Abstract
Microtubules (MTs) are post-translationally modified, but the functions of post-translational modifications (PTMs) have in many cases remained unknown. Most PTMs, such as polyglutamylation, occur on the protruding C-terminal tail (CTT) of tubulins, are reversible, and have been proposed to play a role in regulation of MT-associated proteins (MAPs), molecular motors, and MT-severing proteins. Several PTM enzymes have been identified, including a carboxypeptidase in mice known as CCP1, which reduces polyglutamylation on the CTT of MTs, and causes cell-specific neurodegeneration when mutated.
Keywords: microtubules, post-translational modification, glutamylation, sensory, cilia, axoneme, male-specific, CCP (cytosolic carboxypeptidase), tubulin, TTLL (tubulin tyrosine ligase-like protein)
Introduction
Cilia are MT-based organelles that protrude from most non-dividing eukaryotic cells and perform essential sensory and motile functions. Cilia are essential for vertebrate development.4,5 Human genetic diseases that cause ciliary defects are called ciliopathies. Due to the near ubiquity of cilia on non-dividing mammalian cells, ciliopathies cause diverse and severe symptoms such as cystic kidneys, blindness, deafness and laterality defects.4
A transport system called intraflagellar transport (IFT) is necessary for both ciliogenesis and ciliary function, and is conserved from protists to humans.5 The canonical IFT machinery consists of a heterotrimeric kinesin-II of the kinesin-2 family and two conserved polypeptide complexes known as IFT-A and IFT-B, which transport cargoes along the ciliary MT cytoskeleton (the axoneme) in the anterograde direction. Ciliary dynein moves IFT-A and IFT-B complexes and cargoes in retrograde IFT.5 The axoneme serves as the track upon which IFT transport runs, but is also built by IFT.5 As virtually all cilia are built by IFT, ciliary axonemes share a basic structural plan, consisting of the typical “9 + 2” or “9 + 0” MT formations (nine outer doublets with two inner singlets, or nine outer doublets with zero inner singlets), but variations do occur.4
PTMs have often been suggested to be markers of stable MTs, although evidence that the PTMs give rise to stability is sparse.6 Most PTMs involve modification of the CTT of tubulins.6 Detyrosination (removal of the terminal tyrosine of α-tubulin), polyglutamylation (covalent attachment of glutamate side-chains), and polyglycylation (covalent attachment of glycine side-chains) are a few examples.7 Axonemal MTs in cilia are especially prone to PTMs, but the functions of ciliary PTMs are, in most cases, unclear.6
PTMs have been proposed to transmit regulatory information to guide motor traffic in a hypothesis called, “The Tubulin Code.”6-8 In this model, MAPs and molecular motors follow “signposts” comprising particular combinations of PTMs.6-8 Because most PTMs are reversible, enzymes that perform opposing functions could modulate the extent of modifications for particular cellular roles.7 For example, polyglutamylation can be added to polymerized tubulins by one enzyme, a tubulin tyrosine ligase-like (TTLL) protein, but reduced or removed by another, a deglutamylating cytosolic carboxypeptidase (CCP) enzyme.6 Therefore, PTMs could provide important regulation of IFT motors in cilia.
In O’Hagan et al.,3 we showed that ccpp-1 mutants are defective in removal of tubulin glutamylation. C. elegans ccpp-1 encodes a carboxypeptidase similar to mammalian CCP1, which functions as a deglutamylase that reduces polyglutamylation of MTs.1 Our results suggest that in ccpp-1 mutants, hyperglutamylation causes cell-specific defects. In male-specific neurons, ccpp-1 mutants have defects in axonemal MTs as well as the function and localization of particular ciliary kinesin motors. In amphid and phasmid neurons, loss of CCPP-1 also causes a progressive dye-filling (Dyf) defect that becomes severe as animals age, suggesting that cilia form but are not maintained.
The progressive Dyf defect was an exciting finding because mice that lack CCP1 exhibit late-onset degeneration of particular populations of neurons.2 We proposed that loss of CCP1 carboxypeptidase might affect nematodes and mammals similarly—causing neuronal dysfunction and ciliary loss in a cell-specific manner. This, in turn, could deprive neurons of cilia-based signal transduction that is necessary for neuronal survival. Therefore, loss of CCP1 in mammals could cause neurodegeneration by a novel form of ciliopathy.
Discussion
A tubulin deglutamylase, CCPP-1, regulates MT-based transport in male-specific sensory cilia
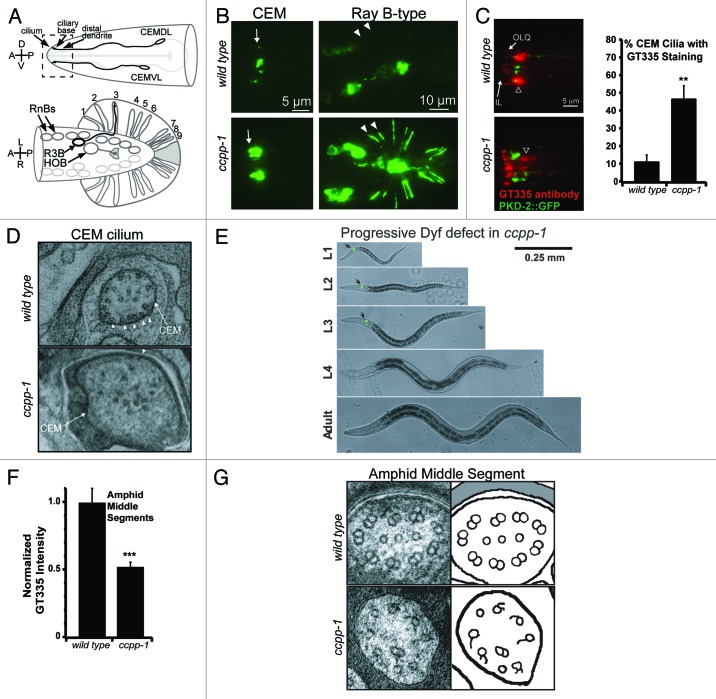
We mapped a mutation that causes defects in PKD-2::GFP localization9 and male mating behavior10,11 to the ccpp-1 locus.3 PKD-2::GFP normally localizes to cell bodies and cilia in male specific CEM, HOB, and ray B-type neurons, but ccpp-1 mutants display PKD-2::GFP accumulations in cilia and dendrites (Fig. 1A and B). GT335, an antibody that detects glutamylation, stains ciliary MTs in CEM neurons in ccpp-1 mutants more often than in wild-type males (Fig. 1C).
Figure 1. Phenotypes of ccpp-1 mutants in neuronal cilia. (A) Diagram of the male-specific PKD-2-expressing neurons; dashed box indicates region of the nose shown in (B). (B) PKD-2::GFP is localized to cell bodies and cilia of CEM and ray B-type male-specific neurons in wild-type males. In ccpp-1 mutants, PKD-2::GFP accumulates in cilia and dendrites in head and tail neurons. (C) Male stained with mAb GT335, which binds glutamylated MTs. CEM cilia are identified by PKD-2::GFP. CEM cilia in ccpp-1 mutants more frequently have detectable GT335 staining. (D) Ultrastructure of CEM cilia in wild-type and ccpp-1 adult males. Wild-type cilia had MT singlets located close to the membrane (arrowheads), while mutants had fewer MTs, which were far from the membrane. (E) ccpp-1 mutants become Dyf with age, indicating progressive structural defects. (F) GT335 staining of amphid channel middle segments (shown by arrowhead in C) was decreased in ccpp-1 mutants. (G) ccpp-1 mutants had defective amphid cilia middle segment MT ultrastructure; B-tubules of doublets were absent or defective. Reproduced from O’Hagan et al.3
We hypothesized that ccpp-1 mutations cause hyperglutamylation of axonemal MTs in CEM cilia, which might affect the function of MT-based kinesin motors. We found that KLP-6::GFP accumulates abnormally in cilia in ccpp-1 mutants. Both the localization and velocity of the IFT-B polypeptide OSM-6::GFP, a cargo of kinesin-II, appear normal in ccpp-1 mutant male-specific CEM cilia.3 Although the localization of the kinesin-2 OSM-3::GFP is normal in ccpp-1 mutant CEM cilia, the average velocity of OSM-3::GFP is abnormally rapid. The increased velocity of OSM-3 motors is particularly striking given that ccpp-1 mutant CEM cilia have fewer MTs (Fig. 1D).
CCPP-1 regulates amphid and phasmid ciliary stability in a TTLL-4-dependent manner
A functional CCPP-1::GFP reporter is expressed in the male-specific CEM, HOB, and Ray B-type neurons, as well as in amphid and IL2 ciliated sensory neurons that are present in both C. elegans males and hermaphrodites.3 As suggested by the expression of CCPP-1::GFP in amphid neurons, ccpp-1 mutants display late-onset Dyf defects (Fig. 1E), and defective osmotic avoidance behavior (Osm) that becomes more severe with age, mirroring the progression of Dyf defects. We propose that cilia may form normally in the absence of CCPP-1, but are not maintained, causing defects in cilia form and function that become more severe as animals age.
To our knowledge, ccpp-1 is the first C. elegans mutant that displays a progressive Dyf and degenerating cilia phenotype. To understand the genetic mechanism by which ccpp-1 acts, we are screening for mutants that display the progressive Dyf phenotype.
Because glutamylation is a reversible PTM, we explored the interaction between CCPP-1 and TTLL-4, an enzyme that had previously been found to be necessary for glutamylation in C. elegans neurons.12 We found that mutation of ttll-4 suppresses both the Dyf and Osm defects of ccpp-1, suggesting that CCPP-1 also reduces polyglutamylation added to amphid and phasmid ciliary MTs. However, we unexpectedly found that ccpp-1 mutant males have decreased (rather than increased) staining by the glutamylation antibody GT335 in amphid cilia middle segments (Fig. 1F). At first glance, this finding seems incompatible with our hypothesis, that CCPP-1 reduces polyglutamylation of ciliary MTs.
Ultrastructural analysis of ccpp-1 amphid channel cilia offers an explanation for the paradoxical reduction in glutamylation in amphid cilia. Compared with wild type, the ccpp-1 mutant amphid channels contain fewer cilia, with remaining cilia displaying abnormal MT doublets with absent or defective B-tubules (Fig. 1G). The B-tubules of MT doublets have previously been found to be the principal substrate of polyglutamylation in cilia.7,13-16 Therefore, the loss of cilia and B-tubules might contribute to the decreased GT335 staining in ccpp-1 mutant amphid cilia.
Cell-specific models of CCPP-1 function
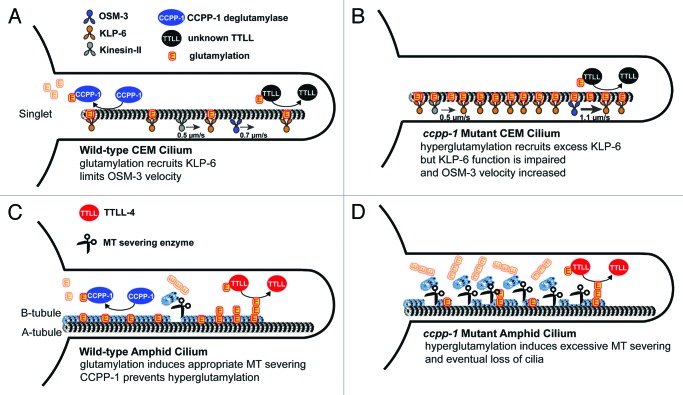
In CEM cilia, OSM-3 moves partially independently from kinesin-II and is slowed by the kinesin-3 KLP-6,17 unlike in amphid cilia middle segments.18,19 Although MT glutamylation could affect both KLP-6 and OSM-3 directly or indirectly, we propose that tubulin glutamylation recruits KLP-6 to cilia, but that KLP-6 function is impaired on hyperglutamylated MTs in ccpp-1 mutants. Because KLP-6 normally slows OSM-3 movement and is required for PKD-2 localization, both the mislocalization of the ciliary receptor PKD-2 and the increased OSM-3 velocity may be explained by dysfunction of KLP-6 on hyperglutamylated CEM ciliary MTs (see Fig. 2 A and B). Our observations also exemplify the Tubulin Code model, because a defect in a single PTM affected some, but not all, kinesins in male-specific neuronal cilia.
Figure 2. Model of cell-specific CCPP-1 deglutamylase function in CEM and amphid channel cilia. (A) Highly schematized diagram of a wild-type CEM cilium. MT singlets in CEM cilia are subject to glutamylation by an unknown enzyme, and deglutamylation by CCPP-1. Normally glutamylated MTs recruit the kinesin-3 KLP-6 and regulate velocity of the kinesin-2 OSM-3. (B) In ccpp-1 mutant hyperglutamylated state, excessive KLP-6 accumulates, perhaps in an inactive form, and OSM-3 moves abnormally fast as if unencumbered by KLP-6.17 The velocity of heterotrimeric kinesin-II is unaffected. (C) Diagram of a wild-type amphid cilium. The antagonistic activities of CCPP-1 and TTLL-4 control MT glutamylation, which in turn, regulates MT severing enzymes. (D) In the absence of CCPP-1, hyperglutamylation leads to excessive severing of B-tubules (although we depict longer glutamylation side-chains in amphids, the side-chain length is unknown in either cilium).
We propose a different model of CCPP-1 function in amphid and phasmid cilia (Fig. 2C and D). In the wild type, a balance of polyglutamylation and deglutamylation regulates MT turnover. While a polyglutamylating enzyme, such as TTLL-4, adds glutamate side-chains, CCPP-1 reduces the length of side-chains to regulate the stability of MTs. When side-chains get too long, microtubule-severing enzymes such as katanin or spastin might prune back MTs.20,21 In the absence of CCPP-1, unopposed activity of TTLL-4 would produce hyperglutamylated MTs, leading to constitutive severing of MT doublets and loss of amphid and phasmid cilia.
Questions for Future Study
In mice as in C. elegans, loss of the deglutamylating carboxypeptidase CCP1 causes cell-specific defects. We propose that the neurodegeneration in mice lacking functional CCP1 might represent a novel ciliopathy in which ciliary degeneration deprives cells of trophic signaling needed for cell survival. For example, defects in IFT have been shown to cause cell death of photoreceptors in Zebrafish22 and in cultured rat astrocytes,23 but a clear molecular pathway from ciliary dysfunction to cell death has not been experimentally elucidated. We hope to use C. elegans to further our understanding of the factors that contribute to the cell-specific effects, such as neurodegeneration and cell death, resulting from loss of CCPP-1.
What factors might determine the cell-specific effects of loss of CCPP-1?
We concluded in O’Hagan et al.3 that CCPP-1 is likely to function as a deglutamylating carboxypeptidase in C. elegans and to regulate ciliary structure and function in a cell-specific manner (Fig. 2). Although loss of TTLL-4 suppresses the amphid Dyf and Osm defects, it does not suppress the defective localization of PKD-2::GFP in ccpp-1 mutant male-specific neurons, suggesting that an unidentified TTLL might oppose CCPP-1 in the CEMs. In other organisms, various TTLL enzymes have tubulin substrate preferences and create distinct polyglutamylation patterns.6 Particular cells might express only a subset of TTLL proteins, which would create specific polyglutamylation patterns on MTs, which might underlie the cell-specific defects seen in ccpp-1 mutants in both mice and C. elegans.
In the Tubulin Code model, PTMs work combinatorially to regulate multiple tubulins, MAPs, and motors. Multicellular organisms such as humans and C. elegans express a diversity of α- and β-tubulins, even the tubulins in specific populations of neurons and their cilia could be quite different.24 Since there are a multitude of tubulins, PTMs, motors, MAPs, and MT-severing enzymes in multicellular organisms, there is much yet to be discovered before we can develop a thorough understanding of how these molecules all function in combination. Future efforts will be directed at studying the effects of ccpp-1 on MT-severing enzymes, other MAPs, and ciliary ultrastructure throughout development.
C. elegans is a good model for understanding the molecular underpinnings of ciliary diversity
The cell-specific defects in ccpp-1 mutants highlight the fact that although virtually all cilia are built by a conserved IFT mechanism and the structure of axonemal MTs is conserved, cilia in metazoans are extremely diverse in form and function.25 Human cilia, such as those that mediate olfaction, the flagella of sperm, and the cilia that mediate phototransduction on the rods and cones of the retina, all display great differences in shape and function.4 Unlike single-celled flagellated protists (which have led to advances in our understanding of cilia in general), C. elegans is a multicellular animal with highly diverse cilia. We suggest that C. elegans will contribute greatly to our understanding of the molecules that allow cilia to become specialized in shape and function for specific tasks.
CCPP-1 may define a molecular link between processes of neurodegeneration and regeneration
CCP1 had previously been identified as a gene induced by sciatic nerve injury in rodents.26 Expression of CCP1 was reported to be associated with re-growth of damaged neurons. Interestingly, CCPP-1 was recently found to be necessary for normal re-growth of axotomized PLM neurons in C. elegans.27 Regulation of polyglutamylation might be important for MT dynamics in maintenance of cilia and neurons as well as regeneration after damage. Therefore, exciting discoveries remain to be made regarding the important roles CCPP-1 plays in neuronal and ciliary integrity.
Acknowledgments
We thank our collaborators on this project: Peter Swoboda, Brian P. Piasecki, Malan Silva, Prasad Phirke, Ken C.Q. Nguyen, and David H. Hall. R.O. is supported by NJCSCR Fellowship 10-2951-SCR-E-0. Work in the Barr laboratory is supported by NIH DK074746 and NIH DK059418.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/19539
References
- 1.Rogowski K, van Dijk J, Magiera MM, Bosc C, Deloulme JC, Bosson A, et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell. 2010;143:564–78. doi: 10.1016/j.cell.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, et al. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science. 2002;295:1904–6. doi: 10.1126/science.1068912. [DOI] [PubMed] [Google Scholar]
- 3.O’Hagan R, Piasecki BP, Silva M, Phirke P, Nguyen KC, Hall DH, et al. The tubulin deglutamylase CCPP-1 regulates the function and stability of sensory cilia in C. elegans. Curr Biol. 2011;21:1685–94. doi: 10.1016/j.cub.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–93. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 5.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–9. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–86. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 7.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–60. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 8.Bulinski JC, Gundersen GG. Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays. 1991;13:285–93. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- 9.Bae YK, Lyman-Gingerich J, Barr MM, Knobel KM. Identification of genes involved in the ciliary trafficking of C. elegans PKD-2. Dev Dyn. 2008;237:2021–9. doi: 10.1002/dvdy.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 11.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–9. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 12.Kimura Y, Kurabe N, Ikegami K, Tsutsumi K, Konishi Y, Kaplan OI, et al. Identification of tubulin deglutamylase among Caenorhabditis elegans and mammalian cytosolic carboxypeptidases (CCPs) J Biol Chem. 2010;285:22936–41. doi: 10.1074/jbc.C110.128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechtreck KF, Geimer S. Distribution of polyglutamylated tubulin in the flagellar apparatus of green flagellates. Cell Motil Cytoskeleton. 2000;47:219–35. doi: 10.1002/1097-0169(200011)47:3<219::AID-CM5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Suryavanshi S, Eddé B, Fox LA, Guerrero S, Hard R, Hennessey T, et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr Biol. 2010;20:435–40. doi: 10.1016/j.cub.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–5. doi: 10.1016/j.cub.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 16.Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123:3447–55. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morsci NS, Barr MM. Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of Caenorhabditis elegans. Curr Biol. 2011;21:1239–44. doi: 10.1016/j.cub.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–13. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- 19.Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–7. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 20.Sharma N, Bryant J, Wloga D, Donaldson R, Davis RC, Jerka-Dziadosz M, et al. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J Cell Biol. 2007;178:1065–79. doi: 10.1083/jcb.200704021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, et al. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol. 2010;189:945–54. doi: 10.1083/jcb.201001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–16. doi: 10.1016/S0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura K, Kawate T, Takeda S. Signaling through the primary cilium affects glial cell survival under a stressed environment. Glia. 2011;59:333–44. doi: 10.1002/glia.21105. [DOI] [PubMed] [Google Scholar]
- 24.Hurd DD, Miller RM, Núñez L, Portman DS. Specific alpha- and beta-tubulin isotypes optimize the functions of sensory Cilia in Caenorhabditis elegans. Genetics. 2010;185:883–96. doi: 10.1534/genetics.110.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–16. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Harris A, Morgan JI, Pecot M, Soumare A, Osborne A, Soares HD. Regenerating motor neurons express Nna1, a novel ATP/GTP-binding protein related to zinc carboxypeptidases. Mol Cell Neurosci. 2000;16:578–96. doi: 10.1006/mcne.2000.0900. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Wang Z, Ghosh-Roy A, Hubert T, Yan D, O’Rourke S, et al. Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron. 2011;71:1043–57. doi: 10.1016/j.neuron.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]