Abstract
Chronic stress plays a role in the etiology of several affective and anxiety-related disorders. Despite this, its mechanistic effects on the brain are still unclear. Of particular interest is the effect of chronic stress on the amygdala, which plays a key role in the regulation of emotional responses and memory consolidation. This review proposes a neuroplasticity model for the effects of chronic stress in this region, emphasizing the roles of glutamate and BDNF signaling. This model provides a review of recent discoveries of the effects of chronic stress in the amygdala and reveals pathways for future research.
Keywords: neuroplasticity, chronic stress, basolateral amygdala (BLA), glutamate, N-methyl-D-aspartate (NMDA) receptor, brain-derived neurotrophic factor (BDNF), small-conductance Ca2+-activated K+ channels (SK channels), fatty acid amide hydrolase (FAAH), anandamide (AEA)
Introduction
Deep in the medial temporal lobes of the brain, two small, almond-shaped nuclei called the amygdalae play critical roles in the establishment of the human emotional experience [1,2] and memory consolidation [3,4,5]. When rodents [6,7] or humans [8,9,10] are exposed to chronic stress, defined as a prolonged period of exposure to potentially threatening or emotionally challenging stimuli, changes in behavior and morphology of the amygdala occur.
Chronic stress and its effects on the amygdala both contribute to the formation of affective and anxiety disorders [11], which represent some of the foremost causes of disability worldwide [12]. Furthermore, studies have demonstrated that amygdalar activity is increased in patients suffering from anxiety and affective disorders [13,14]. While existing treatments benefit a large percentage of the population, there is still a significant need to develop better drug and behavioral therapies [15]. Due to the relationship between chronic stress, amygdalar activity, and the formation of affective and anxiety disorders, it is important to characterize the effects of chronic stress in this region.
This paper will examine the biological basis for chronic-stress induced changes in the amygdala and the hippocampus (HC), as well as illuminate potential targets for future studies. In particular, the basolateral amygdala (BLA), which is critical in anxiety and memory consolidation [1,2,4,5], will be explored. The activity of the HC, a key structure for memory processes, also changes following a period of chronic stress [6,16] and will serve as a point of comparison for the effects on chronic stress in the brain.
These observations will be used to form a “neuroplasticity hypothesis” of the effects of chronic stress in the amygdala. It will be shown that stress increases glutamatergic signaling in the BLA, resulting in enhanced brain-deprived neurotrophic factor (BDNF) expression and dendritic outgrowth. In contrast, chronic stress-induced increases in glutamatergic signaling in the hippocampus are accompanied by decreased BDNF signaling. These changes contribute to changes in BLA and HC morphology and activity, which then result in an enhanced stress response. By contributing to the understanding of chronic stress in the BLA, this hypothesis should aid in the search for better treatments of affective and anxiety disorders.
Chronic Stress Alters Synaptic Plasticity and Amygdala-Dependent Learning
When vertebrates are exposed to chronic stress for prolonged periods of time, a dichotomy appears in the morphology of different brain regions. In the rodent amygdala, chronic restraint stress (CRS) increases dendritic arborization in spiny pyramidal and stellate neurons [6]. In contrast, chronic stress leads to a loss of spines and dendritic branch points in the HC [6,16]. While the morphological changes in the hippocampus CA3 area are reversed within 21 days of the end of chronic stress [7], the changes in the BLA persist during this time.
These changes are important because the hippocampus and amygdala play key roles in regulating the hypothalamic-pituitary-adrenal (HPA) axis [17]. The HPA axis controls the stress response through interactions between the hypothalamus, pituitary gland, and the adrenal gland. These responses regulate body processes such as digestion, the immune system, and mood. Inhibitory inputs from the amygdala and excitatory connections from the hippocampus project to inhibitory neurons in the paraventricular nucleus (PVN) and hypothalamus [18,19]. This implies that increasing input from the amygdala or decreasing input from the hippocampus (as occurs during chronic stress) enhances the net activity of the HPA axis. This dysregulation of the HPA axis is responsible for many of the negative effects of chronic stress on brain functioning and behavior [20,21].
In addition to changes in the HPA axis, chronic stress enhances the consolidation of memories in auditory fear conditioning [1,7], a task that depends on amygdala activity. This is supported by evidence that damage or inactivation of the amygdala impairs fear learning [3,4,5]. This result opposes that for hippocampal-dependent learning, where chronic stress impairs contextual memory in rats [22,23] and declarative memory in humans [24,25,26]. Chronic stress additionally leads to increased anxiety in rats, which has been associated with activity of the basolateral amygdala [1,2].
Chronic Stress Enhances Glutamatergic Signaling
Despite the fact that the morphology of the hippocampus and basolateral amygdala are regulated in opposite ways following chronic stress, glutamate transmission is enhanced in both regions [27,28,29]. Studies of the hippocampus reveal chronic stress or treatment with glucocorticoids up-regulates glutamate transporter expression hippocampal CA3 region glia [30,31]. Furthermore, antidepressant treatment minimizes the transmission of glutamate in the hippocampus [32]. In the BLA, studies have found that repeated activation of the corticotropin releasing factor (CRF) receptor occurs during periods of chronic stress and results in an increased N-methyl-D-aspartate (NMDA) glutamate receptor-mediated calcium influx [33].
The role of glutamate is complicated by its implication in the inhibition of dendritic growth cones in a Ca2+-dependent manner [34,35]. Ca2+ influx through NMDA receptors inhibits the polymerization of the tubulin dimers responsible for microtubule and neurite elongation [36,37,38]. Ca2+ simultaneously triggers the local polymerization of actin that forms filopodial extensions of growth cones [39]. If Ca2+ is sustained at high levels, microtubules and microfilaments are depolymerized to trigger dendritic regression [39,40]. Thus, while the calcium activity from NMDA receptor activation can explain the dendritic atrophy of the hippocampus, some other mechanism must explain the dendritic hypertrophy of the basolateral amygdala.
This does not mean that glutamate does not play a critical role in the morphological changes that occur in the basolateral amygdala. NMDA receptor antagonists in the amygdala reduce anxiety-like behavior [41], and mice with decreased expression of the NR2A subunit of the NMDA receptor have reduced pyramidal dendritic spines in the BLA [42]. The reasons for this apparent discrepancy will be explored in the next section.
The Role of Neurotrophic Factors in the BLA
Calcium release in the cell has a multitude of effects, including triggering mitogen-activated protein kinases (MAPK) such as the extracellular signal-related kinases (ERK) 1 and 2 at the point of calcium entry [43,44], as well as activating protein kinase A (PKA) through the cAMP signaling pathway [45]. These kinases translocate to the nucleus, where they activate the protein cAMP response element-binding protein (CREB), which regulates the expression of proteins including BDNF [46]. Glutamate, through its action at NMDA and non-NMDA receptors, stimulates the expression of BDNF [47].
BDNF and other neurotrophic factors typically increase axonal and dendritic outgrowth [48,49]. It has been hypothesized that this action occurs in a Ca2+-dependent manner through changes in local cytoskeletal dynamics, along with increases in cytoskeletal protein and cell adhesion molecule gene expression [35,50]. Growth factors, therefore, alter the effects of glutamate in the cell and inhibit glutamate-induced dendritic atrophy. BDNF is released from the postsynaptic terminal and binds to TrkB channels in a retrograde fashion on the presynaptic cell, activating intracellular signaling pathways including phospholipase C-γ (PLCγ) and ERK signaling [51,52,53].
BDNF also increases postsynaptic responses to glutamate by triggering NMDA receptor phosphorylation and the rapid delivery of NR2B-containing NMDA receptors to the membrane [54]. This is supported by studies showing inhibition of NMDA receptors containing NR2B results in the loss of BDNF enhancement of glutamatergic transmission in the hippocampus [55]. While glutamate enhances BDNF, it can also shut off CREB expression through the action of glutamate at extrasynaptic NMDA receptors [56]. Glutamate and neurotrophic factors therefore work together to control neurite outgrowth and synaptogenesis.
Chronic stress has been shown to significantly alter BDNF expression in vertebrates. In the basolateral amygdala, chronic stress triggers increased expression of BDNF, while in the hippocampus, BDNF levels are significantly decreased [57]. The duration of BDNF expression in each of these areas mimics the duration of morphological changes, with BDNF returning to baseline in 21 days in the hippocampus but not the amygdala [57]. Furthermore, BDNF heterozygous deletion mutant mice have less dendritic branching in the hippocampus [58]. In clinical populations, atrophy in the hippocampus is associated with a variety of affective and anxiety disorders [59,60,61].
Based on this data, I propose a neuroplasticity hypothesis of chronic stress in which stress leads to increases in glutamatergic signaling in the amygdala, resulting in enhanced BDNF expression and dendritic outgrowth (Figure 1). In contrast, in the hippocampus, a signaling mechanism downstream from the glutamate and upstream of BDNF results in decreased BDNF signaling. This allows glutamate to inhibit microtubule and neurite elongation and trigger dendritic retraction [34,36,37,38,40]. These changes in morphology result in abnormal HPA axis signaling and an enhanced stress response.
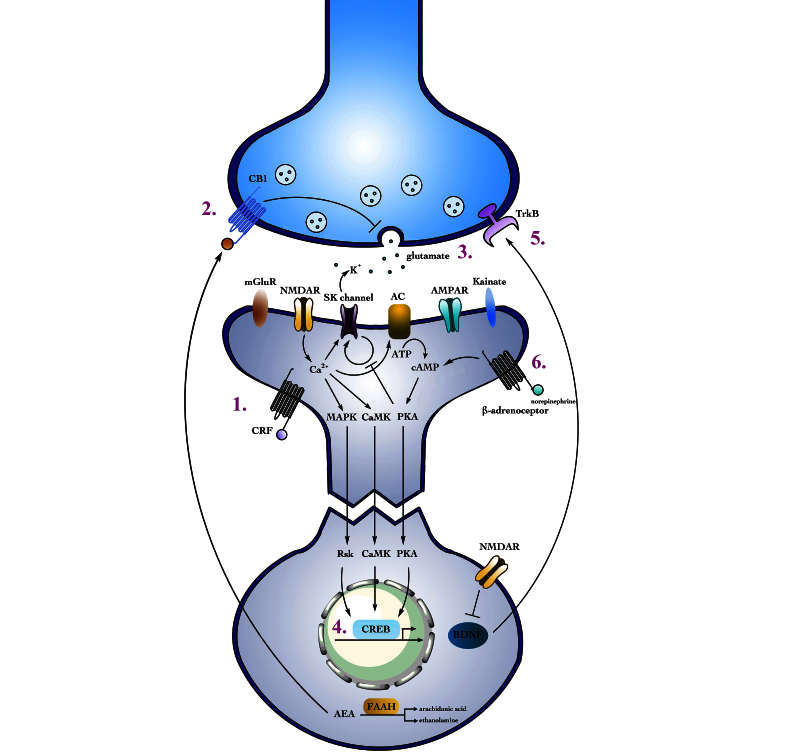
Figure 1.
The effect of chronic stress on glutamate synapses and neurotransmission in the amygdala. (1) Repeated activation of the CRF receptor following chronic stress increases NMDA receptor-mediated calcium influx. (2) FAAH activity increases during chronic stress, thereby decreasing AEA levels and increasing the release of glutamate from the presynaptic terminal. (3) Through the activity of FAAH and other factors, glutamate release increases following chronic stress along with NMDAR activity. (4) Increased calcium influx activates intracellular pathways that in turn activate CREB, which results in an up-regulation of BDNF. (5) BDNF increases axonal and dendritic outgrowth in the amygdala, acting retrogradely to activate intracellular pathways and postsynaptically increasing NMDAR activity. Notably, in the hippocampus BDNF levels are decreased and dendritic atrophy occurs. (6) Norepinephrine release is increased following stress, and its binding to β1-adrenoceptors triggers the cAMP and PKA signaling pathways. These pathways, in turn, disrupt the constitutive cycling of SK channels from the membrane and enhance excitatory neurotransmission in the amygdala.
This model would also suggest that drugs altering BDNF expression nonspecifically in the brain may be ineffective at treating pathologies caused by chronic stress because of the dichotomy between the hippocampus and amygdala. This is supported by recent studies showing transgenic overexpression of BDNF in mice prevents the hippocampal atrophy caused by chronic stress but increases spinogenesis in the BLA [62]. There is, therefore, a need to develop drugs with regional specificity that have BDNF as a molecular target.
The Role of Norepinephrine and SK Channels During Chronic Stress
Along with the morphological changes to the BLA, treatment with glucocorticoids increases the activity of principal glutamatergic neurons at least partially through the altered activity of small-conductance Ca2+-activated K+ channels (SK channels) [63,64]. The compound 1-EBIO, which increases SK channel activity, diminishes excitability of neurons in the lateral amygdala (LA) following chronic stress [63]. Similarly, overexpression of the type-2 SK channel (SK2) causes dendritic retraction in the BLA and reduces anxiety-like behavior [65].
The biological action of the SK channels has now been established. Activation of postsynaptic NMDA receptors results in the opening of SK channels, which are positioned in close proximity to NMDA receptors in the cell membrane [64]. These channels can be activated by release of intracellular Ca2+ stores through metabotropic glutamate receptors activity [64]. SK channels are stabilized by the actin cytoskeleton of the cell and constitutively removed from the membrane through a dynamin-dependent mechanism. The channels are then replaced through actin cytoskeleton-mediated transport [66].
Following a stressful stimuli, release of norepinephrine is increased in the basolateral amygdala [67]. Norepinephrine binds to β adrenoceptors within the basolateral amygdala, and β1 adrenoceptors trigger activation of the cAMP signaling pathway and PKA. PKA then alters the recycling of SK channels in the cell membrane, reducing the number of postsynaptic SK channels [66]. Furthermore, SK channels have been shown to regulate anxiety-like behavior and the secretion of corticosterone, a glucocorticoid involved in the stress response [65]. This theory is supported by studies showing disruption of the actin cytoskeleton results in loss of SK channels and β adrenoceptor-mediated action [66]. Chronic stress thus results in loss of SK channels and enhanced excitatory synaptic transmission in the amygdala.
Endocannabinoids and Glutamatergic Transmission
Endocannabinoids (eCBs) are atypical neurotransmitters in that they are not released vesicularly but are instead synthesized in response to increases in neuronal excitation or intracellular calcium [68]. eCBs act retroactively, binding to cannabinoid type one (CB1) receptors to inhibit neurotransmitter release [69,70,71]. Of the endocannabinoids, anandamide (AEA) is particularly active in the amygdala. AEA activation inhibits the release of glutamate and GABA in the BLA, with evidence that this signaling preferentially targets CB1 receptors on glutamatergic terminals [71]. Fatty acid amide hydrolase (FAAH), localized on pyramidal neurons [72], regulates AEA activity.
A recent study found FAAH activity increases during chronic stress [73], therefore decreasing levels of AEA and modulating the release of glutamate from the presynaptic terminal. It has been suggested that chronic stress, through an increase in FAAH activity, reduces the AEA activation of excitatory inputs to the pyramidal neurons of the BLA and results in abnormal glutamate release. Recent studies indicate that a similar mechanism occurs in the hippocampus [74].
Conclusions and Implications for Treatment
Chronic stress results in persistent and significant changes in brain morphology and functioning and is associated with the formation of a variety of affective and anxiety disorders. In this review, I have proposed the following neuroplasticity-related model (Figure 1): Chronic stress enhances glutamatergic signaling in the amygdala and hippocampus through repeated activation of the CRF receptor and increased FAAH activity. Low BDNF levels indicate that this abnormal glutamate transmission results in dendritic atrophy in the hippocampus [75], while in the amygdale, high BDNF levels alter glutamate signaling to increase synaptogenesis and dendritic outgrowth. Differences in BDNF are caused by a yet-unknown pathway downstream from glucocorticoids and glutamate. Chronic stress also increases the action of norepinephrine, which enhances excitatory transmission in the amygdala by facilitating the removal of SK channels from the membrane.
This model affords many potential therapeutic targets for chronic stress-related disorders. Studies indicate that current antidepressant treatments increase BDNF expression in the hippocampus [76,77], although there is currently no consensus on the actions of antidepressants on BDNF expression in the amygdala [78,79,80,81]. As discussed, returning BDNF expression to normal levels in the brain is complicated by differential expression of BDNF in the amygdala and hippocampus.
Glutamate is another target for drug development. Ketamine, a non-competitive NMDA receptor antagonist, triggers persistent and rapid antidepressant effects [82,83]. A recent study demonstrated that ketamine exerts its influence at least in part by increasing BDNF expression in the hippocampus and deactivating eEF2 kinase [84]. In the hippocampus CA3 region, mice lacking the NMDA receptor did not show evidence of chronic stress-induced dendritic atrophy [85], and co-treatment with the antidepressant fluoxetine or imipramine and the NMDA antagonist amantadine increases BDNF protein levels in the rat hippocampus [86,87]. In the amygdala, blockade of NMDA receptors prevents stress-induced anxiety-like behavior [41].
Finally, SK channels and FAAH represent additional molecular targets. Overexpression of SK2 has been shown to reduce anxiety-like behavior and trigger dendritic retraction in the BLA [65]. Similarly, mice deficient in FAAH or treated with an FAAH inhibitor do not have stress-induced changes in the structure of the amygdala and lack chronic-stress induced anxiety-like behavior [74].
These targets and their associated pathways all represent promising future routes in drug development for chronic stress-induced affective and anxiety disorders. While many studies have focused on the role of the hippocampus, only a fraction has targeted the amygdala. Because of its key role in regulating the HPA axis and its regulation of emotional responses, the amygdala and its role in the neuroplasticity model of stress must be considered in the development of future treatments for affective and anxiety disorders.
Abbreviations
- AEA
anandamide
- BDNF
brain-derived neurotrophic factor
- BLA
basolateral amygdale
- CB1
cannabinoid type one
- CREB
cAMP response element-binding protein
- CRF
corticotropin releasing factor
- CRS
chronic restraint stress
- eCBs
endocannabinoids
- eEF2
eukaryotic elongation factor 2
- ERK
extracellular regulated kinase
- FAAH
fatty acid amide hydrolase
- GLT-1
glutamate transporter
- HC
hippocampus
- HPA
hypothalamic-pituitary-adrenal
- LA
lateral amygdale
- MAPK
mitogen-activated protein kinases
- NMDA
N-methyl-D-aspartate
- PLCγ
phospholipase C-γ
- PKA
protein kinase A
- PVN
paraventricular nucleus
- SK channels
small-conductance Ca2+-activated K+ channels
- SK2
type-2 SK channel
References
- Conrad C, Magariños A, LeDoux J, McEwen B. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113(5):902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Wood G, Young L, Reagan L, McEwen B. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav. 2003;43(1):205–213. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24(9):540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Maren S. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18(11):3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana B, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai A, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann NY Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. N Engl J Med. 1997;337:1889–1895. doi: 10.1056/NEJM199712253372606. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB. et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47(9):769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54(3):200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Murray CCJL. The global burden of disease, 1990-2020. Nat Med. 1998;4(11):1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager T. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D, Meader N, Pilling S, Creed F, Goldberg D. Pharmacological interventions for people with depression and chronic physical health problems: systematic review and meta-analyses of safety and efficacy. Br J Psychiatry. 2011;198(3):179–188. doi: 10.1192/bjp.bp.110.077610. [DOI] [PubMed] [Google Scholar]
- Radley J, Rocher A, Miller M, Janssen W, Liston C, Hof P. et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51(6 Suppl 1):5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- Herman J, Tasker J, Ziegler D, Cullinan W. Local circuit regulation of paraventricular nucleus stress integration: Glutamate–GABA connections. Pharmacol Biochem Behav. 2002;71(3):457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Herman J, Mueller N. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann NY Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. A healthy body in a healthy mind–and vice versa–The damaging power of “uncontrollable” stress. J Clin Endocrinol Metab. 1998;83(6):1842–1845. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuroendocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Sandi C, Merino J, Cordero M, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its phosphorylation, and L1. Neuroscience. 2001;102(2):329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci. 1994;14(4):2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf O, May M, Wippich W, Hellhammer D. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58(17):1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Lupien S, Gaurdreau S, Tchiteya B, Maheu F, Sharma S, Nair N. et al. Stress-induced declarative memory impairment in healthy elderly subjects: a relationship to cortisol reactivity. J Clin Endocrinol Metab. 1997;82(7):2070–2075. doi: 10.1210/jcem.82.7.4075. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH, Wyatt RJ, Tizabi T. Region-selective stress-induced increase of glutamate uptake and release in rat forebrain. Brain Res. 1990;525(2):335–338. doi: 10.1016/0006-8993(90)90886-g. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Gault L, Yamamoto BK. Rapid communication: adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 2006;61(5):1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Fontella FU, Vendite DA, Tabajara AS, Porciúncula LO, Torres ILDS, Jardim FM. et al. Repeated restraint stress alters hippocampal glutamate uptake and release in the rat. Neurochem Res. 2004;29(9):1703–1709. doi: 10.1023/b:nere.0000035805.46592.6c. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Muñoz C, Rothstein J. et al. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci USA. 2004;101(7):2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Grillo CA, Piroli G, Rothstein JD, McEwen BS, Reagan LP. Glucocorticoid regulation of GLT-1 glutamate transporter isoform expression in the rat hippocampus. Neuroendocrinology. 2006;83(5-6):371–379. doi: 10.1159/000096092. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L. et al. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25(13):3270–3279. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24(14):3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Taylor-Hunter A, Kater SB. Neurite outgrowth in individual neurons of a neuronal population is differentially regulated by calcium and cyclic AMP. J Neurosci. 1988;8:1704–1711. doi: 10.1523/JNEUROSCI.08-05-01704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann NY Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg RC. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Haga T, Abe T, Kurokawa M. Polymerization and depolymerization of microtubules in vitro as studied by flow birefringence. FEBS Lett. 1974;39(3):291–295. doi: 10.1016/0014-5793(74)80133-x. [DOI] [PubMed] [Google Scholar]
- Olmsted JB, Borisy GG. Characterization of mictrotubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium as sculptor and destroyer of neural circuitry. Exp Gerontol. 1992;27(1):29–49. doi: 10.1016/0531-5565(92)90027-w. [DOI] [PubMed] [Google Scholar]
- Lankford KL, Letourneau PC. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J Cell Biol. 1989;109(3):1229–1243. doi: 10.1083/jcb.109.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec RE, Burton P, Shallow T, Budgell J. Unilateral block of NMDA receptors in the amygdala prevents predator stress-induced lasting increases in anxiety-like behavior and unconditioned startle--effective hemisphere depends on the behavior. Physiol Behav. 1999;65(4-5):739–751. doi: 10.1016/s0031-9384(98)00225-x. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S. et al. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30(15):5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJL, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4(6):565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294(5541):333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G. et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21(4):869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ Influx Regulates BDNF Transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1988;20(4):709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Zafra F, Castrén E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA. 1991;88(22):10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5(11):1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Horch HW. Local effects of BDNF on dendritic growth. Rev Neurosci. 2004;15(2):117–129. doi: 10.1515/revneuro.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Wong ROL. Regulation of dendritic growth and plasticity by local and global calcium dyanmics. Cell Calcium. 2005;37(5):403–409. doi: 10.1016/j.ceca.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yamagishi S, Adachi N, Matsumoto T, Yokocaku D, Yamada M. et al. Brain-derived neurotrophic factor-induced potentiation of Ca2+ oscillations in developing cortical neurons. J Biol Chem. 2002;277(8):6520–6529. doi: 10.1074/jbc.M109139200. [DOI] [PubMed] [Google Scholar]
- Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su T, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J Biol Chem. 2006;281(18):12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14(23):2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35(2):208–219. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Black IB, Plummier MR. Blockade of NR2B-containing NMDA receptors prevents BDNF enhancement of glutamatergic transmission in hippocampal neurons. Learn Mem. 1999;6(3):257–266. [PMC free article] [PubMed] [Google Scholar]
- Cordero M, Rodriguez J, Davies H, Peddie C, Sandi C, Stewart M. Chronic restraint stress down-regulates amygdaloid expression of polysialylated neural cell adhesion molecule. Neuroscience. 2005;133(4):903–910. doi: 10.1016/j.neuroscience.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Brusés JL, Rutishauser U. Roles, regulation, and mechanism of polysialic acid function during neural development. Biochimie. 2001;83(7):635–643. doi: 10.1016/s0300-9084(01)01293-7. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17(4):879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Djebbara-Hannar Z, Zhang H, Paccaud J, Durbec P, Rougon G. et al. PSA-NCAM modulates BDNF-dependent survival and differention of cortical neurons. Eur J Neurosci. 2001;13(7):1391–1402. doi: 10.1046/j.0953-816x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28(42):10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal BDNF DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45(7):919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Neasta J, Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. J Biol Chem. 2010;285(25):19043–19050. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67(12):1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8(5):635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Mitra R, Ferguson D, Sapolsky RM. SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Mol Psychiatry. 2009;14(9):847–855. doi: 10.1038/mp.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci. 2008;28(43):10803–10813. doi: 10.1523/JNEUROSCI.1796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66(3):253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Deutsch DG. Biochemistry of the endogenous ligands of cannabinoid receptors. Neurobiol Dis. 1998;5(6 Pt B):386–404. doi: 10.1006/nbdi.1998.0214. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Retrograde signaling by endocannabinoids. Handb Exp Pharmacol. 2005;(168):367–383. doi: 10.1007/3-540-26573-2_12. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signaling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81(2):224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32(6):1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Gulyas A, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F. et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20(2):441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM. et al. Disruption of fatty acid amide hydrolysis activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry. 2012 Jul 10; doi: 10.1038/mp.2012.90. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A. et al. Antidepressant activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62(10):1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Kiselycznyk C, Holmes A. Stress-Induced deficits in cognition and emotionality: a role for glutamate. Curr Top Behav Neurosci. 2012 Jan 20; doi: 10.1007/7854_2011_193. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A Neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Hendry RM, Reznikov LR, Piroli GG, Wood GE, McEwen BS. et al. Tianeptine increases brain-derived neurotrophic factor expression in the rat amygdala. Eur J Pharmacol. 2007;565(1-3):68–75. doi: 10.1016/j.ejphar.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Della FP, Abelaira HM, Réus GZ, Ribeiro KF, Antunes AR, Scaini G. et al. Tianeptine treatment induces antidepressive-like effects and alters BDNF and energy metabolism in the brain of rats. Behav Brain Res. 2012;233(2):526–535. doi: 10.1016/j.bbr.2012.05.039. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya V. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45(4):553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS. et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Nakai T, Skamoto K, Nagafusa Y, Higuchi T, Toru N. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT. 2010;26(3):223–227. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Miracle AD, Wellman CL, Nakazawa K. Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience. 2011;174:26–36. doi: 10.1016/j.neuroscience.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogóz Z, Skuza G, Legutko B. Repeated co-treatment with fluoxetine and amantadine induces brain-derived neurotrophic factor gene expression in rats. Pharmacol Rep. 2008;60(6):817–826. [PubMed] [Google Scholar]
- Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P. et al. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav Brain Res. 2011;221(1):166–171. doi: 10.1016/j.bbr.2011.02.024. [DOI] [PubMed] [Google Scholar]