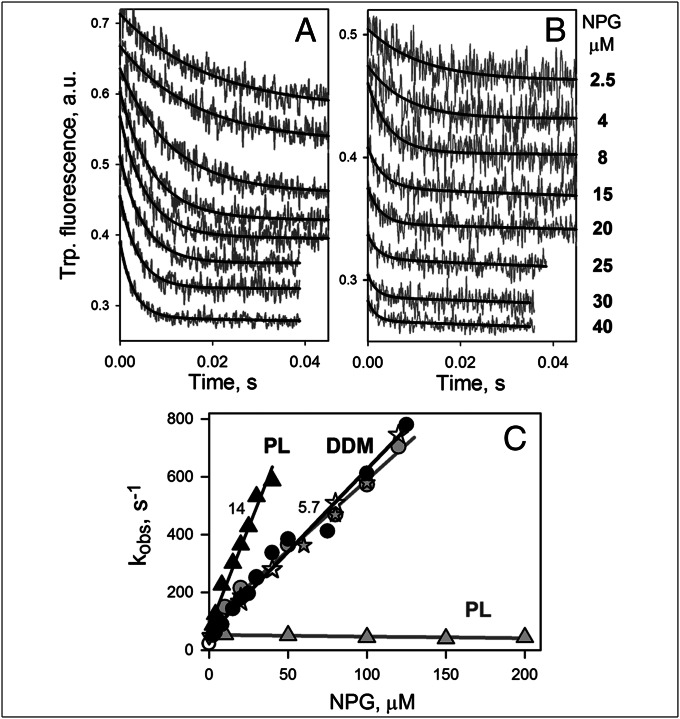
When NPG binding by mutant G46W/G262W is examined by stopped-flow studies, it is apparent that the rate of NPG binding increases with sugar concentration in DDM, as well as in reconstituted proteoliposomes (Fig. 5 A and B, respectively). Binding rates (kobs) estimated from single exponential fits and plotted as a function of NPG concentration exhibit linear dependencies in both cases (Fig. 5C, black symbols and lines). Kinetic parameters estimated for reconstituted protein are koff = 75 s−1, kon = 14 μM−1⋅s−1, and Kd = koff/kon = 5.3 μM. Dissolving the proteoliposomes in DDM restores the kinetic parameters to those observed for the G46W/G262W mutant in DDM: koff = 55 s−1, kon = 5.7 μM−1⋅s−1, and Kd = koff/kon = 9.7 μM. Remarkably, mutant G46W/G262W reconstituted into proteoliposomes exhibits a kon value (14 μM−1⋅s−1 estimated from the slope) that is even higher than the kon value in DDM (5.7 μM−1⋅s−1) and is strikingly different from reconstituted C154G LacY with undisturbed Gly–Gly contacts that binds NPG with a kobs = 50 s−1 independent of NPG concentration (Fig. 5C, gray triangles). In DDM, C154G LacY, which is also a conformationally constrained, nontransporting mutant, binds NPG with the same rates as G46W/G262W (Fig. 5C, gray and black circles). The data clearly demonstrate unrestricted accessibility of the sugar-binding site in the G46W/G262W mutant not only in DDM but also in proteoliposomes, thereby supporting the argument that the periplasmic cavity is completely open.
Fig. 5.
Rates of NPG binding to mutant G46W/G262W measured by Trp→NPG FRET. Stopped-flow traces of Trp fluorescence change (excitation and emission wavelengths 295 and 340 nm, respectively) were recorded after mixing of NPG (at final concentrations indicated) with G46W/G262W (0.5 μM) in DDM (A) or with the same mutant reconstituted into proteoliposomes (B). Sugar-binding rates (kobs) were estimated from single exponential fits (black lines on A and B) and plotted vs. NPG concentrations (C) for NPG binding to the mutant in DDM solution (●), reconstituted into proteoliposomes (PL; ▲) or after dissolving the proteoliposomes in DDM (). Linear fits to the data (kobs = koff + kon[NPG]) are shown as black lines. Numbers near the lines show estimated kon (μM−1⋅s−1) values. Kinetic parameters for NPG binding to mutant G46W/G262W in DDM and in proteoliposomes (data in parentheses) are koff = 55 (75) s−1, kon = 5.7 (14) μM−1⋅s−1, and Kd = 9.7 (5.3) μM. Gray symbols and lines demonstrate kinetics of NPG binding to C154G for comparison. Kinetic parameters measured with C154G in DDM are koff = 100 s−1, kon = 5.0 μM−1⋅s−1, and Kd = 20 μM. Mutant C154G reconstituted into proteoliposomes binds NPG with a kobs = 50 s−1.
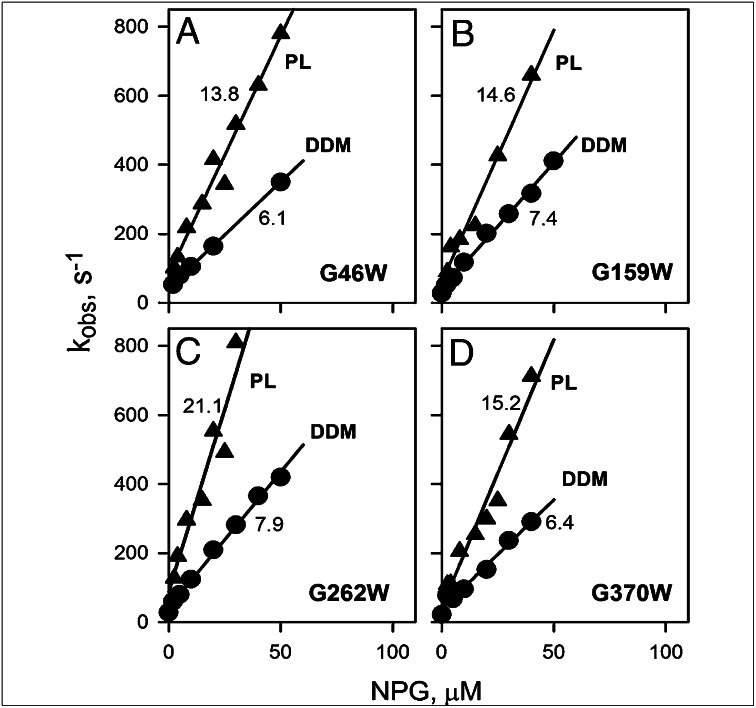
The G46W/G370W mutant, either in DDM or reconstituted into proteoliposomes, displays kinetic parameters for NPG binding that are practically indistinguishable from those obtained with G46W/G262W LacY (Fig. S5). Sugar-binding kinetics were also studied for mutants with single Gly→Trp replacements (Fig. 6 and Fig. S6). Individual Trp replacements for each Gly in the Gly–Gly pairs exhibit similar effects on sugar-binding kinetics, as observed with the double Gly→Trp replacements. In all cases, linear concentration dependencies of kobs are observed in DDM and in proteoliposomes, indicating that a single bulky replacement in the tight interdomain Gly–Gly contacts is sufficient to shift the structural distribution toward an outward-facing conformation.
Fig. 6.
Kinetics of sugar binding to single Gly→Trp mutants. Sugar binding rates (kobs) were estimated from single exponential fits of stopped-flow traces similar to those shown in Fig. 5 A and B after mixing of NPG with each purified mutant solubilized in DDM (●) or reconstituted into proteoliposomes (▲). Stopped-flow traces recorded with reconstituted proteoliposomes are presented in Fig. S6. Kinetic parameters for NPG binding to G46W (A), G159W (B), G262W (C), and G370W (D) were obtained from linear fits to the data (solid lines), as shown in Fig. 5C. Numbers near each line represent kon (μM−1⋅s−1) values. Estimated values of koff were 30–40 s−1 in DDM or 50–80 s−1 in proteoliposomes, resulting in high-affinity binding (Kd = 3–7 μM).
Effect of Protonation on Sugar Binding.
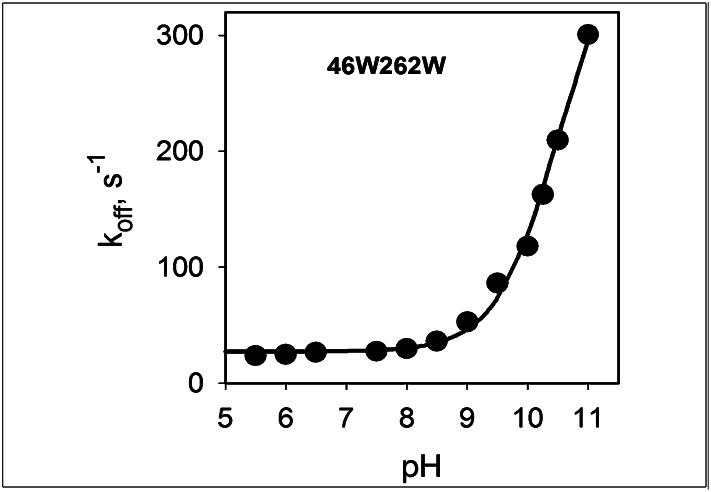
The binding affinity of WT LacY for galactopyranosides decreases sharply at alkaline pH as a result of an increase in koff (Kd = koff/kon), with a pKa of ∼10.5 (34, 36). It follows that over the physiological range of pH, sugar binds to fully protonated LacY. Stabilization of an outward-facing conformation by Trp→Gly replacements may involve changes in the H+-binding site that alter sugar-binding affinity (Fig. S3B). Therefore, the pH dependence of koff was examined for NPG binding to mutant G46W/G262W. Stopped-flow traces of the increase in Trp fluorescence after displacement of bound NPG with an excess of TDG were recorded at pH 5.5–11.0 (Fig. S7A). Single exponential fits provide an estimate of koff values that are plotted as a function of pH (Fig. 7). The data obtained for the G46W/G262W mutant are identical to those obtained for WT and C154G LacY (Fig. S7B) and exhibit a pKa of ∼10.5. Thus, the Gly→Trp mutations that result in an outward-facing conformation of LacY do not significantly perturb the H+-binding site.
Fig. 7.
Effect of pH on NPG dissociation rate (koff) for mutant G46W/G262W in DDM. Rates of displacement of bound NPG by excess of TDG were determined by measuring Trp→NPG FRET at given pH values. Protein preincubated with NPG was rapidly mixed with TDG, and rates of increase in Trp fluorescence measured by stopped-flow were obtained from single exponential fits (see traces in Fig. S7A). Final concentrations were: protein, 0.8–1.6 μM; NPG, 0.2–0.4 mM; and TDG 30–60 mM. Displacement rates (koff) plotted vs. pH and fitted with a sigmoidal equation (SigmaPlot 10.0) indicate a pKa of ∼10.5.
Discussion
As anticipated, Trp substitutions for Gly residues at points of close contact between the periplasmic ends of helices 2 and 11 or helices 5 and 8, which provide tight junctions between the N- and C-terminal six-helix domains of LacY, produce mutants that exhibit the characteristics of proteins with an open periplasmic cavity. The Gly→Trp mutants are expressed at a level comparable to WT LacY, with no apparent adverse effect on cell viability, and the mutants do not catalyze sugar/H+ transport across the membrane (Fig. 2). However, sugar binding remains unaffected, as determined by Trp→NPG FRET with purified mutants in DDM or after reconstitution into proteoliposomes. Mutant G46W/G262W solubilized in DDM exhibits exceptional structural stability, withstanding 1 h incubation at 50°C without significant aggregation and only moderately decreased sugar binding. In contrast, after 10 min at 50°C, WT LacY precipitates heavily and is completely inactivated (Fig. 3). Thermostability is also observed with mutant Cys154→Gly where the structural changes necessary for translocation of bound substrate are also blocked (42). Therefore, the Gly→Trp mutants apparently adopt a conformation that is not sufficiently flexible to catalyze transport but can retain intact ability to bind sugar.
Molecular modeling suggests that bulky Trp residues introduced in place of Gly in the tight Gly–Gly junctions between the N- and C-terminal six-helix domains of LacY should prevent closure of the periplasmic cavity (Fig. 1 C and D). Therefore, opening of the periplasmic cavity in the Gly→Trp mutants was examined by two approaches: site-directed alkylation of a Cys replacement for Asn245 and pre-steady state kinetics of sugar binding with protein solubilized in DDM or reconstituted into proteoliposomes. With respect to site-directed alkylation, it is well documented that position 245 is one of several positions on the periplasmic side of LacY at which Cys replacements exhibit very low reactivity/accessibility in the absence of bound sugar and a dramatic increase in reactivity/accessibility on sugar binding (11–15, 59). Because a six- to 10-fold increase in reactivity/accessibility of Cys replacements on the sealed periplasmic side is mirrored by a six- to 10-fold decrease in reactivity/accessibility of Cys replacements on the open cytoplasmic side of LacY (14), an alternating access mechanism is likely central to the transport process. Moreover, as shown here with the Gly→Trp mutants (Fig. 4 B, D, and F and Fig. S4), a Cys replacement for Asn245 undergoes rapid site-directed alkylation in the absence of sugar, thereby indicating that the periplasmic cavity is constitutively open.
Regarding pre-steady state kinetics of sugar binding, the findings presented here provide more direct evidence for the contention that the Gly→Trp mutants are arrested in an outward-open conformation. Thus, reconstituted WT and C154G LacY, which are oriented physiologically in proteoliposomes with the sealed periplasmic side facing out, exhibit no concentration dependence of sugar binding rates because opening of the periplasmic cavity is the limiting step for binding. In contrast, Gly→Trp mutants with single- or double-Trp replacements reconstituted into proteoliposomes exhibit acute linear dependencies of binding rates on sugar concentrations (Figs. 5C and 6 and Fig. S5A), indicating free access of the binding site to sugar. Remarkably, the sugar- and H+-binding sites in the Gly→Trp mutants are unaffected, as judged from sugar-binding kinetic parameters and the pH dependence of sugar-binding affinity, which are essentially identical to LacY, with unperturbed Gly–Gly interactions between two six-helix domains.
Materials and Methods
Materials.
Oligonucleotides were synthesized by Integrated DNA Technologies. Restriction enzymes were purchased from New England Biolabs. QuikChange II kits were purchased from Stratagene. TDG was obtained from Carbosynth Limited. NPG and buffers were from Sigma-Aldrich. Talon superflow resin was purchased from BD Clontech. Bimane-C3-maleimide was from Life Technologies. DDM and n-octyl-β-d-glucoside (OG) were obtained from Affymetrix. Synthetic 1-palmitoyl-2-oleolyl-sn-glycero-3-phosphoethanolamine and 1-palmitoyl-2-oleolyl-sn-glycero-3-phospho-(1′-rac-glycerol) were from Avanti Polar Lipids. All other materials were of reagent grade and obtained from commercial sources.
Construction of Mutants, Purification of LacY, and Reconstitution into Proteoliposomes.
Construction of mutants, expression in E. coli, and purification of LacY were performed as described (18). All mutants contained a C-terminal 6 His-tag that was used for affinity purification on Talon resin with a typical yield of ∼0.1 mg protein/g wet cells. Purified proteins (10–15 mg/mL) in 50 mM NaPi/0.02% DDM (pH 7.5) were frozen in liquid nitrogen and stored at −80°C until use.
Reconstitution of LacY into proteoliposomes was carried out with synthetic phospholipids (1-palmitoyl-2-oleolyl-sn-glycero-3-phosphoethanolamine/1-palmitoyl-2-oleolyl-sn-glycero-3-phospho-(1′-rac-glycerol); 3:1, wt/wt), using the dilution method (60). Briefly, purified LacY in 0.02% DDM solution was mixed with phospholipids (40 mg/mL dissolved in 1.2% OG), maintaining a lipid-to-protein ratio of 5 (wt/wt). The mixture was kept on ice for 20 min and then quickly diluted in 50 mM NaPi buffer (pH 7.5), so that final concentration of OG was decreased to less than 0.01%. After 5 min stirring at room temperature, the proteoliposomes were collected by centrifugation at 100,000 × g for 1 h, resuspended at a protein concentration of 2 mg/mL, subjected to 2 cycles of freeze-thaw/sonication, and kept at room temperature during the experiment. Where indicated, proteoliposomes were dissolved in 0.3% DDM and kept on ice before use.
Transport Assays.
For active transport, E. coli cells (T184, lacZ−Y−) transformed with each plasmid were grown aerobically at 37°C in Luria-Bertani broth containing ampicillin (100 μg/mL). Fully-grown cultures were diluted 10-fold and grown for another 2 h before induction with 1 mM isopropyl-1-thio-β-D-galactopyranoside. After 2 h, cells were harvested, washed with 100 mM KPi/MgSO4 (pH 7.0), and adjusted to an optical density of 10 at 420 nm (∼0.7 mg protein/mL). Accumulation of [14C]lactose (5 mCi/mmol) was measured at room temperature in 50-μL aliquots of cell suspensions (35 μg total protein), at a final concentration of 0.4 mM lactose. Reactions were terminated at given times by dilution in 3 mL 100 mM KPi/100 mM LiCl (pH 5.5) and assayed by rapid filtration (61). Radioactivity retained on the filters was assayed by liquid scintillation spectrometry. All mutants were expressed similar to WT LacY.
For equilibrium exchange, RSO vesicles were prepared from the same E. coli cells as described (62, 63). Vesicles were washed with 100 mM KPi (pH 7.5), resuspended at protein concentration 35 mg/mL, and equilibrated with 10 mM [14C]lactose (8.5 mCi/mmol) at 4°C overnight, followed by 1 h at room temperature. NEM-treated RSO vesicles were prepared by 5 min incubation of vesicles with 2 mM NEM at room temperature before equilibration with radioactive lactose (39). To initiate exchange, 2 μL aliquots were diluted into 0.4 mL 100 mM KPi buffer (pH 7.5) containing 10 mM nonradioactive lactose. Reactions were terminated at given times by dilution in 3 mL 100 mM KPi/100 mM LiCl buffer (pH 5.5) and assayed by rapid filtration (61).
Fluorescence Measurements.
Steady-state fluorescence was monitored at room temperature on a SPEX Fluorolog 3 spectrofluorometer (Horiba) or an SLM-Aminco 8100 spectrofluorometer in a 2.5-mL cuvette with constant stirring. Stopped-flow measurements were performed at 25°C on a stopped-flow device (dead-time, 2.7 ms), using an SLM-Aminco 8100 spectrofluorometer modified by Olis, or on an SFM-300 rapid kinetic system equipped with TC-50/10 cuvette (dead-time, 1.2–1.5 ms), and MOS-450 spectrofluorometer (Bio-Logic USA), as described (20, 22). Typically, 10–15 stopped-flow traces were recorded for each data point and averaged and fitted with an exponential equation using the built-in Bio-Kine32 software package or by using Sigmaplot 10 (Systat Software Inc.). Experimental errors did not exceed 10%. All given concentrations are final after mixing.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK51131, DK069463, and GM073210, as well as National Science Foundation Grant MCB-1129551 (to H.R.K.).
Footnotes
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306849110/-/DCSupplemental.
References
- 1.Saier MH, Jr, et al. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1(2):257–279. [PubMed] [Google Scholar]
- 2.Saier MH., Jr Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35(4):699–710. doi: 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 3.West IC, Mitchell P. Stoicheiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973;132(3):587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel L, Garcia ML, Kaback HR. Direct measurement of lactose/proton symport in Escherichia coli membrane vesicles: Further evidence for the involvement of histidine residue(s) Biochemistry. 1982;21(23):5805–5810. doi: 10.1021/bi00266a013. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Celma JJ, Smirnova IN, Kaback HR, Fendler K. Electrophysiological characterization of LacY. Proc Natl Acad Sci USA. 2009;106(18):7373–7378. doi: 10.1073/pnas.0902471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smirnova I, Kasho V, Kaback HR. Lactose permease and the alternating access mechanism. Biochemistry. 2011;50(45):9684–9693. doi: 10.1021/bi2014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301(5633):610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 8.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H+ symport in LacY. EMBO J. 2006;25(6):1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci USA. 2007;104(39):15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaptal V, et al. Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition. Proc Natl Acad Sci USA. 2011;108(23):9361–9366. doi: 10.1073/pnas.1105687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaback HR, et al. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci USA. 2007;104(2):491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie Y, Ermolova N, Kaback HR. Site-directed alkylation of LacY: Effect of the proton electrochemical gradient. J Mol Biol. 2007;374(2):356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie Y, Sabetfard FE, Kaback HR. The Cys154—>Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379(4):695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie Y, Kaback HR. Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane. Proc Natl Acad Sci USA. 2010;107(21):9903–9908. doi: 10.1073/pnas.1004515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X, Nie Y, Kaback HR. Site-directed alkylation studies with LacY provide evidence for the alternating access model of transport. Biochemistry. 2011;50(10):1634–1640. doi: 10.1021/bi101988s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X, et al. Evidence for an intermediate conformational state of LacY. Proc Natl Acad Sci USA. 2012;109(12):E698–E704. doi: 10.1073/pnas.1201107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majumdar DS, et al. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci USA. 2007;104(31):12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnova I, et al. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci USA. 2007;104(42):16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci USA. 2008;105(10):3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smirnova IN, Kasho VN, Kaback HR. Direct sugar binding to LacY measured by resonance energy transfer. Biochemistry. 2006;45(51):15279–15287. doi: 10.1021/bi061632m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smirnova I, Kasho V, Sugihara J, Kaback HR. Probing of the rates of alternating access in LacY with Trp fluorescence. Proc Natl Acad Sci USA. 2009;106(51):21561–21566. doi: 10.1073/pnas.0911434106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smirnova I, Kasho V, Sugihara J, Kaback HR. Opening the periplasmic cavity in lactose permease is the limiting step for sugar binding. Proc Natl Acad Sci USA. 2011;108(37):15147–15151. doi: 10.1073/pnas.1112157108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaback HR, Smirnova I, Kasho V, Nie Y, Zhou Y. The alternating access transport mechanism in LacY. J Membr Biol. 2011;239(1-2):85–93. doi: 10.1007/s00232-010-9327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radestock S, Forrest LR. The alternating-access mechanism of MFS transporters arises from inverted-topology repeats. J Mol Biol. 2011;407(5):698–715. doi: 10.1016/j.jmb.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Madej MG, Soro SN, Kaback HR. Apo-intermediate in the transport cycle of lactose permease (LacY) Proc Natl Acad Sci USA. 2012;109(44):E2970–E2978. doi: 10.1073/pnas.1211183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugihara J, Sun L, Yan N, Kaback HR. Dynamics of the L-fucose/H+ symporter revealed by fluorescence spectroscopy. Proc Natl Acad Sci USA. 2012;109(37):14847–14851. doi: 10.1073/pnas.1213445109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.le Coutre J, Kaback HR, Patel CK, Heginbotham L, Miller C. Fourier transform infrared spectroscopy reveals a rigid alpha-helical assembly for the tetrameric Streptomyces lividans K+ channel. Proc Natl Acad Sci USA. 1998;95(11):6114–6117. doi: 10.1073/pnas.95.11.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayeed WM, Baenziger JE. Structural characterization of the osmosensor ProP. Biochim Biophys Acta. 2009;1788(5):1108–1115. doi: 10.1016/j.bbamem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Patzlaff JS, Moeller JA, Barry BA, Brooker RJ. Fourier transform infrared analysis of purified lactose permease: A monodisperse lactose permease preparation is stably folded, alpha-helical, and highly accessible to deuterium exchange. Biochemistry. 1998;37(44):15363–15375. doi: 10.1021/bi981142x. [DOI] [PubMed] [Google Scholar]
- 30.Nie Y, Smirnova I, Kasho V, Kaback HR. Energetics of ligand-induced conformational flexibility in the lactose permease of Escherichia coli. J Biol Chem. 2006;281(47):35779–35784. doi: 10.1074/jbc.M607232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaczorowski GJ, Kaback HR. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 1. Effect of pH on efflux, exchange, and counterflow. Biochemistry. 1979;18(17):3691–3697. doi: 10.1021/bi00584a009. [DOI] [PubMed] [Google Scholar]
- 32.Kaback HR, Sahin-Tóth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2(8):610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 33.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smirnova IN, Kasho V, Kaback HR. Protonation and sugar binding to LacY. Proc Natl Acad Sci USA. 2008;105(26):8896–8901. doi: 10.1073/pnas.0803577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smirnova I, Kasho V, Sugihara J, Vázquez-Ibar JL, Kaback HR. Role of protons in sugar binding to LacY. Proc Natl Acad Sci USA. 2012;109(42):16835–16840. doi: 10.1073/pnas.1214890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smirnova IN, Kasho VN, Sugihara J, Choe JY, Kaback HR. Residues in the H+ translocation site define the pKa for sugar binding to LacY. Biochemistry. 2009;48(37):8852–8860. doi: 10.1021/bi9011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrasco N, Antes LM, Poonian MS, Kaback HR. lac permease of Escherichia coli: Histidine-322 and glutamic acid-325 may be components of a charge-relay system. Biochemistry. 1986;25(16):4486–4488. doi: 10.1021/bi00364a004. [DOI] [PubMed] [Google Scholar]
- 38.Persson B, Roepe PD, Patel L, Lee J, Kaback HR. Site-directed mutagenesis of lysine 319 in the lactose permease of Escherichia coli. Biochemistry. 1992;31(37):8892–8897. doi: 10.1021/bi00152a028. [DOI] [PubMed] [Google Scholar]
- 39.Sahin-Toth M, Kaback HR. Arg-302 facilitates deprotonation of Glu-325 in the transport mechanism of the lactose permease from Escherichiacoli. Proc Natl Acad Sci USA. 2001;98(11):6068–6073. doi: 10.1073/pnas.111139698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menick DR, Sarkar HK, Poonian MS, Kaback HR. cys154 Is important for lac permease activity in Escherichia coli. Biochem Biophys Res Commun. 1985;132(1):162–170. doi: 10.1016/0006-291x(85)91002-2. [DOI] [PubMed] [Google Scholar]
- 41.van Iwaarden PR, Driessen AJ, Lolkema JS, Kaback HR, Konings WN. Exchange, efflux, and substrate binding by cysteine mutants of the lactose permease of Escherichia coli. Biochemistry. 1993;32(20):5419–5424. doi: 10.1021/bi00071a017. [DOI] [PubMed] [Google Scholar]
- 42.Smirnova IN, Kaback HR. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry. 2003;42(10):3025–3031. doi: 10.1021/bi027329c. [DOI] [PubMed] [Google Scholar]
- 43.Ermolova NV, Smirnova IN, Kasho VN, Kaback HR. Interhelical packing modulates conformational flexibility in the lactose permease of Escherichia coli. Biochemistry. 2005;44(21):7669–7677. doi: 10.1021/bi0502801. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Madej MG, Guan L, Nie Y, Kaback HR. 2011. An early event in the transport mechanism of LacY: Interaction between helices V and I. J Biol Chem 286(35):30415--304212.
- 45.Jung K, Jung H, Colacurcio P, Kaback HR. Role of glycine residues in the structure and function of lactose permease, an Escherichia coli membrane transport protein. Biochemistry. 1995;34(3):1030–1039. doi: 10.1021/bi00003a038. [DOI] [PubMed] [Google Scholar]
- 46.Consler TG, Tsolas O, Kaback HR. Role of proline residues in the structure and function of a membrane transport protein. Biochemistry. 1991;30(5):1291–1298. doi: 10.1021/bi00219a019. [DOI] [PubMed] [Google Scholar]
- 47.Kasho VN, Smirnova IN, Kaback HR. Sequence alignment and homology threading reveals prokaryotic and eukaryotic proteins similar to lactose permease. J Mol Biol. 2006;358(4):1060–1070. doi: 10.1016/j.jmb.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senes A, Ubarretxena-Belandia I, Engelman DM. The Calpha ---H...O hydrogen bond: A determinant of stability and specificity in transmembrane helix interactions. Proc Natl Acad Sci USA. 2001;98(16):9056–9061. doi: 10.1073/pnas.161280798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Eilers M, Patel AB, Smith SO. Helix packing moments reveal diversity and conservation in membrane protein structure. J Mol Biol. 2004;337(3):713–729. doi: 10.1016/j.jmb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Weinglass AB, Kaback HR. Conformational flexibility at the substrate binding site in the lactose permease of Escherichia coli. Proc Natl Acad Sci USA. 1999;96(20):11178–11182. doi: 10.1073/pnas.96.20.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinglass AB, Smirnova IN, Kaback HR. Engineering conformational flexibility in the lactose permease of Escherichia coli: Use of glycine-scanning mutagenesis to rescue mutant Glu325—>Asp. Biochemistry. 2001;40(3):769–776. doi: 10.1021/bi002171m. [DOI] [PubMed] [Google Scholar]
- 52.Russell RB, Eggleston DS. New roles for structure in biology and drug discovery. Nat Struct Biol. 2000;7(Suppl):928–930. doi: 10.1038/80691. [DOI] [PubMed] [Google Scholar]
- 53.Kleiger G, Grothe R, Mallick P, Eisenberg D. GXXXG and AXXXA: Common alpha-helical interaction motifs in proteins, particularly in extremophiles. Biochemistry. 2002;41(19):5990–5997. doi: 10.1021/bi0200763. [DOI] [PubMed] [Google Scholar]
- 54.Frillingos S, Sahin-Tóth M, Wu J, Kaback HR. Cys-scanning mutagenesis: A novel approach to structure function relationships in polytopic membrane proteins. FASEB J. 1998;12(13):1281–1299. doi: 10.1096/fasebj.12.13.1281. [DOI] [PubMed] [Google Scholar]
- 55.Weitzman C, Kaback HR. Cysteine scanning mutagenesis of helix V in the lactose permease of Escherichia coli. Biochemistry. 1995;34(29):9374–9379. doi: 10.1021/bi00029a013. [DOI] [PubMed] [Google Scholar]
- 56.He MM, Sun J, Kaback HR. Cysteine scanning mutagenesis of putative helix XII in the lactose permease of Escherichia coli. Biochemistry. 1996;35:12909–12914. doi: 10.1021/bi960876b. [DOI] [PubMed] [Google Scholar]
- 57.Frillingos S, Sun J, Gonzalez A, Kaback HR. Cysteine-scanning mutagenesis of helix II and flanking hydrophilic domains in the lactose permease of Escherichia coli. Biochemistry. 1997;36(1):269–273. doi: 10.1021/bi9618629. [DOI] [PubMed] [Google Scholar]
- 58.Frillingos S, Kaback HR. The role of helix VIII in the lactose permease of Escherichia coli: II. Site-directed sulfhydryl modification. Protein Sci. 1997;6(2):438–443. doi: 10.1002/pro.5560060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkatesan P, Liu Z, Hu Y, Kaback HR. Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: Helix II. Biochemistry. 2000;39:10649–10655. doi: 10.1021/bi0004394. [DOI] [PubMed] [Google Scholar]
- 60.Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- 61.Kaback HR. 1974. Transport in isolated bacterial membrane vesicles. Methods Enzymol 31(Pt A):698–709.
- 62.Kaback HR. 1971. Bacterial Membranes. Methods in Enzymol, eds Kaplan NP, Jakoby WB, & Colowick NP (Elsevier, New York), Vol XXII, pp 99–120. [Google Scholar]
- 63.Short SA, Kaback HR, Kohn LD. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975;250(11):4291–4296. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.