Abstract
A multicenter survey of 11 cancer centers was performed to determine the rate of hospital-onset Clostridium difficile infection (HO-CDI) and surveillance practices. Pooled rates of HO-CDI in patients with cancer were twice the rates reported for all US patients (15.8 vs 7.4 per 10,000 patient-days). Rates were elevated regardless of diagnostic test used.
In 2011, an estimated 1.6 million people underwent treatment for cancer.1 Clostridium difficile is the most common bacterial cause of healthcare-associated diarrhea in persons receiving chemotherapy for cancer. Several risk factors in this population raise the risk of C. difficile infection (CDI).2,3 In the last decade, with the emergence of BI/NAP1 strain the incidence and severity of CDI increased across North America and Europe, and the need for widespread surveillance became more important than ever.4
At Memorial Sloan Kettering Cancer Center (MSKCC), CDI rates are highest among hematopoietic stem cell transplant (HSCT) recipients and those undergoing chemotherapy for leukemia (unpublished data; Table 1). Studies from other cancer centers have also reported a higher rate of CDI compared to the general population, with strikingly high rates among allogeneic HSCT recipients.5
TABLE 1.
Cancer-Specific Rates of CDI
| Rate | Cases | Total patients | Percentage |
|---|---|---|---|
| Allogeneic HSCTa | 83 | 307 | 27 |
| Autologous HSCT | 27 | 290 | 9.0 |
| Leukemia/MDS | 53 | 426 | 12.4 |
| Lymphoma | 37 | 1,189 | 3.1 |
| Solid tumors | 317 | 22,889 | 1.4 |
NOTE. Clostridium difficile infection (CDI) rates by underlying cancer at Memorial Sloan Kettering Cancer Center, 2008–2009. HSCT, hematopoietic stem cell transplant; MDS, myelodysplastic syndrome.
Includes adult and pediatric cases.
With the advent of public reporting for CDI, comparison of rates across centers will occur and may not take into account differences in patient populations. Therefore, we sought to determine the rate of hospital-onset (HO)-CDI and surveillance practices in a population of HSCT recipients and patients with cancer. Establishment of a benchmark for this large but unique patient group will assist both infection control practitioners and concerned consumers as they compare rates across states and hospitals.
METHODS
In sum, 10 of 11 participants were members of the Comprehensive Cancer Center’s Infection Control Group (C3IC network). The participating centers included MSKCC, Fox Chase Cancer Center, Roswell Park Cancer Institute, Moffitt Cancer Center, MD Anderson Cancer Center, Barnes-Jewish Hospital, James Cancer Hospital at Ohio State University Medical Center, Dana-Farber Cancer Institute, Barbara Ann Karmanos Cancer Institute, Thornton Hospital, University of California–San Diego, and New York University Langone Medical Center. Data were collected electronically using a secure website and were considered exempt from institutional review board.
Participating centers provided specific information in response to the C. difficile surveillance questionnaire. Information submitted included (1) oncology-specific hospital characteristics, including number of oncology and bone marrow transplant (BMT) beds; (2) laboratory method of C. difficile detection—enzyme immunoassay (EIA), cytotoxin assay (CTA), or polymerase chain reaction (PCR); (3) surveillance definition for (a) HO-CDI and (b) definition of relapse versus second new infection; (4) most recent rates of HO-CDI (annual rate for 2010 or YTD rate for 2011). Rates were calculated as the number of HO-CDI cases per oncology-specific patient-days. Additional queries included information on duration of isolation practice for C. difficile cases.
RESULTS
A total of 11 centers participated in the survey. Hospital characteristics are shown in Table 2. Among the centers, the number of oncology beds ranged from 22 to 600 (median, 100 beds); HSCT beds, 6–80 (median, 26 beds). PCR was the most common detection method (6), followed by EIA (4) and CTA (1). Six centers are located in states where C. difficile is a reportable healthcare-associated infection (HAI).
TABLE 2.
Hospital Characteristics of Participating Centers
| Characteristic | Hospital
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | |
| No. of beds | |||||||||||
| Oncology | 435 | 109 | 100 | 24 | 600 | 27 | 185 | 125 | 56 | 26 | 22 |
| Transplant | 22 | 14 | 0 | 30 | 68 | 18 | 24 | 32 | 28 | 80 | 6 |
| Diagnostic test | PCR | EIA | CTA | PCR | EIA | PCR | CTA/PCRa | EIA | PCR | EIA | PCR |
| Surveillance definition HO-CDI (positive test from time of admission), hours | >48 | >48 | >48 | >72 | >48 | >72 | >72 | >72 | >48 | >72 | >72 |
| Second new infection (time from index episode in weeks) | 12 | NA | NA | 8 | 8 | 8 | 8 | 8 | 8 | 6 mo | 8 |
| Duration of isolation, days | |||||||||||
| No. of days (minimum) | 7 | ||||||||||
| Until resolution of symptoms | Yes (2 days) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Minimum treatment duration | 7 | Complete | |||||||||
| Entire hospitalization | Yes | Yes | |||||||||
NOTE. CTA, cytotoxin assay; EIA, enzyme immunoassay; HO-CDI, hospital-onset Clostridium difficile infection; NA, not available; PCR, polymerase chain reaction.
Center with recent transition from CTA to PCR; rates were reported while CTA was in use.
Rates of HO-CDI
A case of HO-CDI was defined as a positive result of a laboratory assay for C. difficile toxin A and/or B following in-patient admission. The cutoff used was >48 hours at 5 centers and >72 hours at 6 centers.
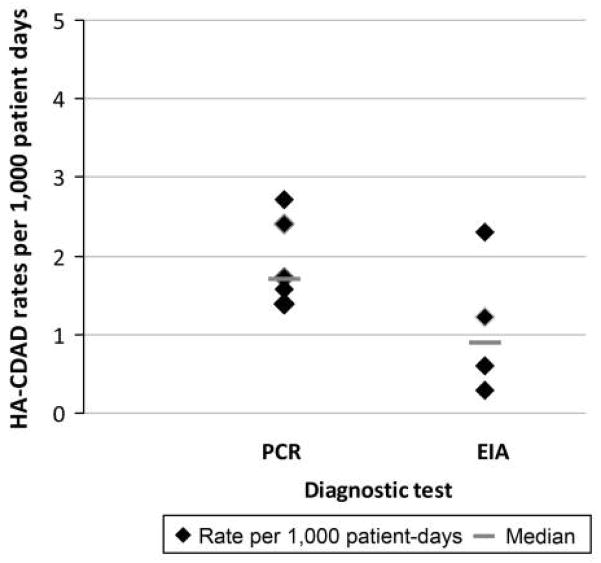
Centers using PCR as detection method had a higher median HO-CDI rate (1.72 per 1,000 patient-days) compared to EIA (0.9 per 1,000 patient-days; Figure 1). Among the centers that use PCR, the median HO-CDI rate was highest when the 48-hour cutoff from admission was used to define an HO-CDI case: 2.2 per 1,000 patient-days (more than 48 hours) and 1.57 per 1,000 patient-days (more than 72 hours).
FIGURE 1.
Hospital-onset Clostridium difficile infection rates (per 1,000 patient-days) among participating centers stratified by diagnostic test used. EIA, enzyme immunoassay; HA-CDAD, hospital-acquired C. difficile–associated diarrhea; PCR, polymerase chain reaction.
Relapse versus second new infection
Most centers followed the ad hoc C. difficile surveillance working group’s criteria for recurrent infection.6 In total, 7 of 9 centers that track recurrent cases consider an episode occurring more than 8 weeks after the index episode as a second new infection. One center uses 12 weeks as the interval and another center only considers a recurrent episode occurring at least 6 months after the index episode as second new infection.
Duration of isolation
Isolation practice for C. difficile varied widely across all centers. Two of 11 centers isolated patients with CDI for the entire duration of hospitalization. The remaining centers isolated patients until resolution of symptoms. Duration of treatment was used as criterion in addition to symptom resolution at 2 centers, each requiring at least 7 days of treatment and complete therapy in addition to resolution of diarrhea.
DISCUSSION
We found the rate of HO-CDI in a large group of cancer patients to be well above the reported rate for all US patients (New York 2010, 0.82; California 2010–2011, 0.70; Ohio 2006, 0.7–0.8 per 1,000 patient-days).7–9 The rate was elevated regardless of diagnostic test used. More recently, NHSN reported pooled hospital rate of HO-CDI of 7.4 per 10,000 patient-days. In this report 33% of centers used nucleic acid amplification test as the diagnostic assay. The pooled rate of HO-CDI in our study is more than twice the NHSN rate (15.8 per 10,000 patient-days), despite a comparable breakdown of diagnostic assays used.10
Persons with cancer are at high risk of CDI. In addition, other factors make surveillance particularly challenging in this population. First, most cancer centers have transitioned to molecular-based testing for C. difficile in contrast to low-sensitivity detection methods such as EIA.11 The proposed surveillance definitions do not take into account the higher sensitivity of the newer tests, which has been reported to increase rates of HO-CDI by 2-fold in some studies.6,12,13 Second, surveillance definitions are not universally applicable because of the frequent healthcare-related exposure during treatment for cancer, as well as likely higher frequency of testing. Finally, rates of CDI among cancer patients are likely to rise with better survival and the growing use of intensive chemotherapeutic regimens and HSCT, especially among older adults, a group at heightened risk for CDI.
Although previous reports in immunocompromised hosts have shown elevation of CDI rates, this study is novel in its examination of the rate across a large number of hospitals and patients. There are several possible explanations for this finding: CDI is a well-recognized complication of antineoplastic chemotherapy; healthcare exposure and antibiotic use (especially fluoroquinolones) may be greater in the population and for patients with HSCT; length of stay is substantially longer.
Another substantial contributor is selection of diagnostic test. EIAs for the detection of C. difficile toxins are the most widely used tests for diagnosis of CDI. The poor sensitivity of these tests is particularly problematic for control efforts. As a result, molecular-based testing methods for CDI are now approved by the Food and Drug Administration and increasingly being implemented at many places. For institutions making such a transition in states where CDI reporting is mandatory, such as Cleveland Clinic (Ohio) and MSKCC (New York), the rates of HO-CDI have doubled in the absence of epidemiologic evidence of transmission, including lack of clustering and homology by molecular typing of the isolated strains.12,13 In our study, the majority of participants used PCR for diagnosis. Despite the small numbers, the impact of diagnostic test on HO-CDI rates is apparent (Figure 1).
Our study has several limitations, including small number of participating centers from widespread geographic areas. To improve compliance our survey included information for rates only, patient-days that were not reported, and testing for statistical significance not possible.
We report several important findings. First, we found a high incidence of HO-CDI among patients undergoing treatment for cancer. Second, we demonstrate the impact of diagnostic tests on HO-CDI rates, suggesting the urgent need for standardization of methodology prior to interhospital comparison or widespread public access to HAI-related information. Finally, we demonstrate the strength of specialized infection control groups such as the C3IC network in combining data to develop HAI benchmarks for specialized populations.
As the Centers for Disease Control and Prevention and designated organizations devise surveillance strategies, it is crucial to recognize the multitude of factors that affect the occurrence of HAI in persons undergoing treatment for cancer and its impact in the context of public reporting and the looming risk of financial penalties. Targets for CDI should be based on rates derived from homogenous populations that employ similar surveillance and diagnostic strategies.
Acknowledgments
Financial support. M.K. was supported by NIH/NIAID grant K23-AI0838880.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
References
- 1.American Cancer Society. http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2011.
- 2.Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17(1):109–113. doi: 10.1093/clinids/17.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Gorschluter M, Glasmacher A, Hahn C, et al. Clostridium difficile infection in patients with neutropenia. Clin Infect Dis. 2001;33(6):786–791. doi: 10.1086/322616. [DOI] [PubMed] [Google Scholar]
- 4.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile–associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 5.Bobak D, Arfons LM, Creger RJ, Lazarus HM. Clostridium difficile–associated disease in human stem cell transplant recipients: coming epidemic or false alarm? Bone Marrow Transplantation. 2008;42(11):705–713. doi: 10.1038/bmt.2008.317. [DOI] [PubMed] [Google Scholar]
- 6.McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile–associated disease. Infect Control Hosp Epidemiol. 2007;28(2):140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 7.New York State Department of Health. http://www.health.ny.gov/statistics/facilities/hospital/hospital_acquired_infections/2010/docs/hospital_acquired_infection.pdf.
- 8.California Department of Public Health. http://www.cdph.ca.gov/programs/hai/Pages/CDI-Report.aspx.
- 9.Ohio Department of Public Health. http://www.odh.ohio.gov/~/media/ODH/ASSETS/Files/news/cdifffinalreport.ashx.
- 10.Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61:157–162. [PubMed] [Google Scholar]
- 11.Deshpande A, Pasupuleti V, Rolston DD, et al. Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected Clostridium difficile infection: a meta-analysis. Clin Infect Dis. 2011;53(7):e81–e90. doi: 10.1093/cid/cir505. [DOI] [PubMed] [Google Scholar]
- 12.Fong KS, Fatica C, Hall G, et al. Impact of PCR testing for Clostridium difficile on incident rates and potential on public reporting: is the playing field level? Infect Control Hosp Epidemiol. 2011;32(9):932–933. doi: 10.1086/661789. [DOI] [PubMed] [Google Scholar]
- 13.Kaltsas A, Simon M, Unruh LH, et al. Clinical and laboratory characteristics of Clostridium difficile infection in patients with discordant diagnostic test results. J Clinical Microbiol. 2012;50(4):1303–1307. doi: 10.1128/JCM.05711-11. [DOI] [PMC free article] [PubMed] [Google Scholar]