Abstract
Chemokine-like receptor 1 (CMKLR1) ligands chemerin and resolvin E1 are suggested to have a role in non-alcoholic fatty liver disease (NAFLD). Here, expression of CMKLR1 in liver cells and NAFLD was studied. CMKLR1 was detected in primary human hepatocytes (PHH), Kupffer cells, bile-duct cells and hepatic stellate cells. In human and rodent fatty liver and in fibrotic liver of mice fed a methionine–choline deficient diet CMKLR1 was reduced. Hepatocytes are the major cells in the liver and effects of adipokines, cytokines and lipids on CMKLR1 in PHH were analyzed. Increased cellular triglyceride or cholesterol content, lipopolysaccharide, IL-6, TNF and leptin did not influence CMKLR1 levels in PHH whereas profibrotic TGFβ tended to reduce CMKLR1. Adiponectin strongly upregulated CMKLR1 mRNA and protein in PHH and hepatic CMKLR1 when injected into wild type mice. Further, CMKLR1 was suppressed in the liver of adiponectin deficient mice. These data indicate that low CMKLR1 in NAFLD may partly result from reduced adiponectin activity.
Keywords: Adipokine, Hepatic steatosis, Chemerin receptor, Liver
1. Introduction
Non-alcoholic fatty liver disease is becoming the most common cause of chronic liver diseases in westernized countries (Clark, 2006). Obesity is frequently accompanied by NAFLD and altered metabolic function and increased production of inflammatory adipokines are suggested to contribute to hepatic steatosis and progressive liver disease (Clark, 2006; Schaffler et al., 2005). IL-6 is preferentially released from visceral fat and upregulates suppressor of cytokine signaling 3 in the liver which causes hepatic insulin resistance (Fontana et al., 2007; Sabio et al., 2008; Wiest et al., 2011). Leptin is mainly produced by subcutaneous adipocytes, and obesity is characterized by elevated systemic levels. Leptin prevents lipid accumulation in the liver, and animal studies proved that leptin directly promotes fibrogenesis (Biddinger et al., 2006; Wang et al., 2009a). Systemic adiponectin which lowers hepatocyte lipid storage and protects from progressive liver damage is reduced in obesity and patients with fatty liver disease independent of body mass index (Polyzos et al., 2010; Schaffler et al., 2005; Wang et al., 2009b). Antiinflammatory effects of adiponectin include inhibition of TNF activity, a cytokine involved in the pathophysiology of metabolic liver injury (Tilg, 2010).
Chemokine-like receptor 1 (CMKLR1) is expressed by immune cells and is downregulated by TNF and LPS in macrophages (Zabel et al., 2006). TGFβ which deactivates macrophages induces CMKLR1 (Zabel et al., 2006). Mice with deficiency of CMKLR1 exhibit increased recruitment of inflammatory cells in the lung upon LPS challenge indicating antiinflammatory effects of CMKLR1 (Luangsay et al., 2009).
Chemerin is one of the ligands of CMKLR1 and circulating levels are increased in obesity (Meder et al., 2003; Weigert et al., 2010). Serum chemerin positively correlates with BMI, Homeostasis Model Assessment of Insulin Resistance, triglycerides, systolic blood pressure, leptin, resistin, C-reactive protein, TNF and IL-6 and negatively with HDL cholesterol suggesting a function of chemerin in metabolic disturbances associated with obesity (Bozaoglu et al., 2007; Ernst et al., 2010; Parlee et al., 2010; Stejskal et al., 2008; Weigert et al., 2010). In morbidly obese patients with non-alcoholic steatohepatitis (NASH) chemerin is further elevated (Sell et al., 2010). Chemerin is an attractant for immune cells and pro-and antiinflammatory effects of chemerin have been described (Ernst and Sinal, 2010). More recently it has been shown that chemerin impairs insulin signaling in adipocytes and skeletal muscle cells (Becker et al., 2010; Kralisch et al., 2009; Sell et al., 2009).
CMKLR1 also serves as a receptor for resolvin E1 which is derived from omega 3 polyunsaturated fatty acids at inflammatory sites (Arita et al., 2007). Resolvin E1 mediates insulin-sensitizing and antisteatotic effects in rodent models of obesity (Arita et al., 2007; Gonzalez-Periz et al., 2009).
CMKLR1 is expressed in the liver (Ernst et al., 2010; Parlee et al., 2010) and so far it has been shown that mRNA levels are not altered in the liver of ob/ob mice compared to wild type animals. It was hypothesized that CMKLR1 is also expressed by hepatocytes and that its level may be altered in NAFLD. Therefore, we intended to more closely analyse the expression of CMKLR1 in human liver cell populations, human and rodent fatty liver and rodent NASH.
2. Materials and methods
2.1. Material
Dulbecco's modified eagle medium (DMEM) was from PAA (Karlsruhe, Germany), RNeasy Mini Kit was from Qiagen (Hilden, Germany) and oligonucleotides were synthesized by Metabion (Planegg-Martinsried, Germany). LightCycler FastStart DNA Master SYBR Green I was purchased from Roche (Mannheim, Germany). The ACAT inhibitor Sandoz 58-035, LPS (Escherichia coli serotype 055:B5), palmitic acid and oleic acid were ordered from Sigma (Deisenhofen, Germany). Acetylated LDL was from Invitrogen GmbH (Darmstadt, Germany). GAPDH antibody was from New England Biolabs GmbH (Frankfurt, Germany). CMKLR1 antibody raised in rabbits was ordered from Abcam (Cambridge, UK). Recombinant full-length human adiponectin, leptin, TNF, TGFβ and IL-6 and mouse adiponectin were from R&D Systems (Wiesbaden-Nordenstadt, Germany).
2.2. Isolation of primary liver cells
Human liver tissue for cell isolation was obtained from liver resections of patients undergoing partial hepatectomy for metastatic liver tumors of colorectal cancer. Experimental procedures were performed according to the guidelines of the charitable state controlled foundation Human Tissue and Cell Research (HTCR), with the informed patient's consent approved by the local ethical committee of the University of Regensburg (Thasler et al., 2003). Primary human hepatocytes were isolated and cultivated in serum-free medium (DMEM supplemented with 4.5 g/l glucose, 0.4 ng/ml hydrocortisone, 0.415 mU/ml insulin, 2 mM glutamine, and 100 U/ml penicillin/streptomycin) as previously described (Weiss et al., 2003). Contaminating cells using the standard isolation protocol are mainly Kupffer cells and endothelial cells and are less than 2% as examined by light microscopy and RT-PCR studies (Jeschke et al., 2008). Isolation and culture of hepatic stellate cells (HSC) was performed as described (Steiling et al., 2004; Wanninger et al., 2009). Purity of the cells was examined by immunohistochemistry and cytological analysis and was about 90% (unpublished data).
Human Kupffer cells (KC) were obtained within the process of hepatocyte isolation using a modified two-step EGTA/collagenase perfusion procedure. Briefly, tissue samples were perfused with EGTA/collagenase solution at 37 °C, followed by dissection of the digested tissue. The minced tissue in solution was filtered through different meshes (210 and 70 μm) and centrifuged at 72g (2×) to separate hepatocytes (pellet) and non-parenchymal cell fraction containing KC. The non-parenchymal cell fraction was washed with HBSS buffer and centrifuged at 650g for 7 min at 4 °C. Cell pellets were resuspended in HBSS buffer and centrifuged on a density cushion of Percoll (50% and 25%) at 1800g for 15 min at 4 °C. The KC fraction was collected, centrifuged at 650g for 7 min, resuspended again in buffer and plated using culture media without FCS (Weiss et al., 2003). Purity of the cells was examined by immunohistochemistry and cytological analysis and was about 80–85% (unpublished data).
The non-parenchymal cell fractions were also used to isolate endothelial cells and bile duct cells. Density barrier centrifugation step using 20% Nycodenz (Sigma, Germany) was performed, and cells in the pellet were resuspended. To purify endothelial cells CD31 MicroBead (Miltenyi Biotec, Bergisch Gladbach, Germany) were used as recommended by the manufacturer of the kit. To purify bile duct cells anti-human EpCAM (CD326), clone HEA125, and subsequently goat anti-mouse MicroBeads (Miltenyi Biotec) were used. Purity of the cells was examined by immunohistochemistry and cytological analysis and was about 90% in endothelial cells and 90–95% in bile duct cells (unpublished data).
Human monocytes were isolated as described using anti CD14 MicroBeads (Bauer et al., 2011a) and differentiated for 3 d in RPMI medium supplemented with 10% autologous serum.
2.3. Human steatotic and control liver tissues
Liver tissues for immunoblot analysis were obtained of 7 patients (4 females, 3 males) without and 7 patients (2 females, 5 males) with biopsy proven steatosis. Surgery was done because of hepatic metastases of extrahepatic tumors and only healthy tissue was used for the studies. Here, neither inflammation nor fibrosis were detected in the livers by the pathologist. Age (58 ± 13 and 62 ± 15 years) and BMI (26.8 ± 3.7 and 29.3 ± 3.2 kg/m2) were similar between controls and patients with hepatic steatosis. BMI of one control was not known.
2.4. Animal models
Adiponectin deficient mice have already been described in more detail (Shibata et al., 2005). Liver from male mice, 5–7 months old and fed a standard chow were used. Body weight of the six adiponectin deficient mice was 30.7 ± 1.9 g and similar to the six wild type animals with 30.7 ± 1.4 g. All animal procedures were approved by the committee on animal research and complied with the UFAW `Handbook on the care and management of laboratory animals' 1999.
MCD diet causes hepatic steatosis (triglycerides were significantly induced, data not shown), inflammation (significant induction of TNF mRNA, data not shown) and fibrosis (confirmed by Sirius red staining and α-smooth muscle actin immunoblot, data not shown) as has already been described (Schattenberg and Galle, 2010). Adiponectin was increased in the four male mice fed a MCD diet to 3.8 ± 0.5 μg/ml (p = 0.029 compared to the four control mice where serum adiponectin was 2.4 ± 0.3 μg/ml) and body weight was reduced to 13.8 ± 0.6 g (p = 0.029 compared to control mice with 18.3 ± 0.5 g body weight).
Further, male C57/BL6 mice fed a high fat diet for 16 weeks and with a final bodyweight of 42 ± 6 g were fasted for 2 h. Then 6.5 μg recombinant murine adiponectin per gram bodyweight or PBS were intraperitoneally injected (3 mice each). After 6 h mice were killed and the liver was immediately removed and stored at −80 °C. Serum was also collected to measure adiponectin which was 2 to 4-fold increased in the animals with adiponectin injection compared to PBS injected mice.
Seven week old male C57/BL6 mice were kept on a high fat diet (HFD, 5 mice) or standard chow (SD, 4 mice) for 14 weeks. Final body weight was 37.6 ± 6.5 g in the HFD group and 26.2 ± 1.1 g (p = 0.016) in the SD group. Adiponectin was 5.6 ± 1.0 μg/ml in the HFD and 7.6 ± 2.6 μg/ml in the SD group. Adiponectin tended to be lower in the HFD group but because of one outlier in the SD group this difference did not become significant. Mice on a HFD develop hepatic steatosis whereas inflammation and fibrosis are not increased compared to SD fed animals (data not shown).
2.5. Monitoring of gene expression by real-time RT-PCR
Real-time RT-PCR was performed as recently described (Neumeier et al., 2007, 2006a,b). The primers for human CMKLR1 were CMKLR1 uni: 5′-ACC TGC ATG GGA AAA TAT CCT-3′ and CMKLR1 rev: 5′-GAG GTT GAG TGT GTG GTA GGG-3′ For β-actin: β-actin uni: 5′-CTA CGT CGC CCT GGA CTT CGA GC-3′, and β-actin rev: 5′-GAT-GGA GCC GCC GAT CCA CAC GG-3′ were used. Primers for murine CMKLR1 were CMKLR1m uni: 5′-CTT CTC CCC TAA TCC CCT CA-3′ and CMKLR1m rev: 5′-GGG GTG AGT GAG CCA TTT T-3′. For normalization cyclophilin A expression which was similar in the mice kept on the SD and HFD diets was used and was amplified with cyclophilin Am uni: 5′-AAC ACA AAC GGT TCC CAG TT-3′ and rev: 5′-TTG AAG GGG AAT GAG GAA AA-3′.
2.6. Immunohistochemistry (IHC)
Immunohistochemical studies for the expression of CMKLR1 utilized the EnVision+ Kit (DAKO, Glostrup, Denmark) based on a HRP labeled polymer which is conjugated with a secondary antibody. Three micrometers sections were cut from formalin-fixed and paraffin-embedded human liver tissues. After deparaffinization for 15 min in Histol, tissue sections were rehydrated in descending ethanol series following antigen retrieval (microwave oven for 20 min at 800 W in sodium citrate buffer). Endogenous peroxidase activity was eliminated by subsequent incubation with 0.3% hydrogen peroxide for 10 min. After washing in TBS, 0.5% Tween 20 slides were incubated for 1 h in a protein-blocking solution (DAKO). Incubation with the CMKLR1 antibody (1:100-fold diluted, Abcam) was performed overnight at 4 °C in a humid chamber. After thorough washing with TBS, 0.5% Tween 20, tissue sections were incubated with anti-mouse HRP labeled polymer for 30 min. Staining was completed by incubation with DAB substrate chromogen (DAKO) according to the manufacturer's instructions.
2.7. SDS-PAGE and immunoblotting
Proteins (20 μg) were separated by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes (Bio-Rad, Munich, Germany). Incubations with antibodies were performed in 1.5% BSA in PBS, 0.1% Tween. Detection of the immune complexes was carried out with the ECL Western blot detection system (Amersham Pharmacia, Deisenhofen, Germany).
2.8. Statistical analysis
Data are presented as box plots indicating median, lower and upper quartiles and range of the values. Statistical differences were analyzed by two-tailed Mann-Whitney U Test or Student's t-test, and a value of p < 0.05 was regarded as statistically significant. The Pearson's correlation was calculated using the PASW statistics 17.0 programme.
3. Results
3.1. CMKLR1 expression in primary human liver cells
CMKLR1 mRNA was detected in primary human hepatocytes (PHH), hepatic stellate cells (HSC), endothelial cells, Kupffer cells (KC) and bile duct cells (Fig. 1A). CMKLR1 protein was analyzed in PHH, KC and HSC and was expressed by all of these cells. Although identical concentrations of the different protein lysates were used GAPDH protein was higher abundant in HSC, KC and macrophages compared to PHH (Fig. 1B). To confirm similar amounts of protein in the different lysates Coomassie stained membrane is also shown. Highest levels of CMKLR1 were found in HSC (Fig. 1B). Immunohistochemistry confirmed CMKLR1 protein in PHHs, KC and bile duct cells (Fig. 1C). CMKLR1 was highly expressed in the hepatoma cell lines Hep3B, PLC and Huh7 (Fig. 1D).
Fig. 1.

Expression of CMKLR1 in human liver cells. (A) CMKLR1 mRNA in primary human hepatocytes (PHH_1, PHH_2 indicates cells from two different donors), bile duct cells, hepatic stellate cells (HSC), endothelial cells (EC) and Kupffer cells (KC). (B) CMKLR1 protein in primary human macrophages, KC and PHH (PHH_1 to PHH_4 isolated from liver tissue of four different donors). Because GAPDH was found to be unequally expressed in the different cell lysates Coomassie stained membrane is shown to confirm equal protein concentrations. (C) Immunohistochemistry of CMKLR1 in human liver confirming expression in PHH, KC and bile duct cells (upper left corner). Control staining was done in almost the same manner but without CMKLR1 antibody. (D) CMKLR1 in PHH and hepatoma cell lines.
3.2. CMKLR1 is reduced in human fatty liver
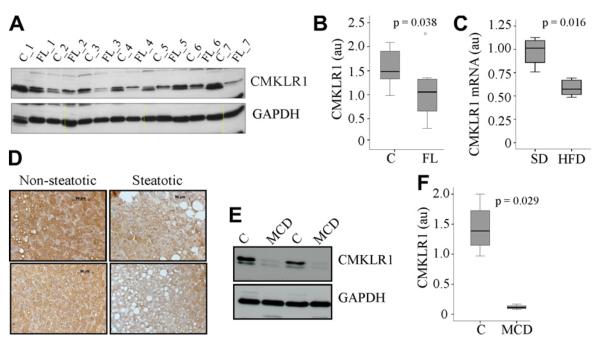
Analysis of CMKLR1 in non-steatotic and steatotic human liver revealed significant reduction of CMKLR1 protein in the latter (Fig. 2A and B). Liver CMKLR1 did not correlate with age or BMI of the patients (data not shown). Serum of these patients was not available to measure adiponectin. To analyse correlation of CMKLR1 expression and serum adiponectin levels CMKLR1 mRNA expression was determined in the liver of mice kept on a standard chow or a high fat diet, and was found significantly reduced in the latter (Fig. 2C). CMKLR1 mRNA tended to negatively correlate with body weight (r = 0.672, p = 0.05) but not with serum adiponectin (data not shown).
Fig. 2.
CMKLR1 in human and mouse fatty liver and rodent NASH liver. (A) CMKLR1 was analyzed in liver tissues of 7 patients without (C_1 to C_7) and 7 patients with histological defined hepatic steatosis (FL_1 to FL_7). (B) Quantification of CMKLR1 immunoblot shown in A. (C) CMKLR1 mRNA was determined in the liver of 4 mice kept on a standard chow (SD) and 5 mice kept on a high fat diet (HFD). (D) Immunohistochemistry of CMKLR1 in human non-steatotic (2 patients) and steatotic liver (2 patients). (E) CMKLR1 in the liver of mice fed a methionine–choline deficient diet (MCD) and control animals. (F) Quantification of CMKLR1 immunoblot partly shown in C in liver lysates of 4 control and 4 MCD fed animals.
Hepatocytes are the major cell population in the liver suggesting that reduced CMKLR1 in hepatic steatosis is due to lower levels in these cells. To proof this suggestion, immunohistochemistry using human non-steatotic (4 patients) and steatotic liver (4 patients) was performed. Hepatocytes in fatty liver were found to have reduced CMKLR1 (Fig. 2D and data not shown). Further, CMKLR1 was analyzed in the liver of mice fed a methionine-choline deficient diet (MCD) for 6 weeks. This kind of diet is commonly used a model for non-alcoholic steatohepatitis and induces marked hepatic steatosis, inflammation and fibrosis (Schattenberg and Galle, 2010; Wanninger et al., 2011b). CMKLR1 was lower in the liver of four mice fed a MCD diet (Fig. 2E and F) compared to four control fed animals (Fig. 2E and F).
3.3. Effect of excess lipid storage, LPS, leptin and TGFβ on CMKLR1 levels in PHH
Hepatocyte CMKLR1 is diminished in fatty liver and to find out which of the alterations associated with NAFLD may suppress CMKLR1 PHH were incubated with 0.3 mM palmitic or oleic acid for 24 h to significantly elevate triglyceride storage (data not shown) but CMKLR1 protein (Fig. 3A) and mRNA levels (data not shown) were not suppressed. To increase free cholesterol which is elevated in fatty liver PHH were incubated with acyl coenzyme A:cholesterol acyltransferase (ACAT) inhibitor and acetylated LDL (Nagelkerke et al., 1983). In PHH a 24 h incubation with an ACAT inhibitor (10 μg/ml), with 50 μg/ml AcLDL or both did not affect CMKLR1 (data not shown). Similarly LPS (1, 10 μg/ml), leptin (10, 20 ng/ml), IL-6 (5 ng/ml) and TNF (0.5, 2 ng/ml) did not alter CMKLR1 levels within 24 h of incubation (Fig. 3B and data not shown). TGFβ (4 ng/ml) tended to reduce CMKLR1 protein (Fig. 3B and C).
Fig. 3.

Regulation of CMKLR1 by fatty acids, adipokines and cytokines. (A) CMKLR1 in PHH incubated with 0.3 mM palmitic acid (PA) or oleic acid (OA) for 24 h. (B) CMKLR1 in PHH incubated with LPS (1, 10 μg/ml), leptin (10, 20 ng/ml) or TGFβ (4 ng/ml) for 24 h. (C) Quantification of the results of four independent experiments partly shown in B indicate a trend to reduced CMKLR1 in TGFβ incubated PHH.
3.4. Adiponectin upregulates CMKLR1 in PHH
Hepatocytes express both adiponectin receptors, AdipoR1 and AdipoR2, and human and rodent hepatocytes respond to adiponectin (Kaser et al., 2005; Miller et al., 2011; Wanninger et al., 2011a,b). Immunoblot analysis revealed that adiponectin induced CMKLR1 protein (Fig. 4A) and this effect was significant when data of four independent experiments were calculated (p = 0.02). Adiponectin also increased CMKLR1 mRNA (Fig. 4B). Analysis of CMKLR1 in the liver of adiponectin-deficient mice and suitable control animals demonstrated significantly reduced CMKLR1 protein in the knock-out mice (Fig. 4C and D). To further analyse the relationship of adiponectin and CMKLR1 mice were fed a high fat diet for 4 months. These animals have increased liver triglycerides compared to animals kept on a standard chow (data not shown). Intraperitoneal injection of adiponectin increased systemic adiponectin (data not shown) and hepatic CMKLR1 protein was significantly higher when analyzed 6 h later (Fig. 4E and F).
Fig. 4.
Regulation of CMKLR1 by adiponectin. (A) CMKLR1 in the cell lysate of PHH incubated with adiponectin (APN, 10 μg/ml) for 24 h. (B) CMKLR1 mRNA in PHH of four different donors incubated with adiponectin (APN, 10 μg/ml) for 24 h. (C) CMKLR1 in the liver of wild type (WT) and adiponectin-deficient mice (KO). (D) Quantification of CMKLR1 immunoblot partly shown in C in liver lysates of 6 mice each. (E) CMKLR1 in liver of wild type mice 6 h after injection of PBS (control_1 and _2) or adiponectin (APN_1, and APN_2). (F) Quantification of the data of 3 PBS and 3 adiponectin-injected mice partly shown in E.
4. Discussion
In the current study we show that CMKLR1 protein is expressed by hepatocytes, hepatic stellate cells, bile duct epithelial cells and Kupffer cells. All of these cells express CMKLR1 mRNA but it has to be considered that primary cells are contaminated with other liver cells to some degree and this may give false positive results. To solve this problem in situ hybridisation to detect cellular mRNAs has to be performed. CMKLR1 protein is highly expressed in hepatoma cell lines suggesting that these cells may respond to CMKLR1 ligands.
Ligands of this receptor described so far are chemerin and resolvin E1 (Arita et al., 2007; Meder et al., 2003; Wittamer et al., 2003). Chemerin is well known to stimulate cell migration and phagocytosis of macrophages (Yoshimura and Oppenheim, 2008) and may have similar effects in Kupffer cells. However, its function in nonimmune cells has not been studied in more detail. In adipocytes and skeletal muscle cells chemerin impairs insulin signaling, and LDL-receptor deficient mice with chronically increased serum chemerin have reduced skeletal muscle insulin sensitivity (Becker et al., 2010; Kralisch et al., 2009; Sell et al., 2009). Nevertheless, hepatic insulin signaling is not disturbed (Becker et al., 2010). In endothelial cells proinflammatory cytokines induce CMKLR1. Here, chemerin stimulates angiogenesis (Bozaoglu et al., 2010; Kaur et al., 2010), matrix metalloproteinase (MMP) activity and cell survival (Kaur et al., 2010). In chondrocytes which also express CMKLR1 chemerin induces production of proinflammatory cytokines and MMP13 (Berg et al., 2010). However, the effect of chemerin on hepatocytes has not been studied so far. Processing of chemerin by cysteine proteases generates peptides which are biologically effective and also signal through CMKLR1 (Cash et al., 2008). These peptides are most likely generated at sites of inflammation and exert antiinflammatory activities (Cash et al., 2008).
Resolvin E1 is also a ligand of CMKLR1 and protective and anti-inflammatory effects in various diseases have been described (Arita et al., 2007; Seki et al., 2009). This lipid reduces hepatocyte fat storage, hepatocyte death and macrophage number in the liver of ob/ob mice (Gonzalez-Periz et al., 2009). Whether these are direct effects of resolvin E1 in liver cells or secondary to increased adiponectin synthesis in the fat tissues of these mice has not been evaluated in more detail (Gonzalez-Periz et al., 2009).
In human and rodent fatty liver and in rodent NASH CMKLR1 is significantly reduced. Hepatocytes are the main cell population in the liver and in fatty liver CMKLR1 protein is reduced in the parenchymal cells. In contrast to endothelial cells and macrophages (Kaur et al., 2010; Zabel et al., 2006) CMKLR1 is not regulated by proinflammatory mediators in PHH. TGFβ even tends to reduce CMKLR1 protein in hepatocytes whereas an upregulation is observed in macrophages (Zabel et al., 2006). Lipid loading of hepatocytes does not affect CMKLR1 levels similar to adipocytes where enhanced triglyceride accumulation has no effect on this receptor (Bauer et al., 2011b).
Adiponectin is well described as a hepatoprotective adipokine lowering hepatic lipid accumulation and liver fibrosis (Schaffler et al., 2005; Tilg, 2010). Adiponectin upregulates CMKLR1 mRNA and protein in PHH and CMKLR1 is found significantly reduced in the liver of adiponectin-deficient mice. These findings suggest that adiponectin is an important inductor of CMKLR1 in vivo and recombinant adiponectin increases CMKLR1 in the liver of mice. Nevertheless, there is no direct correlation of systemic adiponectin and hepatic CMKLR1 expression. Serum adiponectin consists of trimers, hexamers and high molecular weight forms whereas recombinant adiponectin used in the current study does not contain trimers (Neumeier et al., 2006a). Different adiponectin isoform have distinct biologic activites (Neumeier et al., 2006a) and this may explain why there is no correlation of total adiponectin and hepatic CMKLR1. Nevertheless, it is also likely that other cytokines and adipokines regulate CMKLR1 expression.
Adiponectin deficient mice are more susceptible to high fat diet mediated hepatic triglyceride storage, hepatic insulin resistance and inflammation (Asano et al., 2009; Yano et al., 2008), and further studies using CMKLR1 deficient mice are needed to define the role of this receptor in NAFLD. Adiponectin effects are transduced by two receptors, AdipoR1 and AdipoR2, which are both expressed in hepatocytes (Wanninger et al., 2011a; Yamauchi et al., 2007). AMP activated protein kinase and peroxisome proliferator activated receptor α are downstream signaling molecules of AdipoR1 and AdipoR2, respectively. More recently adiponectin effects which are independent of these receptors have been described (Awazawa et al., 2011). Adiponectin is well known to induce IL-6 synthesis in monocytes/macrophages and upregulation of IL-6 in adipose tissue resident macrophages has been shown to activate hepatic signal transducer and activator of transcription 3 (STAT3) (Awazawa et al., 2011; Neumeier et al., 2006a; Schober et al., 2007). Importantly IL-6 is only weakly induced in Kupffer cells indicating that adipose tissue released IL-6 affects STAT3 in the liver (Awazawa et al., 2011). However, as is shown herein, recombinant IL-6 does not induce CMKLR1 in PHH suggesting that this pathway may not explain increased CMKLR1 in mice injected with adiponectin. Of note upregulation of CMKLR1 by adiponectin is already observed in vitro using purified human hepatocytes.
Systemic adiponectin is reduced in patients with hepatic steatosis and NASH and may contribute to lower CMKLR1 protein in the liver of these patients (Kamada et al., 2008; Polyzos et al., 2010; Schaffler et al., 2005). Impaired hepatic adiponectin activity has been described in mice fed a MCD diet (Larter et al., 2008; Tomita et al., 2008) where systemic adiponectin is significantly higher because of lower body weight. Here, adiponectin resistance and TGFβ which is increased in fibrotic liver may contribute to lower CMKLR1.
In conclusion the current study provides evidence that adiponectin activity is connected to hepatic CMKLR1 abundance. CMKLR1 is reduced in NAFLD and further studies have to address the role of this receptor in liver physiology and metabolic liver disease.
Acknowledgments
The technical assistance of Yvonne Hader is greatly appreciated. The study was supported by a Grant from the Deutsche Forschungsgemeinschaft (BU 1141/3–3, BU 1141/7–1) to C. Buechler and a grant from the Regensburger Forschungsförderung (ReForm C) to C. Buechler, C. Hellerbrand and T.S. Weiss.
Footnotes
Disclosure The authors declare that they have no conflicts of interest related to this work.
References
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Asano T, Watanabe K, Kubota N, Gunji T, Omata M, Kadowaki T, Ohnishi S. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J. Gastroenterol. Hepatol. 2009;24:1669–1676. doi: 10.1111/j.1440-1746.2009.06039.x. [DOI] [PubMed] [Google Scholar]
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, Kobayashi M, Iwane A, Sasako T, Okazaki Y, Ohsugi M, Takamoto I, Yamashita S, Asahara H, Akira S, Kasuga M, Kadowaki T. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13:401–412. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Bauer S, Wanninger J, Neumeier M, Wurm S, Weigert J, Kopp A, Bala M, Schaffler A, Aslanidis C, Buechler C. Elevated free fatty acids and impaired adiponectin bioactivity contribute to reduced SOD2 protein in monocytes of type 2 diabetes patients. Exp. Mol. Pathol. 2011a;90:101–106. doi: 10.1016/j.yexmp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Bauer S, Wanninger J, Schmidhofer S, Weigert J, Neumeier M, Dorn C, Hellerbrand C, Zimara N, Schaffler A, Aslanidis C, Buechler C. Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces chemerin in hypertrophic adipocytes. Endocrinology. 2011b;152:26–35. doi: 10.1210/en.2010-1157. [DOI] [PubMed] [Google Scholar]
- Becker M, Rabe K, Lebherz C, Zugwurst J, Goke B, Parhofer KG, Lehrke M, Broedl UC. Expression of human chemerin induces insulin resistance in the skeletal muscle but does not affect weight, lipid levels, and atherosclerosis in LDL receptor knockout mice on high-fat diet. Diabetes. 2010;59:2898–2903. doi: 10.2337/db10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg V, Sveinbjornsson B, Bendiksen S, Brox J, Meknas K, Figenschau Y. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21–157) Arthritis Res. Ther. 2010;12:R228. doi: 10.1186/ar3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Miyazaki M, Boucher J, Ntambi JM, Kahn CR. Leptin suppresses stearoyl-CoA desaturase 1 by mechanisms independent of insulin and sterol regulatory element-binding protein-1c. Diabetes. 2006;55:2032–2041. doi: 10.2337/db05-0742. [DOI] [PubMed] [Google Scholar]
- Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, Morrison S, Carless M, Dyer TD, Cole SA, Goring HH, Moses EK, Walder K, Cawthorne MA, Blangero J, Jowett JB. Chemerin, a novel adipokine in the regulation of angiogenesis. J. Clin. Endocrinol. Metab. 2010;95:2476–2485. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 2006;40(Suppl. 1):S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- Ernst MC, Issa M, Goralski KB, Sinal CJ. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151:1998–2007. doi: 10.1210/en.2009-1098. [DOI] [PubMed] [Google Scholar]
- Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, Titos E, Martinez-Clemente M, Lopez-Parra M, Arroyo V, Claria J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke MG, Klein D, Thasler WE, Bolder U, Schlitt HJ, Jauch KW, Weiss TS. Insulin decreases inflammatory signal transcription factor expression in primary human liver cells after LPS challenge. Mol. Med. 2008;14:11–19. doi: 10.2119/2007-00062.Jeschke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J. Gastroenterol. 2008;43:811–822. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem. Biophys. Res. Commun. 2010;391:1762–1768. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- Kralisch S, Weise S, Sommer G, Lipfert J, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul. Pept. 2009;154:102–106. doi: 10.1016/j.regpep.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM, Williams J, Bell-Anderson KS, Farrell GC. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J. Hepatol. 2008;49:407–416. doi: 10.1016/j.jhep.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 2009;183:6489–6499. doi: 10.4049/jimmunol.0901037. [DOI] [PubMed] [Google Scholar]
- Meder W, Wendland M, Busmann A, Kutzleb C, Spodsberg N, John H, Richter R, Schleuder D, Meyer M, Forssmann WG. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 2003;555:495–499. doi: 10.1016/s0014-5793(03)01312-7. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chu Q, Le Lay J, Scherer PE, Ahima RS, Kaestner KH, Foretz M, Viollet B, Birnbaum MJ. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1–AMPK signaling. J. Clin. Invest. 2011;121:2518–2528. doi: 10.1172/JCI45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke JF, Barto KP, van Berkel TJ. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J. Biol. Chem. 1983;258:12221–12227. [PubMed] [Google Scholar]
- Neumeier M, Sigruener A, Eggenhofer E, Weigert J, Weiss TS, Schaeffler A, Schlitt HJ, Aslanidis C, Piso P, Langmann T, Schmitz G, Scholmerich J, Buechler C. High molecular weight adiponectin reduces apolipoprotein B and E release in human hepatocytes. Biochem. Biophys. Res. Commun. 2007;352:543–548. doi: 10.1016/j.bbrc.2006.11.058. [DOI] [PubMed] [Google Scholar]
- Neumeier M, Weigert J, Schaffler A, Wehrwein G, Muller-Ladner U, Scholmerich J, Wrede C, Buechler C. Different effects of adiponectin isoforms in human monocytic cells. J. Leukoc. Biol. 2006a;79:803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- Neumeier M, Weigert J, Schaffler A, Weiss TS, Schmidl C, Buttner R, Bollheimer C, Aslanidis C, Scholmerich J, Buechler C. Aldehyde oxidase 1 is highly abundant in hepatic steatosis and is downregulated by adiponectin and fenofibric acid in hepatocytes in vitro. Biochem. Biophys. Res. Commun. 2006b;350:731–735. doi: 10.1016/j.bbrc.2006.09.101. [DOI] [PubMed] [Google Scholar]
- Parlee SD, Ernst MC, Muruganandan S, Sinal CJ, Goralski KB. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-{alpha} Endocrinology. 2010;151:2590–2602. doi: 10.1210/en.2009-0794. [DOI] [PubMed] [Google Scholar]
- Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2010;12:365–383. doi: 10.1111/j.1463-1326.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler A, Scholmerich J, Buchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue – emerging role in nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2:273–280. doi: 10.1038/ncpgasthep0186. [DOI] [PubMed] [Google Scholar]
- Schattenberg JM, Galle PR. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig. Dis. 2010;28:247–254. doi: 10.1159/000282097. [DOI] [PubMed] [Google Scholar]
- Schober F, Neumeier M, Weigert J, Wurm S, Wanninger J, Schaffler A, Dada A, Liebisch G, Schmitz G, Aslanidis C, Buechler C. Low molecular weight adiponectin negatively correlates with the waist circumference and monocytic IL-6 release. Biochem. Biophys. Res. Commun. 2007;361:968–973. doi: 10.1016/j.bbrc.2007.07.106. [DOI] [PubMed] [Google Scholar]
- Seki H, Tani Y, Arita M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 2009;89:126–130. doi: 10.1016/j.prostaglandins.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Sell H, Divoux A, Poitou C, Basdevant A, Bouillot JL, Bedossa P, Tordjman J, Eckel J, Clement K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 2010;95:2892–2896. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- Sell H, Laurencikiene J, Taube A, Eckardt K, Cramer A, Horrighs A, Arner P, Eckel J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes. 2009;58:2731–2740. doi: 10.2337/db09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiling H, Muhlbauer M, Bataille F, Scholmerich J, Werner S, Hellerbrand C. Activated hepatic stellate cells express keratinocyte growth factor in chronic liver disease. Am. J. Pathol. 2004;165:1233–1241. doi: 10.1016/S0002-9440(10)63383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskal D, Karpisek M, Hanulova Z, Svestak M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population – a pilot study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2008;152:217–221. doi: 10.5507/bp.2008.033. [DOI] [PubMed] [Google Scholar]
- Thasler WE, Weiss TS, Schillhorn K, Stoll PT, Irrgang B, Jauch KW. Charitable state-controlled foundation human tissue and cell research: ethic and legal aspects in the supply of surgically removed human tissue for research in the academic and commercial sector in Germany. Cell Tissue Bank. 2003;4:49–56. doi: 10.1023/A:1026392429112. [DOI] [PubMed] [Google Scholar]
- Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig. Dis. 2010;28:179–185. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- Tomita K, Oike Y, Teratani T, Taguchi T, Noguchi M, Suzuki T, Mizutani A, Yokoyama H, Irie R, Sumimoto H, Takayanagi A, Miyashita K, Akao M, Tabata M, Tamiya G, Ohkura T, Hibi T. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458–473. doi: 10.1002/hep.22365. [DOI] [PubMed] [Google Scholar]
- Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, George J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009a;137:713–723. doi: 10.1053/j.gastro.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhou M, Lam KS, Xu A. Protective roles of adiponectin in obesity-related fatty liver diseases: mechanisms and therapeutic implications. Arq. Bras. Endocrinol. Metabol. 2009b;53:201–212. doi: 10.1590/s0004-27302009000200012. [DOI] [PubMed] [Google Scholar]
- Wanninger J, Neumeier M, Hellerbrand C, Schacherer D, Bauer S, Weiss TS, Huber H, Schaffler A, Aslanidis C, Scholmerich J, Buechler C. Lipid accumulation impairs adiponectin-mediated induction of activin A by increasing TGFbeta in primary human hepatocytes. Biochim. Biophys. Acta. 2011a;1811:626–633. doi: 10.1016/j.bbalip.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Wanninger J, Neumeier M, Weigert J, Bauer S, Weiss TS, Schaffler A, Krempl C, Bleyl C, Aslanidis C, Scholmerich J, Buechler C. Adiponectin-stimulated CXCL8 release in primary human hepatocytes is regulated by ERK1/ERK2, p38 MAPK, NF-kappaB, and STAT3 signaling pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G611–G618. doi: 10.1152/ajpgi.90644.2008. [DOI] [PubMed] [Google Scholar]
- Wanninger J, Walter R, Bauer S, Eisinger K, Schaffler A, Dorn C, Weiss TS, Hellerbrand C, Buechler C. MMP-9 activity is increased by adiponectin in primary human hepatocytes but even negatively correlates with serum adiponectin in a rodent model of non-alcoholic steatohepatitis. Exp. Mol. Pathol. 2011b;91:603–607. doi: 10.1016/j.yexmp.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Weigert J, Neumeier M, Wanninger J, Filarsky M, Bauer S, Wiest R, Farkas S, Scherer MN, Schaffler A, Aslanidis C, Scholmerich J, Buechler C. Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin. Endocrinol. (Oxf.) 2010;72:342–348. doi: 10.1111/j.1365-2265.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- Weiss TS, Pahernik S, Scheruebl I, Jauch KW, Thasler WE. Cellular damage to human hepatocytes through repeated application of 5-aminolevulinic acid. J. Hepatol. 2003;38:476–482. doi: 10.1016/s0168-8278(02)00454-3. [DOI] [PubMed] [Google Scholar]
- Wiest R, Weigert J, Wanninger J, Neumeier M, Bauer S, Schmidhofer S, Farkas S, Scherer MN, Schaffler A, Scholmerich J, Buechler C. Impaired hepatic removal of interleukin-6 in patients with liver cirrhosis. Cytokine. 2011;53:178–183. doi: 10.1016/j.cyto.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- Yano W, Kubota N, Itoh S, Kubota T, Awazawa M, Moroi M, Sugi K, Takamoto I, Ogata H, Tokuyama K, Noda T, Terauchi Y, Ueki K, Kadowaki T. Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr. J. 2008;55:515–522. doi: 10.1507/endocrj.k08e-093. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Oppenheim JJ. Chemerin reveals its chimeric nature. J. Exp. Med. 2008;205:2187–2190. doi: 10.1084/jem.20081736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel BA, Ohyama T, Zuniga L, Kim JY, Johnston B, Allen SJ, Guido DG, Handel TM, Butcher EC. Chemokine-like receptor 1 expression by macrophages in vivo: regulation by TGF-beta and TLR ligands. Exp. Hematol. 2006;34:1106–1114. doi: 10.1016/j.exphem.2006.03.011. [DOI] [PubMed] [Google Scholar]