Abstract
Information processing in individuals with autism is marked by a unique interplay of strengths and weaknesses that in concert distinguishes social cognition in autism from individuals with typical-functioning brains. In autism, difficulties with higher cognitive processing and enhancement of low-level visuospatial processing, such as in visual search tasks, may lead to diminished central coherence, which has the potential to hinder how an individual functions in social interactions where integration of components such as intention, emotion, and context paints the global picture necessary for social processing. A more thorough understanding of the cognitive and neural processes in autism is important for the advancement of intervention programs. The intention of this review is to discuss the implications of neuroimaging and behavioral studies that have analyzed the higher cognitive functions in individuals with high-functioning autism, with a particular emphasis on studies that have investigated visuospatial processing.
Keywords: autism, visuospatial processing, fMRI, weak central coherence, functional connectivity, functional underconnectivity, mindblindness
Introduction
According to estimates from the Center for Disease Control (CDC), one in 88 children are identified as having an Autism Spectrum Disorder (ASD), with the prevalence in males being five times greater than that in females [1]. The terms ASD and autism are often used interchangeably in the current clinical and research settings due to the historic challenges in segmenting the population because of the overlapping symptoms among the multiple Autism Spectrum Disorders. In the fourth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [2], ASD in fact is designated as an umbrella term under which there are five distinct pervasive developmental disorders: Rett syndrome, Childhood Disintegrative Disorder (CDD), autistic disorder, Pervasive Developmental Disorder Not Otherwise Specified (PDD-NOS), and Asperger syndrome. Rett syndrome is a disorder associated with a genetic mutation, and it mostly affects females. It is characterized by a series of events that includes normal childhood maturation and achievement of developmental milestones up until around 5 months of age, after which there is a significant regression in development that results in severe impairment of the child’s psychomotor skills, social engagement, and language abilities. Features of Rett syndrome include dementia, characteristic hand movements (e.g., handwringing), and deceleration of cranial growth (in contrast to accelerated head growth seen in autism). CDD is an exceptionally rare syndrome in which children develop normally for at least 2 years and then experience a dramatic regression of the previously acquired skills, much like Rett syndrome, which leaves the children with severely affected language, social, and motor skills. However, CDD is also associated with seizures and metabolic disorders [3]. Autistic disorder, PDD-NOS, and Asperger syndrome are phenotypically most similar and the most difficult to dissociate. Individuals with autism have marked difficulty with social interaction, certain cognitive skills, and language ability. Asperger syndrome, which is considered a milder variant, similarly affects cognitive and social functioning, but in contrast to autism, there is no generalized impairment of language development. Individuals who fall under the category of PDD-NOS display difficulties with language, social interaction, and cognition, similar to autism and Asperger syndrome, but do not share the full extent of the symptoms of either disorder [2]. Although the causes and/or inciting factors of these various Autism Spectrum Disorders have yet to be definitively isolated, there is currently a basic understanding that the etiologies of these disorders are genetic, neurological, and cognitive in nature [4].
Autistic disorder, more commonly known as autism, is defined as a pervasive development disorder marked by impairments in social interaction, language, and communication and is characterized by restricted interests and repetitive behaviors [5]. Accordingly, individuals with autism may display a narrow range of interest, often presenting at a young age with a preoccupation with particular objects or toys. Repetitive behavior can involve spending hours doing a specific task, for example, a child lining up his/her toys in order of size. A majority of individuals with autism have a developmental delay in verbal communication. Language and communication abilities, if they develop at all, are often affected by pronoun reversal and echolalia, i.e., the involuntary repetitive imitation of vocalizations made by another individual. About 20 percent of individuals with autism display average to above average intellectual ability relative to individuals with typically functioning brains and are referred to as having high-functioning autism [4,5]. For these individuals with high-functioning autism, performance of tasks involving simple language, memory, arithmetic, and rule-learning are in essence unimpaired. However, difficulty with more complex problem-solving, language, and memory and concept formation tasks impedes full-functioning in society [6]. For example, in these high-functioning individuals, communication is hampered by the inability to respond appropriately to social cues, especially with regard to emotional context and common social expressions. Current treatment of individuals with autism centers on behavioral therapy to improve functioning in activities of daily living as well as enhancement of communication and interpersonal skills.
Although not part of the triad of symptoms, notable aspects of information processing in individuals with autism are increased reliance on the visuospatial method of processing and, correspondingly, a perceived advantage in that realm. Temple Grandin, an individual with high-functioning autism, has written in her book, Thinking in Pictures, that she attributes much of her cognitive functioning to dependence on visuospatial processing and that she, as many others with high-functioning autism have described, sees the world through pictorial representations [7]. This greater command of visual cues over social and lingual cues in day-to-day operations may even help to explain some of the more archetypal characteristics of individuals with autism. It is still unclear, though, whether the dependence on visuospatial information processing causes the typically observed disconnect between visuospatial function and social cognition or if, instead, enhancement of visuospatial processing results as compensation for autonomous functioning of visuospatial brain regions that are disengaged from brain areas that typically perform executive functions. Regardless, the distinct pattern of behavior in autism points clearly to an atypical neural circuitry with alternate mechanisms of information processing. The distinct schemas of information processing at the cognitive and neural levels have been examined as a base from which the characteristic features of autism stem. Behavioral and neuroimaging studies have been successful in beginning to uncover the neural bases of autism.
How Does Information Processing in Autism Differ from the Typically Functioning Brain?
A multitude of theories have been proposed to characterize information processing in autism, which is typically marked by the dichotomy of an individual exhibiting difficulties in certain higher-cognitive processing tasks along with a simultaneous enhancement of lower-level visuospatial processing. One such higher-cognitive process that is impaired in autism is described as “mindblindness” [8]. An impairment of theory of mind, mindblindness is the inability to attribute thoughts, feelings, emotions, and other mental states to oneself and others. Without the ability to understand that others are capable of formulating their own thoughts, it is impossible to interpret action and intention. This leaves individuals with autism incapable of “mind-reading,” a skill that is imperative for facilitating social interaction among typically functioning individuals. As in a common example used to describe this phenomenon, looking down at a watch during a meeting implies an individual’s desire to know the time and perhaps signals to the other parties in attendance to consider that time is running out, that the meeting may be winding down, that the watch-holder is busy and may have another meeting coming up, or is bored and waiting for the meeting to end. In order to have an effective social exchange, one must interpret the meaning behind such a simple action by getting into the mindset of another individual and attempting to predict that person’s intentions. The various components in such a scenario must be integrated together and holistically understood before a contextually appropriate reaction can take place, e.g., accelerating the pace of the meeting, concluding the exchange, moving on to another more interesting topic, etc. Without the ability to see “the big picture,” individuals with autism often struggle with formulating a suitable response in this type of social exchange.
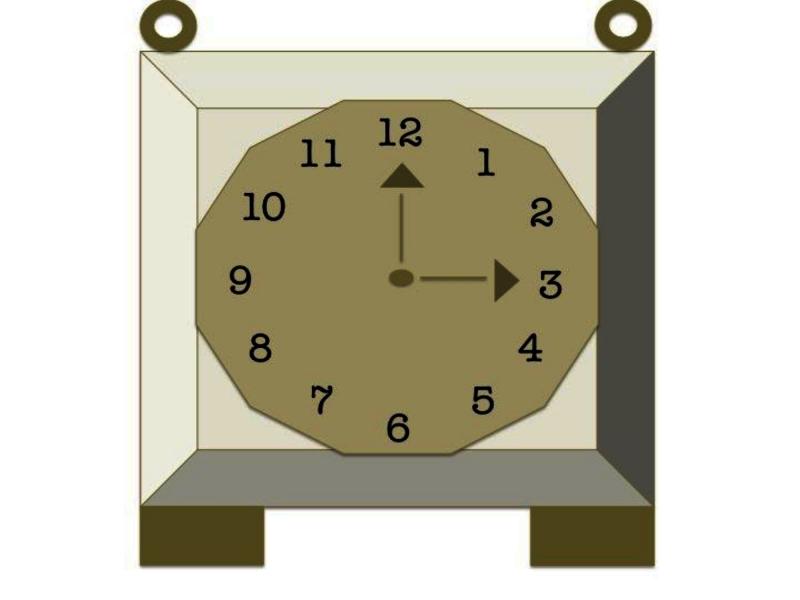
One of the cognitive theories of autism, the Weak Central Coherence (WCC) theory [9], discusses this tendency in autism to “miss seeing the forest for the trees.” The WCC theory offers two models to explain how this detail-focused bias in individuals with autism is both a disadvantage (the deficit model) and an advantage (the strength model), depending on the information processing tasks at hand. The WCC theory suggests that the impairment of social and cognitive functioning in autism is due to difficulties in putting information together to form context and hence failure to see “the big picture.” This is explained as a result of an individual’s inability to see the relationship between multiple components of a depiction [9] or a detail-oriented bias [10]. This is evident in tests, such as the homograph test [11], in which it has been observed that autistic individuals have difficulty distinguishing the appropriate pronunciation of homonyms when given two different contexts, e.g., there was a tear in her dress vs. there was a tear in her eye. Another example of the deficit model is with regard to social cognition and social interaction. As discussed earlier, in these instances, autistic individuals display an inability to holistically integrate the components of a social situation in order to comprehend its greater meaning. As such, it is more difficult for people with autism to distinguish emotions, respond appropriately to others’ emotions, or to comprehend the relevance of emotions in social situations. On the other hand, the “strength” model of the WCC theory proposes that individuals with autism simply have a superior ability to process features, rather than an inferior ability to process a global pattern [12,13]. The increased focus on details in autism has been found to be advantageous in tasks such as the Embedded Figures Task (EFT) and the Block Design (BD) component of the Wechsler IQ test. In an EFT, subjects are asked to identify a simple image concealed within a more intricate image. For example, the subject is asked to find a triangle hidden within a more complex drawing of a clock (Figure 1). In the BD task, the subject is given blocks that individually have different color patterns and is asked to arrange the blocks according to a given pattern. Performance in such tasks requires ignoring the global aspects and focusing on the details, and subjects with autism exhibited intact or superior performance when compared to their typically functioning counterparts [14,15].
Figure 1.
The Embedded Figures Task (EFT) is a visual search task that asks participants to identify a simple (local) image hidden in a more complex (global) image. For example, a participant may be presented with the image in the figure and asked to identify a triangle.
The WCC theory also relates to the Gestalt principles of psychology, which emphasize the human tendency to see things in global form. However, the difference between global and Gestalt stimuli must be explained. Global stimuli are recognized without regard to the specificity of the local element (Figure 2), while the Gestalt stimuli are apparent by the precise orientation of particular local features [16,17,18]. For example, a global figure might be composed of nine small triangles oriented to construct a greater triangle, while a Gestalt figure might include three circles placed in the shape of a triangle with precise pie slice-shaped exclusions that when seen in the cohesive “whole” form induces the mind to re-organize the seemingly unrelated components of the figure to perceive a triangle where one has not been drawn (Figure 3). A parallel can be made between this Gestalt processing and social cognition, which also requires the integration of seemingly unconnected stimuli to derive the nature of a given situation. Examination of Gestalt perception in high-functioning individuals with autism, similar to investigations of global processing, has revealed inferior Gestalt perceptual ability [19].
Figure 2.
The image in the figure is an example of a global/local task. A participant may be asked to identify the larger image (square) in the global task and identify the smaller image (circle) in the local task. Note that the global image of a larger rectangle only requires specific placement of the local features and does not require specific local features in order to be perceived.
Figure 3.
According to the Gestalt Principle of psychology, this image is perceived to include a triangle and is dependent on the precise orientation of specific local features. At the local level, the partial circles are perceived. When integrated together at the Gestalt level, it almost looks like there are three circles in the background that are partially covered up by a white triangle in the forefront.
There is conflict in the literature concerning the performance of autistic individuals on tasks that require global and local visuospatial information processing compared to typically functioning individuals. Performance is compared based on accuracy and reaction time of the task completed. While some studies find superior performance by the autism group on tasks that require participants to focus on the details and some find inferior performance of individuals with autism on tasks that require attention to the global picture, other studies find that there is no significant difference in accuracy or reaction time between the two groups. The “hierarchization deficit model” [20] provides an explanation of the differences in performance in visuospatial tasks and contrasts the WCC model by reasoning that individuals with autism are equally capable of performing global and local tasks. This theory proposes that the default way of information processing in typical individuals is global and the default in people with autism is local. Accordingly, individuals with autism are capable of performing equally to control participants on visuospatial processing tasks, but display a preference for local processing over global processing. On the other hand, the variation in results across the behavioral studies may be explained by the inconsistency in methodological and analysis techniques used [21]. Additionally, the heterogeneity within autism itself may make it difficult to analyze the extent of the detail-focused bias. Interestingly, White et al. [21] elucidated how studies that demonstrated a considerable difference in performance on global versus local tasks used lower-functioning groups than the studies that did not discover a significant difference in performance. The severity of ASD may be positively correlated with the intensity of the difference in central coherence, thereby supporting the case for the Weak Central Coherence theory [21].
Minshew et al. [6] suggests that performance capability of individuals with autism on mental tasks, including visuospatial tasks, is dependent on the complexity of the task and proposes that autism is characterized by a disorder of complex information processing rather than a detail-oriented bias. Similarly, Bertone et al. [22] proposes a complexity-specific account that characterizes visuospatial information processing in individuals with autism. The study by Bertone et al. suggested that high-functioning autistic participants had enhanced performance in less complex image discrimination tasks (first order or luminance-defined processing) and impaired performance in tasks that were more complex (second-order or texture-defined processing). In typically functioning participants, the less complex image discrimination task is associated with a single visual area (V1) versus in the more complex image discrimination task, which is associated with recruitment of multiple visual areas (V1+V2/V3). A proposed “superior when autonomous, inferior when synchronized” theory explains the difference in performance of more complex versus less complex visuospatial processing in autism. This theory suggests a processing pathway that is efficient and superior when recruited in isolation, as in the less complex task. In contrast, the more complex image discrimination task that requires multiple visual areas to work in sync are less efficient and perform inferiorly in high-functioning autism.
Cortical Correlates
Most of the previous studies that have investigated visuospatial information processing in high-functioning adults with autism used mainly behavioral tasks that gathered data such as accuracy and reaction time to make inferences about cognitive processing styles. There are only a few neuroimaging studies that target global and local processing in autism. Functional magnetic resonance imaging (fMRI) scanners have been a prominent tool used in gathering information about autism. fMRI studies have been used to measure brain activation in cortical gray matter regions during performance of behavioral tasks and, through group analysis techniques that subtract intensity of cortical activation, allow examiners to identify how cortical activation patterns differ between participants with autism and typically functioning controls. Data gathered from the scanner along with data collected on accuracy and reaction time help to develop a fuller story of how visuospatial processing in autism is different. The Embedded Figures Task has been used commonly in fMRI studies to evaluate cortical activation in global and local aspects of static visuospatial information processing tasks. In an fMRI study in which subjects performed the EFT, Ring et al. [23] found that there were differences between the autism and control groups in the cortical regions recruited for executing the task and suggested that the control group’s mechanism for task completion is reliant on working memory systems in contrast to the autism group, which demonstrated a different activation pattern that may suggest a greater dependence on visual systems for visuospatial reasoning. In particular, this study found a lack of frontal activation in autism and greater reliance on the inferior temporal lobe for complex visual search tasks. In a similar fMRI study utilizing the EFT, Damarla et al. [24] identified differences in activation in several distinct cortical regions, with autism observed to have reduced activation in the left dorsolateral prefrontal and inferior parietal areas, and enhanced activation in bilateral superior parietal extending to the inferior parietal and right occipital regions when compared to typically developing controls. In an additional study utilizing the EFT, Manjaly et al. [25] found that individuals within the ASD group had greater activation in the right primary visual cortex and bilateral extrastriate areas, and the control group of typically developing individuals recruited the left parietal and premotor regions to complete the task. The general interpretation of these findings is that during visuospatial tasks, the ASD groups display greater dependence on visual processing areas, while the control groups demonstrate a significant dependence on regions of the brain that are traditionally thought of as executive functioning regions. In another fMRI study, Lee et al. [26] discovered that during EFT performance, control participants had activation in the left dorsolateral, medial, and dorsal premotor regions of the frontal cortex and bilateral superior parietal and occipital cortical regions. ASD participants, in contrast, activated only the dorsal premotor, left superior parietal, and right occipital regions. Lee et al. proposes that the left dorsolateral prefrontal recruitment observed in the control group suggests involvement in verbal working memory, indicating that typically functioning individuals were reliant on additional verbal strategies for working through the complex visual EFT. Generally, across these multiple fMRI studies, the ASD group had greater involvement of the visual processing areas of the cortex during performance of the EFT task compared to the control group (Table 1). As discussed earlier, the behavioral analysis in many of the studies did not reveal a significant difference in accuracy or response time; however, the activation patterns indicate a greater suppression of the global bias in typically functioning individuals and a more concise pattern of involvement in ASD individuals in local processing, which is consistent with the Weak Central Coherence theory [26].
Table 1. Cortical activation differences between autism and control groups in four major fMRI studies utilizing the Embedded Figures Task to examine visuospatial processing..
|
Areas in which the control group showed more activation than the
autism group
| ||
| Study | Cortical Activation | General Function of Cortical Region, Relevant to EFT Performance |
| Ring et al. [23] | Prefrontal cortical areas | Executive function |
| Damarla et al. [24] | Left dorsolateral prefrontal cortex | Executive function |
| Left superior medial frontal gyrus | Executive function | |
| Left inferior parietal areas | Sensory information interpretation | |
| Manjaly et al. [25] | Left parietal areas | Sensory information integration |
| Left premotor areas | Planning movement | |
| Lee et al. [26] | Left dorsolateral prefrontal | Executive function, Verbal strategy |
| Left medial premotor | Planning movement | |
| Left dorsal premotor regions | Motor responses, Eye movement | |
| Bilateral parietal and occipital cortices | Visual working memory | |
| Bilateral ventral temporal | Visuospatial processing | |
|
| ||
|
Areas in which the autism group showed more activation than the
control group
| ||
| Study | Cortical Activation | General Function of Cortical Region, Relevant to EFT Performance |
|
| ||
| Ring et al. [23] | Ventral occipitotemporal regions | Visuospatial processing |
| Damarla et al. [24] | Bilateral superior parietal region extending to inferior parietal and right occipital | Visuospatial processing |
| Manjaly et al. [25] | Right primary visual cortex | Visuospatial processing |
| Bilateral extrastraite area | Visuospatial processing | |
| Lee et al. [26] | Dorsal premotor regions | Planning movement |
| Left superior parietal | Visuospatial processing | |
| Right occipital regions | Visuospatial processing | |
In contrast, the control groups demonstrated greater activation in cortical regions responsible for higher-order thought processes. According to Friston et al. [27], in typically functioning individuals, the brain generates global inferences by making predictions of how sensory information fits in with prior knowledge and situational context and evaluates its significance based on a developed theory of mind. This inference-making ability is important for formulation of proper responses to stimuli in the surrounding world. When there is a mismatch between prediction developed in executive functioning regions and sensory information from visuospatial processing cortical regions, there is greater error in inference-making ability and disabled formation of proper responses. The activation and behavioral patterns identified in the various studies discussed earlier suggest that there is a lack of coordination between executive functioning areas and visuospatial sensory processing in autism, and as supported by Loth et al. [28] and Soulieres et al. [29], this altered top-down influence may consequently produce deficient modulation of visuospatial information by context and prior knowledge. This disruption in autism in multiple level analyses may explain the inability to access the global meaning developed by integrating sensory information, prior knowledge, context, and theory of mind.
While the WCC theory explains the cognitive aspects of information processing in autism, the cortical underconnectivity theory [30,31] provides a compelling related neural model of autism. Functional connectivity is defined as the collaboration between brain areas through synchronization. Functional underconnectivity refers to the lower levels of synchronization of these brain areas in the performance of certain cognitive tasks. The theory of functional underconnectivity helps to explain the consistent discrepancy in mechanism of task completion between autism and control groups, suggesting that individuals with autism are more reliant on posterior regions of the brain to work in autonomy rather than collaborating with the frontal areas to perform functions of visuospatial as well as cognitive tasks. Functional underconnectivity in autism has been demonstrated in several higher cognitive functions, such as sentence comprehension [30,31,32] and theory of mind [33]. The postulates of underconnectivity theory are consistent with the WCC theory. While WCC theory talks about failure of integrating cognitive information to form coherence, the underconnectivity theory provides the neural bases of weak coherence in autism. In autism, the brain areas (especially frontal and posterior) do not work as a team to overcome increased computational demand that accompanies more complex tasks. In other words, such underconnectivity among brain regions might be the reason behind the difficulty faced by individuals with autism when performing highly demanding global processing tasks, such as social cognition, problem solving, inhibition, and language comprehension. Just et al. [33] elaborates that the decreased communication between frontal and parietal areas is further demonstrated by decreased communication bandwidth, meaning the maximal rate of data transmission, between the regions. This impairment when integrating information across cortical regions is supported by the “superior when autonomous, inferior when synchronized” theory suggested as an explanation of the impairment in more-complex tasks and enhancement in less-complex tasks. Additionally, Just et al. suggests that in autism, there is a preference to complete tasks using posterior cortical regions. The study also demonstrated that in autism there is a greater dependence on the posterior cortical areas to perform executive functions independently of the frontal regions. This may lead individuals with autism to process content in a more visual or graphic context due to a decreased dependence on frontal areas [34]. The important causal relationship between connectivity and perception is only starting to be mapped out.
Conclusion
A comprehensive understanding of how the trends obtained from behavioral studies compliment cortical activation patterns derived from imaging studies is important for developing insight into how information processing differs in individuals with ASD. This perspective is crucial for the advancement of intervention programs and designing recommendations for health care providers on specific treatment approaches for both children and adults with autism. Additionally, this understanding of how individuals with autism process the stimuli in the world around them is important for parents and caretakers. For example, understanding that individuals with autism have a detail-focused bias and preference for pictorial representations of concepts suggests that parents could use picture books or other simple, static visual media over oral reasoning or descriptions to explain ideas to help facilitate their child’s educational and social development.
Further studies need to be conducted in order to advance our understanding of the neural circuitry that supports cognitive functioning and how these circuits are differentially engaged in people with autism relative to typically functioning individuals. Perhaps an experiment that examines the detail-focused bias in Gestalt processing can help draw a parallel to the disruptions in social cognition. Continued analysis has the potential of developing valuable clues that can help in cultivating more tailored intervention programs to suit the needs of the unique cognitive styles in autism.
Acknowledgments
I would like to thank Rajesh K. Kana, PhD, of the Department of Psychology at the University of Alabama at Birmingham for his assistance in editing this manuscript and providing critical advice.
Abbreviations
- CDC
Centers for Disease Control
- ASD
Autism Spectrum Disorder
- CDD
Childhood Disintegrative Disorder
- PDD-NOS
Pervasive Developmental Disorder Not Otherwise Specified
- WCC
Weak Central Coherence
- EFT
Embedded Figures Task
- BD
Block Design
- V1
single visual area
- fMRI
functional magnetic resonance imaging
References
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators, Centers for Disease Control and Prevention. Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-IV. Washington: American Psychiatric Association; 1994. [Google Scholar]
- Davis AS, editor. The Handbook of Pediatric Neuropsychology. New York: Springer Publishing Company; 2010. [Google Scholar]
- Kana RK, Libero LE, Moore MS. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys Life Rev. 2011;8(4):410–437. doi: 10.1016/j.plrev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Volkmar FR. Handbook of Autism and Pervasive Developmental Disorders. New York: John Wiley & Sons; 2010. [Google Scholar]
- Minshew NJ, Goldstein G. Autism as a disorder of complex information processing. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:129–136. [Google Scholar]
- Grandin T. Thinking in pictures. New York: Vintage Press; 2006. [Google Scholar]
- Baron-Cohen S. Mindblindness. Cambridge: MIT Press; 1995. [Google Scholar]
- Frith U. Autism: explaining the enigma. Oxford: Blackwell; 1989. [Google Scholar]
- Happé FG. Autism: cognitive deficit or cognitive style. Trends Cogn Sci. 1999;3(6):216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Happé FG. An advanced test of theory of mind: understanding of story characters' thoughts and feelings by able autistics, mentally handicapped and normal children and adults. J Autism Dev Disord. 1994;24(2):129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Plaisted K, Saksida L, Alcantara J, Weisblatt E. Towards an understanding of the mechanisms of weak central coherence effects: experiments in visual configural learning and auditory perception. Philos Trans R Soc Lond B Biol Sci. 2003;358(1430):375–386. doi: 10.1098/rstb.2002.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high functioning autism: Evidence from Multiple Paradigms. J Child Psychol Psychiatry. 2003;44(6):904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. An islet of ability in autistic children: a research note. J Child Psychol Psychiatry. 1983;24(4):613–620. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Shah A, Frith U. Why do autistic individuals show superior performance on the block design task? J Child Psychol Psychiatry. 1993;34(8):1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Pomerantz JR. Global and local precedence: Selective attention in form and motion perception. J Exp Psychol Gen. 1983;112(4):516–540. doi: 10.1037//0096-3445.112.4.516. [DOI] [PubMed] [Google Scholar]
- Kimchi R, Palmer SE. Form and texture in hierarchically constructed patterns. J Exp Psychol Hum Percept Perform. 1982;8(4):521–535. doi: 10.1037//0096-1523.8.4.521. [DOI] [PubMed] [Google Scholar]
- Kimchi R. Primacy of wholistic processing and global/local paradigm: A critical view. Psychol Bull. 1992;112(1):24–38. doi: 10.1037/0033-2909.112.1.24. [DOI] [PubMed] [Google Scholar]
- Bolte S, Holtmann M, Poustka F, Scheurich A, Schmidt L. Gestalt perception and local-global processing in high-functioning autism. J Autism Dev Disord. 2007;37(8):1493–1504. doi: 10.1007/s10803-006-0231-x. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- White SJ, Saldana D. Performance of children with autism on the embedded figures test: a closer look at a popular task. J Autism Dev Disord. 2011;41:1565–1572. doi: 10.1007/s10803-011-1182-4. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulis complexity. Brain. 2005;128(Pt 10):2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Ring HA, Baron-Cohen S, Wheelwright S, Williams SC, Brammer N, Andrew C. et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of the embedded figures task performance. Brain. 1999;122(Pt 7):1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL, Minshew NJ. et al. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res. 2010;3(5):273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjaly ZM, Bruning N, Neufang S, Stephan KE, Brieber S, Marshall JC. et al. Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. Neuroimage. 2007;35(1):283–291. doi: 10.1016/j.neuroimage.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Foss-Feig J, Henderson JG, Kenworthy LE, Gilotty L, Gaillard WD. et al. Atypical neural substrates of Embedded Figures Task performance in children with Autism Spectrum Disorder. Neuroimage. 2007;38(1):184–193. doi: 10.1016/j.neuroimage.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Prediction, perception and agency. Int J Psychophysiol. 2012;83(2):248–252. doi: 10.1016/j.ijpsycho.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth E, Carlos Gomez J, Happe F. Detecting changes in naturalistic scenes: contextual inconsistency does not influence spontaneous attention in high-functioning people with autism spectrum disorder. Autism Res. 2008;1(3):179–188. doi: 10.1002/aur.19. [DOI] [PubMed] [Google Scholar]
- Soulieres I, Mottron L, Saumier D, Larochelle S. Atypical categorical perception in autism: autonomy of discrimination? J Autism Dev Disord. 2007;37(3):481–490. doi: 10.1007/s10803-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(Pt 9):2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI Investigation of Working Memory for Faces in Autism: Visual Coding and Underconnectivity with Frontal Areas. Cereb Cortex. 2008;18(2):289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VK, Kana RK, Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]