Abstract
Necrosis, one of the two main types of cell death, contributes critically in many devastating pathological conditions in human, including stroke, ischemia, trauma and neurodegenerative diseases. However, unlike apoptosis, the molecular mechanisms underlying necrotic cell death and neurodegeneration are poorly understood. Caenorhabditis elegans offers a powerful platform for a thorough and systematic dissection of the molecular basis of necrotic cell death. Similarly to humans, neuronal necrosis can be induced by several well-characterized genetic lesions and by adverse environmental conditions in the nematode. The availability of precisely-defined C. elegans neurodegeneration models provides a unique opportunity for comprehensive delineation of the cellular and molecular mechanisms mediating necrotic cell death. Through genetic dissection of such models, we recently uncovered an unexpected requirement for specific proteins involved in endocytosis and intracellular trafficking, in the execution of necrosis. Moreover, initiation of necrotic cell death is accompanied by a sharp increase in the formation of early and recycling endosomes, which subsequently disintegrate during the final stage of cell death. These findings implicate endocytic and intracellular trafficking processes in the cellular destruction during necrosis. Indeed, endocytosis synergizes with two other essential cellular processes, autophagy and lysosomal proteolysis to facilitate necrotic neurodegeneration. In this commentary, we consider the contribution of endocytosis and intracellular trafficking to cell injury and discuss the crosstalk between these processes and other molecular mechanisms that mediate necrosis.
Keywords: autophagy, C. elegans, calcium homeostasis, calpain, clathrin, excitotoxicity, lysosomal proteolysis, necrosis
Introduction
Cell death is a major biological process that plays pivotal roles in normal development, and homeostasis but, when either inappropriately implemented or blocked, contributes to severe pathological conditions. Cells may die by genetically defined programmed cell death (apoptosis), an active process that involves signaling pathways and ordered cellular dismantling. Cells may also die by necrosis upon traumatic injury. Cell death mechanisms may be invoked during development to redistribute cellular space or contents, or during pathogenesis to isolate and remove infected cells. For example, apoptosis in animals is involved in developmental processes such as the sculpting of organs during embryogenesis, and in adults during normal tissue turnover and as part of the immune response. Both necrotic and apoptotic cell death have been intimately associated with devastating human pathologies including ischemic, infectious, neurodegenerative and autoimmune diseases. However, unlike apoptosis, which has been studied in great detail and involves a highly regulated network of biochemical events and cascades, our knowledge of necrotic/non-apoptotic cell death mechanisms is limited.1-3 In humans, necrotic cell death generally occurs in response to severe changes of physiological conditions such as hypoxia and ischemia following stroke, hypoglycemia, trauma and epilepsy. Neurodegenerative syndromes and aging-associated diseases including Alzheimer, Huntington, Parkinson and amyotrophic lateral sclerosis (ALS) also involve necrosis.4 Exposure to toxins, reactive oxygen metabolites and extreme temperature changes can also induce necrosis. Focal necrosis, due to oxygen and nutrient deprivation, is often seen at the center of tumor nodules.5 It is therefore striking that, despite the profound impact of necrotic/non-apoptotic cell death on human health, the molecular events that transpire during cellular necrosis still remain vague. One reason has been the lack of tractable genetic models of necrosis similar to those that accelerated research on apoptosis.
We have developed and extensively characterized genetic models of necrotic cell death and neurodegeneration in the nematode Caenorhabditis elegans. The establishment of such models provides the unique opportunity to utilize a genetically tractable organism such as C. elegans to dissect the process of necrotic cell death at the molecular level. The advantages of using C. elegans as a model for understanding the basis of necrosis and neurodegeneration have been widely recognized.6,7 The nematode provides the capacity for a holistic and systematic attack on the basic biochemistry of necrotic cell death that is not feasible in higher model organisms. The precise anatomical arrangement of the 959 somatic cells of the animal, the complete cell lineage, a simple nervous system with only 302 neurons and a known wiring diagram coupled with the ability to obtain viable nervous system mutants are only some of the features that render C. elegans uniquely suitable for dissecting the molecular mechanisms of cell death. Various cellular insults, including hyperactivation of ion channels, expression of the human β-amyloid or α-synuclein proteins implicated in Alzheimer and Parkinson disease respectively, constitutive activation of certain G proteins and the aging process itself can trigger necrosis in C. elegans.8 By utilizing these versatile worm models of necrosis, we have recently found that endocytosis and intracellular trafficking contribute to cellular destruction during necrotic cell death and neurodegeneration.9
Implicating Endocytosis and Intracellular Trafficking in Necrotic Neurodegeneration
The first evidence pointing to the involvement of the endocytotic mechanisms in the execution of necrotic cell death came from early electron microscopy studies with dying nematode neurons expressing the hyperactive, cytotoxic form of the MEC-4 degenerin ion channel subunit.10 This analysis revealed that the first detectable abnormality of the dying neuron is the formation of small electron-dense whorls adjacent to the plasma membrane. These whorls are subsequently internalized, forming large multi-layered membranous structures. Such ultrastructural features suggest that neurodegeneration may involve enhanced endocytosis at the plasma membrane and increased intracellular trafficking of membrane components and vesicles. Notably, similar structures have also been detected in mouse models for degenerative conditions, such as the Motor Neuron Degeneration (MND) mouse (for neuronal ceroid lipofuscinosis see refs. 11 and 12), and the WOBBLER mouse (for amyotrophic lateral sclerosis see ref. 13).
Accumulating, circumstantial indications further implicate disrupted intracellular trafficking in neurodegenerative diseases such as ALS, and the Alzheimer or Huntington diseases. For example, mutations in proteins such as SOD1 (Cu/Zn superoxide dismutase-1) in ALS patients, affect intracellular trafficking along neuronal axons.14 In Alzheimer disease patients, neurofibrillary tangles and amyloid plaques containing β-amyloid aggregates (Aβ), disrupt intracellular transport of various cargoes such as mitochondria.15-17 Conversely, elimination of the light chain of kinesin 1 impairs intracellular trafficking toward synapse, including the transport of the amyloid protein precursor (APP), increasing β-amyloid production,18 which may exacerbate development of the disease. Moreover, in pyramidal neurons of Alzheimer patients, early endosomes appear to be up to 32 times larger than endosomes in neurons of healthy individuals. Enlarged endosomes have a morphology that is similar to endosomes in cells with increased levels of endocytosis. This intriguing finding indicates a substantial increase in endocytotic activity in neurons of Alzheimer disease patients.19,20 Consistent with these observations, the endocytic protein endophilin I is enriched in their brains of mouse models of Alzheimer disease.21 Finally, genome-wide screens for modifiers of Aβ toxicity in yeast identified several genes encoding endocytotic factors. These factors interfered with Aβ toxicity both in glutamatergic neurons of C. elegans and in primary rat cortical neurons.22
In Drosophila and mammalian neuron models of Huntington disease, expression of mutant huntingtin blocks intracellular trafficking. This effect likely originates from the association of huntingtin with the light chain of kinesin 1 (KLC1)23 and the p150Glued subunit of dynactin.24 Combined, the above accruing evidence suggests a link between endocytosis and intracellular trafficking defects and the development of neurodegenerative disorders. However, these findings do not establish a clear requirement or a causative role of these processes in cellular pathology associated with neurodegenerative disorders. To directly address the role of endocytosis and intracellular trafficking in neurodegeneration, we examined the involvement of key components of these processes in necrotic cell death.
Requirement for Clathrin-Mediated Endocytosis and Intracellular Trafficking in Necrotic Neurodegeneration
Both endocytosis and intracellular trafficking are essential cellular processes. Clathrin-mediated endocytosis (CME), the major route for endocytosis in most cells including neurons, requires the coordinated function of several different proteins and is orchestrated in five steps: nucleation, cargo selection, clathrin coat assembly, vesicle scission and uncoating/clathrin component recycling. Neurons are particularly dependent on both endocytosis and trafficking, owing to their highly differentiated/elongated morphology and specialized physiology.25 Most proteins, including ion channels, neurotransmitters and components of synaptic vesicles are synthesized in the soma and then transferred to their final destination by motor proteins, such as kinesin and dynein. The travel distance is longer than in any other cell type (e.g., more than 1 m for human motor neurons). In addition, the process of neurotransmission relies on endocytosis for the recycling of synaptic vesicle components after neurotransmitter release, and the maintenance of synaptic vesicle pools. Therefore, neurons are particularly sensitive to perturbations of endocytosis and intracellular trafficking.
We reasoned that if endocytosis is required for necrosis, then depletion of key proteins mediating different endocytosis steps may interfere with neurodegeneration triggered by various necrotic insults. Indeed, we found that downregulation or depletion of three important endocytosis factors, synaptotagmin, endophilin and dynamin, significantly ameliorates necrosis induced both by genetic and environmental stimuli. Moreover, dysfunction of kinesin motor proteins involved in intracellular trafficking similarly protects neurons against necrotic death.9 Consistent with these findings, suppression of synaptotagmin reduces brain damage in neonatal rats after ischemic insults.26 Therefore, endocytosis plays an evolutionarily conserved role in cellular destruction during necrosis in organisms as diverse as nematodes and mammals. This suggests that interfering with induction of endocytosis may reduce cell damage during acute neurodegenerative episodes such as ischemia, epilepsy and stroke.
Upregulation of Endocytosis During Neurodegeneration
In addition to being required for neurodegeneration, endocytosis becomes transiently upregulated during cell death. We find that early endosome formation is significantly induced upon triggering neurodegeneration, in C. elegans.9 What is the molecular basis of early endosome increase during the initial phase of necrosis? A common denominator of necrotic cell death inflicted by multiple insults is a sharp increase of intracellular Ca2+ concentration.3 For example, neuronal hyperexcitation observed in neurodegenerative disorders such as ischemia, epilepsy, stroke, trauma and Alzheimer disease results in massive Ca2+ influx. In turn, excessive calcium elevation may signal aberrant induction of endocytosis by promoting oligomerization of clathrin and the synthesis of PI(4,5)P2, which mediates formation of the clathrin lattice.27,28 Indeed, calcium modulates both the assembly and disassembly of clathrin adaptor complexes, such as AP2, that are necessary for the formation of the clathrin coat.29 Elevated cytoplasmic Ca2+ level also causes neurotransmitter exocytosis, followed by clathrin-mediated endocytosis of synaptic vesicle proteins. In addition, the GTPase dynamin interacts with calcineurin in a calcium-dependent manner30 and the disruption of this interaction can block endocytosis. Endocytosis is also regulated by suppressive phosphorylation events.31 Phosphorylation of dynamin and synaptojanin blocks their binding to amphiphysin, whereas phosphorylation of amphiphysin blocks its association with AP2 and clathrin. By contrast, Ca2+-dependent dephosphorylation induces endocytosis. Ca2+ ions are also known to bind clathrin light chain, stimulating the formation of clathrin triskelia.27,32 Ca2+ is also important for processes downstream of endosome formation. For example, Ca2+ binds to calmodulin, which interacts with EEA1 (early endosome antigen 1) and induces the fusion of early endosomes.33 Moreover, calcium regulates fusion between late endosomes and lysosomes and re-formation of lysosomes from hybrid organelles.34 Thus, deregulation of calcium homeostasis during neurodegeneration may underlie the transient increase in endocytic activity observed early during cell death. Calcium fluctuations also impact intracellular trafficking. Low calcium levels induce transport of cargoes from organelles, such as Golgi, whereas too high Ca2+ concentration (> 100 nM) blocks transport.35
Crosstalk Between Molecular Mechanisms Mediating Necrotic Cell Death
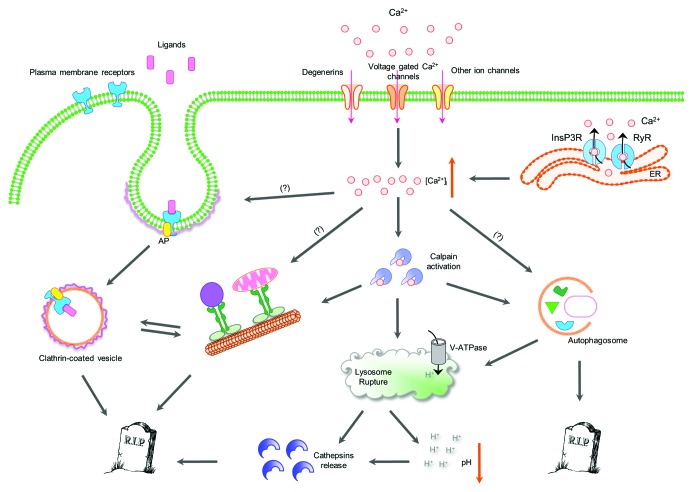
Several previous studies have identified molecular mechanisms and specific proteins that are involved in the destruction of the cell during necrosis.1 As noted above, one of the early events in the necrotic cascade is an increase in intracellular Ca2+ concentration, either because of Ca2+ influx through plasma membrane channels or due to Ca2+ efflux from intracellular store sites (endoplasmic reticulum, Golgi, mitochondria). Ca2+ elevation leads to the activation of cytoplasmic calpain proteases that subsequently attack lysosomal membrane proteins, compromising lysosome integrity. In turn, this results in the release of lysosomal hydrolytic cathepsin proteases, and concomitant acidification of the cytoplasm.36-39 Moreover, autophagy is induced and contributes to necrotic cell destruction.40-42
In addition to the lysosome, cathepsin proteases are also localized in both early and late endosomes.43 Interestingly, cathepsins B and D were found in early endosomes in pyramidal neurons of Alzheimer disease patients.44 Clathrin-coated vesicles lose their coat and fuse with early endosomes during the last steps of clathrin-mediated endocytosis. The V-ATPase proton pump is required for the gradual acidification of early endosomes during their maturation to late endosomes. V-ATPase also acidifies early endosomes, facilitating dissociation of endocytosed receptors from their ligands, which is necessary for receptor release and recycling. Consistently, we find that endocytosis functions in the same pathway with lysosomal proteolysis and V-ATPase acidification mechanisms to mediate neurodegeneration in C. elegans.9 Moreover, combined inactivation of endocytosis and cytoplasmic calpain proteases implicated in necrosis revealed that they also contribute in the same cell destruction pathway (Fig. 1). This notion is further supported by previous studies showing that calpain activity is involved in endocytosis and/or exocytosis, activated by Ca2+-dependent membrane fusion events.45 Calpain proteases have been detected on the membrane of clathrin-coated vesicles in the bovine brain, where they bind in a Ca2+-dependent manner.45 Components of clathrin-coated vesicles, such as the clathrin light chain, amphiphysin and adaptins are calpain substrates. Indeed, calpain blocks vesicle endocytosis by cleaving the endocytotic protein amphiphysin I.46 Recently, calpain proteases were also found to cleave α- and β2-adaptins, epsin and AP180 in the brain of Alzheimer patients after ischemia.47 These observations suggest that Ca2+ signaling may interface with endocytic processes via calpain protease activity during necrotic cell death. Consistent with the role of calpain in moderating endocytosis, we observed a reduction of clathrin-coated vesicles and endosomes during late stages of necrotic cell death.9
Figure 1. Crosstalk between necrotic cell death mechanisms. Diverse necrotic insults (both genetic and environmental) converge to increase intracellular Ca2+ levels via two main routes. First, by calcium influx from extracellular pools through various plasma membrane channels, such as voltage-gated receptors or sodium/calcium exchangers (NCX). Second, by calcium efflux from subcellular organelles with substantial Ca2+ stores, such as the endoplasmic reticulum via the ryanodine (RyR) and the 1,4,5-inositol triphosphate receptors (Ins(1,4,5)P3R). Ca2+ ions then activate cytoplasmic calpain proteases that attack lysosomal membrane proteins, compromising the integrity of lysosomes and causing the release of hydrolytic enzymes, such as cathepsin proteases. Vacuolar H+ ATPase (V-ATPase) -mediated lysosomal acidification is important for subsequent acidification of the cytoplasm and enhancement of cathepsin activity, upon rupture of lysosomes. In addition, autophagy is induced during necrosis, either directly or through calpain activation and synergizes with lysosomal cathepsin proteases to facilitate cellular destruction. Moreover, both clathrin-mediated endocytosis and intracellular trafficking are required for cell death and become upregulated by necrosis-triggering insults. Thus, necrotic cell death is the outcome of synergistic contributions by otherwise essential cellular processes that become aberrantly activated. [Ca2+]i, cytoplasmic calcium; InsP3R, inositol trisphosphate receptor; RyR, ryanodine receptor; ER, endoplasmic reticulum; AP, adaptor proteins for clathrin-mediated endocytosis; V-ATPase, vacuolar H+ ATPase.
Our genetic analysis also revealed that combined inactivation of endocytosis and autophagy enhances cytoprotection against toxic necrotic insults. This result indicates that these two processes act in parallel during necrosis (Fig. 1). Recently, it was shown that autophagosome maturation requires fusion with early endosomes.48 Thus, one likely scenario is that increase in the cytoplasmic Ca2+ concentration during necrosis induces both the autophagic and endocytic pathways which subsequently converge for autophagosome maturation. Similarly, interfering with both intracellular trafficking and autophagy reveals that they synergize to protect neurons from necrotic insults. These findings expose a complex and extensive crosstalk between different subroutines that interact to contribute in cellular destruction during necrosis. Particular insults may engage specific combinations of these subroutines to elicit cell death.
Concluding Remarks
We exploited a range of well-characterized and nematode neurodegeneration models to dissect the involvement of endocytosis and intracellular trafficking in cellular destruction during necrotic death. Our findings, in their totality, reveal a novel requirement for both endocytosis and trafficking in neuron necrosis, and uncover novel aspects of the cellular changes that transpire during neurodegeneration. Downregulation of endocytosis or kinesin-mediated trafficking by interfering with key proteins regulating these processes significantly suppresses necrosis and protects neurons against degeneration. Such information could be effectively utilized toward identifying candidate common intervention targets, in an effort to battle related pathological conditions in humans. A potential caveat of implementing strategies that interfere with essential cellular processes such as endocytosis and trafficking relates to the likelihood of such manipulations causing pleiotropic adverse effects. For example, suppression of necrosis in C. elegans might be a secondary consequence, originating from non-specific metabolic alterations caused by downregulation of endocytosis and trafficking. Nevertheless, several genetic lesions that affect animal energy metabolism either directly or indirectly (for example, in genes regulating insulin/IGF-1 signaling and mitochondrial metabolism),9 do not suppress necrosis, arguing that cytoprotection is not merely the cumulative outcome of cellular dysfunction due to reduced endocytosis and intracellular trafficking.
Investigation of the cellular and molecular events that transpire during necrotic cell death in a simple animal model such as the nematode has effectively broken new ground and laid the foundation for the characterization of mechanisms underlying cellular degeneration in numerous human disorders. While substantial progress has been made toward this direction, a comprehensive understanding of all processes involved in necrosis has yet to be attained. Several important questions still remain. For example, although calcium has been recognized as a major death signaling molecule, the diverse mechanisms by which calcium triggers cellular demise are not fully known. Similarly, the identification of the enzymatic activities that enact death by mediating cellular destruction during necrosis is still incomplete. Nevertheless, analysis of necrotic cell death in simple model organisms such as C. elegans holds promise for providing significant insights toward addressing these gaps.
Acknowledgments
Work in the authors’ laboratory is funded by grants from the European Research Council (ERC), the European Commission 7th Framework Programme and the Greek Ministry of Education.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/20457
References
- 1.Kourtis N, Tavernarakis N. Non-developmentally programmed cell death in Caenorhabditis elegans. Semin Cancer Biol. 2007;17:122–33. doi: 10.1016/j.semcancer.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Syntichaki P, Tavernarakis N. Death by necrosis. Uncontrollable catastrophe, or is there order behind the chaos? EMBO Rep. 2002;3:604–9. doi: 10.1093/embo-reports/kvf138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syntichaki P, Tavernarakis N. The biochemistry of neuronal necrosis: rogue biology? Nat Rev Neurosci. 2003;4:672–84. doi: 10.1038/nrn1174. [DOI] [PubMed] [Google Scholar]
- 4.Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–80. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 5.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 6.Artal-Sanz M, de Jong L, Tavernarakis N. Caenorhabditis elegans: a versatile platform for drug discovery. Biotechnol J. 2006;1:1405–18. doi: 10.1002/biot.200600176. [DOI] [PubMed] [Google Scholar]
- 7.Markaki M, Tavernarakis N. Modeling human diseases in Caenorhabditis elegans. Biotechnol J. 2010;5:1261–76. doi: 10.1002/biot.201000183. [DOI] [PubMed] [Google Scholar]
- 8.Vlachos M, Tavernarakis N. Non-apoptotic cell death in Caenorhabditis elegans. Dev Dyn. 2010;239:1337–51. doi: 10.1002/dvdy.22230. [DOI] [PubMed] [Google Scholar]
- 9.Troulinaki K, Tavernarakis N. Endocytosis and intracellular trafficking contribute to necrotic neurodegeneration in C. elegans. EMBO J. 2011;31:654–66. doi: 10.1038/emboj.2011.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall DH, Gu G, García-Añoveros J, Gong L, Chalfie M, Driscoll M. Neuropathology of degenerative cell death in Caenorhabditis elegans. J Neurosci. 1997;17:1033–45. doi: 10.1523/JNEUROSCI.17-03-01033.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper JD, Messer A, Feng AK, Chua-Couzens J, Mobley WC. Apparent loss and hypertrophy of interneurons in a mouse model of neuronal ceroid lipofuscinosis: evidence for partial response to insulin-like growth factor-1 treatment. J Neurosci. 1999;19:2556–67. doi: 10.1523/JNEUROSCI.19-07-02556.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz ML, Johnson GS. Mouse gene knockout models for the CLN2 and CLN3 forms of ceroid lipofuscinosis. Eur J Paediatr Neurol. 2001;5(Suppl A):109–14. doi: 10.1053/ejpn.2000.0445. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt-John T, Drepper C, Mussmann A, Hahn P, Kuhlmann M, Thiel C, et al. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat Genet. 2005;37:1213–5. doi: 10.1038/ng1661. [DOI] [PubMed] [Google Scholar]
- 14.De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720–8. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiruma H, Katakura T, Takahashi S, Ichikawa T, Kawakami T. Glutamate and amyloid beta-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms. J Neurosci. 2003;23:8967–77. doi: 10.1523/JNEUROSCI.23-26-08967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–49. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 17.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–7. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–8. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 19.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–28. doi: 10.1016/0092-8674(92)90306-W. [DOI] [PubMed] [Google Scholar]
- 20.de Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, et al. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 21.Ren Y, Xu HW, Davey F, Taylor M, Aiton J, Coote P, et al. Endophilin I expression is increased in the brains of Alzheimer disease patients. J Biol Chem. 2008;283:5685–91. doi: 10.1074/jbc.M707932200. [DOI] [PubMed] [Google Scholar]
- 22.Treusch S, Hamamichi S, Goodman JL, Matlack KE, Chung CY, Baru V, et al. Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer’s disease risk factors in yeast. Science. 2011;334:1241–5. doi: 10.1126/science.1213210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–9. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- 24.Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, et al. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. 1997;6:2205–12. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- 25.Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26:373–82. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Omae T, Yoshioka H, Tanaka T, Nagai H, Saji M, Noda K, et al. Antisense in vivo knockdown of synaptotagmin I by HVJ-liposome mediated gene transfer attenuates ischemic brain damage in neonatal rats. Brain Dev. 2008;30:313–20. doi: 10.1016/j.braindev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Näthke I, Hill BL, Parham P, Brodsky FM. The calcium-binding site of clathrin light chains. J Biol Chem. 1990;265:18621–7. [PubMed] [Google Scholar]
- 28.Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, et al. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/S0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 29.Alberdi A, Jimenez-Ortiz V, Sosa MA. The calcium chelator BAPTA affects the binding of assembly protein AP-2 to membranes. Biocell. 2001;25:167–72. [PubMed] [Google Scholar]
- 30.Lai MM, Hong JJ, Ruggiero AM, Burnett PE, Slepnev VI, De Camilli P, et al. The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis. J Biol Chem. 1999;274:25963–6. doi: 10.1074/jbc.274.37.25963. [DOI] [PubMed] [Google Scholar]
- 31.Slepnev VI, Ochoa GC, Butler MH, Grabs D, De Camilli P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–4. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- 32.Mooibroek MJ, Michiel DF, Wang JH. Clathrin light chains are calcium-binding proteins. J Biol Chem. 1987;262:25–8. [PubMed] [Google Scholar]
- 33.Colombo MI, Beron W, Stahl PD. Calmodulin regulates endosome fusion. J Biol Chem. 1997;272:7707–12. doi: 10.1074/jbc.272.12.7707. [DOI] [PubMed] [Google Scholar]
- 34.Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000;149:1053–62. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen JL, Ahluwalia JP, Stamnes M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J Biol Chem. 2002;277:35682–7. doi: 10.1074/jbc.M204157200. [DOI] [PubMed] [Google Scholar]
- 36.Artal-Sanz M, Samara C, Syntichaki P, Tavernarakis N. Lysosomal biogenesis and function is critical for necrotic cell death in Caenorhabditis elegans. J Cell Biol. 2006;173:231–9. doi: 10.1083/jcb.200511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artal-Sanz M, Tavernarakis N. Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett. 2005;579:3287–96. doi: 10.1016/j.febslet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 38.Syntichaki P, Samara C, Tavernarakis N. The vacuolar H+ -ATPase mediates intracellular acidification required for neurodegeneration in C. elegans. Curr Biol. 2005;15:1249–54. doi: 10.1016/j.cub.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 39.Syntichaki P, Xu K, Driscoll M, Tavernarakis N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature. 2002;419:939–44. doi: 10.1038/nature01108. [DOI] [PubMed] [Google Scholar]
- 40.Samara C, Syntichaki P, Tavernarakis N. Autophagy is required for necrotic cell death in Caenorhabditis elegans. Cell Death Differ. 2008;15:105–12. doi: 10.1038/sj.cdd.4402231. [DOI] [PubMed] [Google Scholar]
- 41.Samara C, Tavernarakis N. Autophagy and cell death in Caenorhabditis elegans. Curr Pharm Des. 2008;14:97–115. doi: 10.2174/138161208783378770. [DOI] [PubMed] [Google Scholar]
- 42.Tóth ML, Simon P, Kovács AL, Vellai T. Influence of autophagy genes on ion-channel-dependent neuronal degeneration in Caenorhabditis elegans. J Cell Sci. 2007;120:1134–41. doi: 10.1242/jcs.03401. [DOI] [PubMed] [Google Scholar]
- 43.Stein M, Zijderhand-Bleekemolen JE, Geuze H, Hasilik A, von Figura K. Mr 46,000 mannose 6-phosphate specific receptor: its role in targeting of lysosomal enzymes. EMBO J. 1987;6:2677–81. doi: 10.1002/j.1460-2075.1987.tb02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cataldo AM, Barnett JL, Pieroni C, Nixon RA. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J Neurosci. 1997;17:6142–51. doi: 10.1523/JNEUROSCI.17-16-06142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato K, Saito Y, Kawashima S. Identification and characterization of membrane-bound calpains in clathrin-coated vesicles from bovine brain. Eur J Biochem. 1995;230:25–31. doi: 10.1111/j.1432-1033.1995.tb20529.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Liang S, Oda Y, Ohmori I, Nishiki T, Takei K, et al. Truncations of amphiphysin I by calpain inhibit vesicle endocytosis during neural hyperexcitation. EMBO J. 2007;26:2981–90. doi: 10.1038/sj.emboj.7601741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudinskiy N, Grishchuk Y, Vaslin A, Puyal J, Delacourte A, Hirling H, et al. Calpain hydrolysis of alpha- and beta2-adaptins decreases clathrin-dependent endocytosis and may promote neurodegeneration. J Biol Chem. 2009;284:12447–58. doi: 10.1074/jbc.M804740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Razi M, Chan EY, Tooze SA. Early endosomes and endosomal coatomer are required for autophagy. J Cell Biol. 2009;185:305–21. doi: 10.1083/jcb.200810098. [DOI] [PMC free article] [PubMed] [Google Scholar]