Abstract
We recently published work demonstrating that ROS (reactive oxygen species) generated by the dual oxidase, Ce-Duox1/BLI-3, in response to infection in Caenorhabditis elegans activates the transcription factor SKN-1, initiating a protective response. Moreover, we showed that the crucial innate immune pathway, p38 MAPK signaling, was responsible for relaying the activating signal. In this commentary, we speculate on the signaling pathway upstream of Ce-Duox1/BLI-3 that triggers its activity. Specifically, we hypothesize that a G-protein signaling pathway comprising Gαq - PLCβ - TPA-1 - DKF-2 activates Ce-Duox1/BLI-3. Our rationale is based on work showing that these components are connected to p38 MAPK signaling and innate immunity in the worm, and investigations in other organisms demonstrating that some of these components are involved in dual oxidase activation.
Keywords: C. elegans, Reactive oxygen species, dual oxidase, innate immunity, signaling
Coordinated regulation of immunity is not only crucial for fighting invading pathogens, but also vital for safeguarding host tissues from injury due to excessive reactions. Unchecked immune responses can lead to tissue damage, disease and sometimes death of the host. An example of an immune response that can potentially harm host tissue is the purposeful generation of reactive oxygen species (ROS) by NADPH oxidase enzymes.
The first characterized NADPH oxidase was the phagocytic gp91phox/Nox2. It produces ROS in the phagolysozomes of neutrophils and other immune cells, contributing to the destruction of invading microbes.1,2 The robust consumption of molecular oxygen and the generation of superoxide anions by this enzyme was termed the oxidative burst. Six other homologs of Nox2 (Nox1, Nox3, Nox4, Nox5, Duox1 and Duox2) were subsequently identified in the human genome and found to be expressed in a wide range of tissues, giving rise to the Nox/Duox (NADPH oxidase/dual oxidase) family of proteins. All members of this family of proteins retain a catalytic C-terminal domain comprising NADPH and FAD binding sites and two, membrane-bound heme moieties. In addition to the C-terminal catalytic domain, calcium binding EF hand motifs are found in dual oxidases and Nox5. Dual oxidases also possess an N-terminal heme-containing peroxidase domain. Nox and Duox enzymes occur in plants, algae, fungi, amoeba, nematodes, echinoderms, urochordates, insects, fish, reptiles, birds and mammals, but are absent in prokaryotes.1 Therefore, a variety of systems can be used to study these proteins.
The genome of C. elegans contains two genes that encode for dual oxidases, Ce-Duox1/BLI-3 and Ce-Duox2, but lacks genes encoding for Nox enzymes.3 However, Ce-Duox2 may be a pseudo-gene because it does not appear to be expressed, and a deletion mutant has no phenotype.4,5 In initial studies, a functional role for Ce-Duox1/BLI-3 in the biogenesis of the cuticle was described, and it was localized to the hypodermis.3 Ce-Duox1/BLI-3 was postulated to generate hydrogen peroxide by the C-terminal catalytic domain for use by the N-terminal perioxidase domain. Specifically, the model proposes that the peroxidase domain uses hydrogen peroxide as an electron donor to generate radical tyrosine molecules that react with one another, creating protein crosslinks that stabilize and strengthen the cuticle. Consistent with this model, loss of Ce-Duox1/BLI-3 results in blistering and bubbling of the cuticle.3
Another role for Ce-Duox1/BLI-3, which others and we have established, is the protective generation of ROS in the intestine in response to infection.4,6 Though overall this response was beneficial, there was evidence that the elevated levels of host-generated ROS caused cellular damage.7,8 We speculated that to maintain redox homeostasis during infection, the worm might simultaneously engage oxidative stress response programs, such as the phase II detoxification response, to scavenge free radicals and other reactive molecules using glutathione.9 Phase II detoxification of xenobiotic and chemically induced oxidative stress in C. elegans has been extensively studied and shown to be regulated by SKN-1, which is distantly related to the human Nuclear factor erythroid related factor (Nrf).10-12 In mice, Nrf2 mediated regulation of redox status was shown to modulate immune responses during inflammation.13 However, a role for SKN-1 in responding to infection in the worm had not been demonstrated. Additionally, a link between ROS produced by NOX/DUOX enzymes and the activation of the Nrf2/SKN-1 family of transcription factors had not been established in any organism.
In van der Hoeven et al., we demonstrated, using a variety of techniques, that SKN-1 is activated in the intestine of the worm in response to the human pathogens Enterococcus faecalis and Pseudomonas aeruginosa.9 For example, we observed increased transcription of several SKN-1 dependent genes, such as gcs-1, gst-4, gst-5, gst-7 and gst-10, by qRT-PCR and promoter fusions to gfp, in some cases. Moreover, we established that ROS produced by Ce-Duox1/BLI-3 activates SKN-1 through the p38 MAPK signaling pathway, similarly to that previously shown for chemically induced oxidative stress.14 The p38 MAPK signaling pathway is central to C. elegans innate immune response and is comprised of the Toll/IL-1 receptor domain protein, TIR-1, the MAPKKK, NSY-1, the MAPKK, SEK-1, and the MAPK, PMK-1.15,16 Our analysis of the p38 MAPK pathway revealed that components NSY-1, SEK-1 and PMK-1 are required for activation of SKN-1, while TIR-1, which is essential in responding to pathogens, is not required.9 Finally and most importantly, we showed that SKN-1 positively impacts survival during infection. Loss of skn-1 decreased resistance to the pathogens, whereas overexpression resulted in enhanced survival. Overall, SKN-1 is activated via signaling through the p38 MAPK pathway in response to the oxidative burst generated by Ce-Duox1/BLI-3 during infection of the worm intestine.9
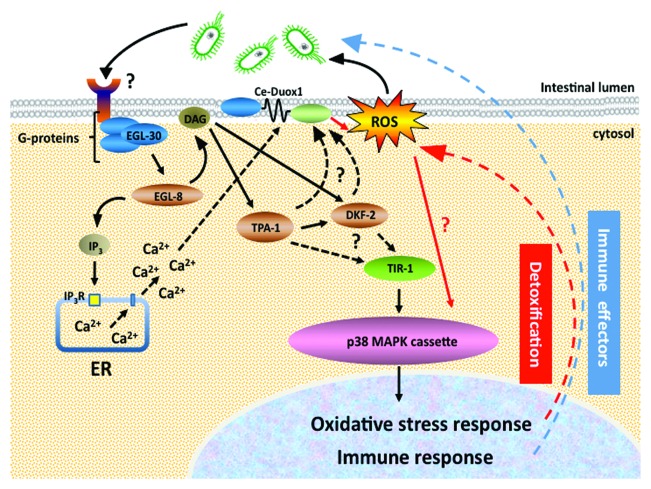
Currently, we are focused on understanding how Ce-Duox1/BLI-3 is triggered to produce ROS in response to pathogen. Interestingly, the p38 MAPK pathway was shown to upregulate DUOX gene expression in Drosophila.17 However by western analysis, we see no increase in Ce-Duox1/BLI-3 protein levels in infected, as compared with uninfected, C. elegans (Garsin lab, unpublished data), leading us to speculate that activation of Ce-Duox1/BLI-3 happens post-translationally. Genetic evidence in Drosophila has shown the Gαq-phospholipase Cβ (PLCβ) pathway utilizes secondary messengers, Inositol triphosphate (Ins(1,4,5)P3) and Ca2+, to regulate DUOX activity.18 It was proposed that a microbe-derived ligand triggers a G-protein coupled receptor, leading to the release of Gαq, which subsequently activates PLCβ to hydrolyze phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) into inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol (DAG). Next, Ins(1,4,5)P3 binds to the Ins(1,4,5)P3 receptor (Ins(1,4,5)P3R), located on the endoplasmic reticulum (ER) membrane, causing the induction and release of intracellular calcium. The released calcium is thought to bind to the EF hands, modulating the activity of DUOX.17,18 In another study using mammalian cell lines derived from the intestinal epithelium, Duox activity was shown to be dependent on intracellular calcium levels and to be regulated by protein kinase C and protein kinase A.19 DAG released by the hydrolysis of PtdIns(4,5)P2 binds to protein kinase C, while protein kinase A is activated by the binding of cAMP synthesized by adenyl cyclase. The study highlights that phosphorylation of Duox at specific serine residues enhances its sensitivity to calcium, further modulating its activity.19 Boots et al. demonstrated activation of Duox1 in immortalized human bronchial epithelial (HBE1) cells by extracellular ATP and purinergic receptor stimulation.20 Based on this information, we postulate that these components also may regulate Ce-Duox1/BLI-3.
Though no evidence currently supports a role for the above-mentioned components in ROS generation in the worm, many do appear to affect susceptibility to pathogen, as would be predicted if they did regulate Ce-Duox1/BLI-3. For example, work by Kawli et al. showed that Gαq (EGL-30) and PLCβ (EGL-8), in C. elegans, regulated both pathogen immune responses and oxidative stress responses through the p38 MAPK pathway.21 DAG, released by PLCβ hydrolysis of membrane lipids, also regulates p38 MAPK activity in the intestine. DAG can additionally interact with C1 domain containing proteins TPA-1 (protein kinase Cδ) and DKF2 (protein kinase D2), which are expressed in the C. elegans intestine.22-24 Interestingly, the TPA-1 - DKF2 module induces the production of immune effectors and the oxidative stress response, via the p38 MAPK pathway. Furthermore, worms lacking DFK-2 and TPA-1 were hypersensitive to killing by pathogenic bacteria.23 Taken together, there is good evidence that the Gαq - PLCβ - TPA-1 - DKF-2 pathway activates the p38 MAPK pathway by DAG signaling. Considering that we have established ROS generated by Ce-Duox1 works through the p38 MAPK pathway to activate the SKN-1-dependent oxidative stress response, it is plausible that a Gαq - PLCβ - TPA-1 - DKF-2 pathway could regulate Ce-Duox1/BLI-3 activity in the worm, and that the ROS generated by BLI-3 acts as a signaling molecule to link the upstream G-protein signaling pathway to the downstream p38 MAPK signaling module (Fig. 1). Validating this hypothesis would enable us to explore the possibility of identifying G-protein coupled receptors that regulate this response by screening for differences in pathogen-triggered ROS production.
Figure 1. Hypothetical model depicting the activation of Ce-Duox1/BLI-3 and the p38 MAPK pathway in C. elegans during infection.
In conclusion, we have established a connection between ROS produced by Ce-Duox1/BLI-3 and the activation of the oxidative stress transcription factor SKN-1, in a p38 MAPK dependent manner, during C. elegans infection. The next challenge is to identify the regulatory network that controls Ce-Duox1/BLI-3 activity in response to pathogen invasion.
Acknowledgments
This work was supported by the National Institutes of Health grant R01AI076406 to D.A.G.
Glossary
Abbreviations:
- ROS
reactive oxygen species
- NOX
NADPH oxidase
- DUOX
dual oxidase
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/19767
References
- 1.Aguirre J, Lambeth JD. Nox enzymes from fungus to fly to fish and what they tell us about Nox function in mammals. Free Radic Biol Med. 2010;49:1342–53. doi: 10.1016/j.freeradbiomed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol. 2001;154:879–91. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavez V, Mohri-Shiomi A, Garsin DA. Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infect Immun. 2009;77:4983–9. doi: 10.1128/IAI.00627-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill AA, Hunter CP, Tsung BT, Tucker-Kellogg G, Brown EL. Genomic analysis of gene expression in C. elegans. Science. 2000;290:809–12. doi: 10.1126/science.290.5492.809. [DOI] [PubMed] [Google Scholar]
- 6.Jain C, Yun M, Politz SM, Rao RP. A pathogenesis assay using Saccharomyces cerevisiae and Caenorhabditis elegans reveals novel roles for yeast AP-1, Yap1, and host dual oxidase BLI-3 in fungal pathogenesis. Eukaryot Cell. 2009;8:1218–27. doi: 10.1128/EC.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chávez V, Mohri-Shiomi A, Maadani A, Vega LA, Garsin DA. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics. 2007;176:1567–77. doi: 10.1534/genetics.107.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohri-Shiomi A, Garsin DA. Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. J Biol Chem. 2008;283:194–201. doi: 10.1074/jbc.M707956200. [DOI] [PubMed] [Google Scholar]
- 9.van der Hoeven R, McCallum KC, Cruz MR, Garsin DA. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 2011;7:e1002453. doi: 10.1371/journal.ppat.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–93. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, et al. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci U S A. 2005;102:16275–80. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–38. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 2010;244:43–56. doi: 10.1016/j.taap.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–83. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297:623–6. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- 16.Liberati NT, Fitzgerald KA, Kim DH, Feinbaum R, Golenbock DT, Ausubel FM. Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc Natl Acad Sci U S A. 2004;101:6593–8. doi: 10.1073/pnas.0308625101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, et al. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat Immunol. 2009;10:949–57. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- 18.Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, et al. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009;16:386–97. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Rigutto S, Hoste C, Grasberger H, Milenkovic M, Communi D, Dumont JE, et al. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J Biol Chem. 2009;284:6725–34. doi: 10.1074/jbc.M806893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A, van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem. 2009;284:17858–67. doi: 10.1074/jbc.M809761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawli T, Wu C, Tan MW. Systemic and cell intrinsic roles of Gqalpha signaling in the regulation of innate immunity, oxidative stress, and longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107:13788–93. doi: 10.1073/pnas.0914715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng H, Ren M, Chen L, Rubin CS. Properties, regulation, and in vivo functions of a novel protein kinase D: Caenorhabditis elegans DKF-2 links diacylglycerol second messenger to the regulation of stress responses and life span. J Biol Chem. 2007;282:31273–88. doi: 10.1074/jbc.M701532200. [DOI] [PubMed] [Google Scholar]
- 23.Ren M, Feng H, Fu Y, Land M, Rubin CS. Protein kinase D is an essential regulator of C. elegans innate immunity. Immunity. 2009;30:521–32. doi: 10.1016/j.immuni.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler K, Kurz CL, Cypowyj S, Couillault C, Pophillat M, Pujol N, et al. Antifungal innate immunity in C. elegans: PKCdelta links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe. 2009;5:341–52. doi: 10.1016/j.chom.2009.03.006. [DOI] [PubMed] [Google Scholar]