Abstract
Synesthesia, the conscious, idiosyncratic, repeatable, and involuntary sensation of one sensory modality in response to another, is a condition that has puzzled both researchers and philosophers for centuries. Much time has been spent proving the condition’s existence as well as investigating its etiology, but what can be learned from synesthesia remains a poorly discussed topic. Here, synaesthesia is presented as a possible answer rather than a question to the current gaps in our understanding of sensory perception. By first appreciating the similarities between normal sensory perception and synesthesia, one can use what is known about synaesthesia, from behavioral and imaging studies, to inform our understanding of “normal” sensory perception. In particular, in considering synesthesia, one can better understand how and where the different sensory modalities interact in the brain, how different sensory modalities can interact without confusion ― the binding problem ― as well as how sensory perception develops.
Keywords: synesthesia, synaesthesia, cross-modal perception, sensory perception, binding problem, Maurer
Introduction
The word synesthesia has an ancient Greek origin: syn, meaning together, and aesthesis, meaning sensation [1]. This is an apt etymology for a condition whereby stimulation of one sensory pathway of the brain leads to the autonomatic and involuntary sensation of a second pathway. For example, the perception of a vivid red (the inducer) could cause the synesthete to hear a middle C (the concurrent) (Figure 1). The prevalence of synesthesia is debated, but is estimated to be between 1 percent and 5 percent [2].
Figure 1.

An example of synesthetic perception. Diagram demonstrating what a synesthete might see when they look at the above characters.
The etiology of synesthesia is a contentious subject. Today, a debate exists between Ramachandran and Hubbard’s hyperconnectivity hypothesis and Grossenbacher and Lovelace’s disinhibition-unmasking hypothesis. The former describes direct connections between sensory cortical regions, while the latter implicates a loss of inhibitory feedback between the cortical regions [3,4]. Other theories include Calkin’s learned association theory, Cytowic’s awareness theory, and Maurer’s neonatal synaesthesia theory [5,6,7] (Table 1). This essay does not attempt to resolve the parsimonious questions of synesthesia etiology but rather addresses a pressing issue: what can one learn of sensory perception from synesthesia.
Table 1. The various conflicting theories of synaesthesia etiology.
| Theory | Author | Explanation | Additional Notes |
| Hyperconnectivity Theory | Ramachandran and Hubbard | Caused by increased connectivity between cortical regions. | Connectivity is likely caused by failure of cortical pruning of neurons. |
| Disinhibition-unmasking hypothesis | Grossenbacher and Lovelace | Caused by a decreased level of feedback from inhibitory cortical areas. | This theory suggests constitutive inhibitory cortical feedback is present in everyone. |
| Learned association theory | Calkin | Suggests that synesthetic links are caused by learned associations early in life. | Discredited due to genetic component and increased incidence in women. |
| Awareness theory | Cytowic | Suggests that synesthesia is part of a normal perceptual process, and the phenomenon is caused by a failure of our brain to suppress the concurrent which he hypothesizes occurs in everyone. | Implicates the limbic system as important especially the hippocampus. |
| Neonatal synaesthesia | Maurer | This theory suggests that humans are all born with synesthesia-like tendencies, which in “normal” people are lost through age. | Widely refuted. Discussed at length later. |
What can be learned from synesthesia depends on its precise definition; for instance, Grossenbacher’s defined synesthesia as unusual, and this precludes Maurer’s theory that everyone is born with synesthesia [1,3]. For this review, synesthesia is considered a condition that is defined as an inducer causing a conscious, involuntary, idiosyncratic, and stable (repeatable) experience of an atypical concurrent (picturing grass when hearing the word green is not atypical).
Those with synesthesia rarely consider it a disability; as such, the study of synesthesia does not seek a cure but a greater understanding of the brain. With this in mind, one can view synesthesia not as a complex problem but a complex answer to some of the most difficult questions neuroscience posits.
Cross-Modal Perception
Aristotle originally divided senses into five separate modalities according to their individual sense organs. However, he could not have appreciated the extent to which the five senses interact in the brain [9]. As our knowledge of sensory perception has advanced, there is an increased understanding of the integration of the senses, namely via the process of cross-modal perception (CMP).
CMP is perception involving the interaction of two or more sensory modalities and is vital for gaining the most accurate estimate of the surrounding world. The first example of this was given by Köhler (1929), who provided evidence of CMP through the bouba/kiki effect, in which the word “bouba” is associated with curved shapes and “kiki” with angular shapes [8]. Another example is the McGurk effect, an interaction between hearing and vision in speech perception [9]. The McGurk effect describes the phenomenon in which audition is altered by vision, such that seeing someone mouth the sound “Faa” while hearing “Baa” makes it impossible to hear anything but “Faa.” Evidence for the interaction of vision with touch and sound has also been shown [10,11]. It is likely that with further study, CMP will be shown to have an increasing role in everyday sensory perception.
Why is CMP important clinically? Answering the questions posed by CMP could lead to better understanding and treatment of disorder of CMP such as Balint syndrome, characterized by optic ataxia, oculomotor apraxia, and simultanagnosia [12].
This essay will focus on what can be learned about CMP from synesthesia, in particular its location, underlying processes, the binding problem, and its development.
Can Synesthesia Really Inform an Understanding of CMP?
In order for what is known about synesthesia to inform our understanding of CMP, one must assume that there are similarities between the two phenomena and therefore the characteristics of one can inform the other. It has been shown experimentally that synesthesia and CMP show many similarities, and so conclusions regarding synesthesia may therefore inform current thinking regarding CMP.
Ward et al. (2006) showed their similarities by playing 70 tones of varying timbres to 10 synesthetes and 10 controls. Irrespective of timbre, both groups showed an identical trend to associate low pitch with dark colors and high pitch with light colors [13]. This is to be expected, as there is cultural association between pitch and color. Both color and pitch can be thought of as linear scales, and it is logical to assume that high pitch and light colors, presumably both high on a linear scale, should be associated. It is likely that non-synesthetes would make a similar association.
Ward went further to provide evidence of common percepts between synesthetes and non-synesthetes that are not simply based on magnitude. Ward et al. showed similarities that cannot be explained by this linear theory. The group showed that synesthetes with colored hearing, and non-synesthetes reported that timbre affects the saturation of color, with middle C often eliciting the most saturation [14]. This is surprising, as it is not based on a culture association. This finding suggests that there are some underlying similarities between the perception of synesthetes and non-synesthetes. Evidence such as this suggests that synesthesia can truly inform our understanding of CMP.
Where Does CMP Take Place?
The first question considered in this essay is: Where does CMP occur? To date, a range of cortical regions, including the superior temporal sulcus, intraparietal sulcus, and fronto-insula have all been implicated; however, evidence is conflicting and often depends on the experimental paradigms [14]. The precise location of these cortical regions is unimportant, as the focus of this review considers the evidence that high order cortical such as those listed above are more likely to be important in CMP and synesthesia.
The notion of high and low order cortices is not necessarily intuitive. It is believed that sensory processing occurs at a multistage level in the cortex. Low order cortical regions, cortical regions that are early in the processing pathway, such as the primary visual cortical regions, are thought to be involved in simple processing. Higher order cortical regions are thought to be involved in more complex processing [15].
The concept of high and lower order cortical regions was demonstrated by the work of Hubel and Wiesel. They showed that the receptive fields of cat V1 neurons consisted of simple receptive fields. They then showed that neurons from higher order cortical regions responded to not only more complex signals, but that these signals were constituted from an integration of input from lower order V1 cells. Thus, Hubel and Wiesel proposed that visual processing occurs in a hierarchical configuration.
Clavagnier, Falchier, and Kennedy (2004) reviewed this work more than 40 years later [16]. It is now known that sensory processing is not as simple as this, and there is a large amount of both feed-forward and feedback interaction between the high and low order cortical regions. In particular, it is thought that high order cortical regions are able to influence low order cortical regions, which is thought to be one of the neurophysiological processes behind attention [17].
The idea of lower order cortical regions feeding into and being refined by higher order cortical regions was outlined in Damasio’s theory of convergence zones. He suggested that sequential convergence zones correspond to higher and higher order cortical regions that identify objects as well as link with other convergence zones that process the other senses of that object. For instance, the convergence zones involved in visual recognition of an apple will link with the convergence zones responding to the taste of that apple [18].
What evidence is there that synesthetic perception involves high rather than low order cortices? Paulesu et al. (1995) used positron emission tomography (PET) to measure brain activation in six sound-vision synasthetes and six controls [19]. PET involves the subject consuming a radionucleotide tracer and then using a gamma detector to detect where in the body the tracer has accumulated. The tracer uses in an analog of glucose and therefore localizes in areas of high metabolic activity. Areas of the brain that are correlated with high tracer emissions are corresponded to areas of high metabolic activity and therefore neural activity.
When listening to inducing words compared with pure tone controls, the synesthetes had an increased activation in the occipital and parietal cortical regions, the bilateral inferior temporal gyrus, and the left lingual gyrus. This increase in activity in these high order cortical regions suggests that they have more of a role in synesthetic perception than they do in normal auditory perception. Interestingly, no change was found in V1 or V4, considered low order cortical regions. This suggests that their activity and thus function differs little between synesthetes and normal individuals, indicating that V1 and V4 are not as important in the synesthetic precept. This also suggests that higher order cortical regions are more important, although this experiment could never hope to exclude low order cortical regions from having a role in synesthesia. Additional evidence regarding this conclusion comes from Esterman et al., who showed that transcranial magnetic stimulation (TMS) disruption over the right posterior parietal lobe but not V1 or the left posterior parietal cortex reduced synesthetic binding [20]. TMS uses electromagnets to create electric currents within the subject’s brain. TMS has been used in a variety of neuroscience experiments for its ability to selectively deactivate areas of the brain, thus providing a quick and reversible alternative to lesion experiments, i.e., electrical ablation of areas of the cortex.
Another experiment by Aleman and colleagues did show lower order cortical region activation [21]. The experiment involved taking functional magnetic resonance imaging (fMRI) readings from a synesthete who, on hearing a word, visualized the word in a particular colour. fMRI also aims to detect metabolic activity by measuring the ratio between oxygen rich and oxygen poor blood.
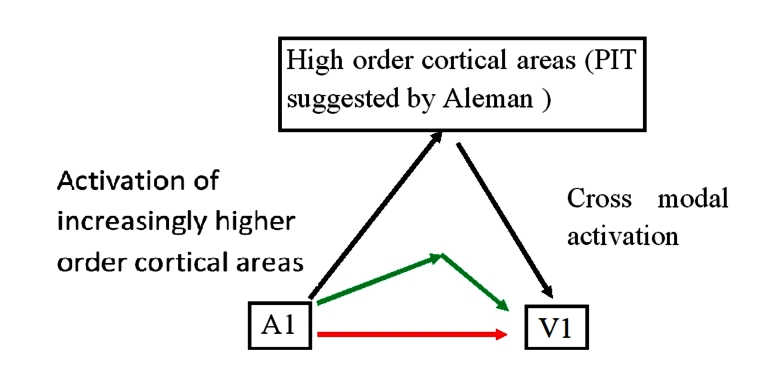
When there was no visual stimulus, the group showed activation of the primary visual cortex. The patient showed significantly more activation during her synesthetic perceptions compared with controls, hearing and responding to non-inducer tones. The group suggests that this is evidence of feedback links between the visual cortical regions and higher order cortical regions (Figure 2).
Figure 2.
Cortical processing schematic. This schematic shows the hypothesis of Aleman and colleagues. They hypothesize a role for high order cortical areas to activate cross-modal cortical areas. What is not clear is to what extent activation of primary sensory cortical regions are able to act more directly, via direct links (red arrow) and via other cortical areas, e.g., V4 (green arrow).
This experiment both supports and contributes to the theory advanced above about the importance of higher order cortical areas. The evidence does not, however, show a definitive mechanism for synesthesia and thus cross-modal perception; it presents a working hypothesis for future experiments to build on, which unfortunately may be the extent of our knowledge regarding the question of where CMP takes place.
One of the difficulties in drawing firm conclusions in this area of neuroscience is due to the excessive reliance on functional imaging, due in part to its ease of use in humans and limited number of synesthetic test subjects. More definitive conclusions may rely on different types of experiments such as lesion studies.
Lesion studies, both through mechanical ablation and TMS, affirm the importance of high order cortical areas. Damage to the angular gyrus was shown to interfere with cross-modal stimuli-matching as shown with the aforementioned bouba/kiki test [22]. Additionally, a TMS study showed that disruption of the PPC attenuated visual-tactile CMP in normal individuals [23]. What is not clear but would be an interesting experiment is whether disruption of lower order cortical regions such as V1 in the Aleman study interferes with synesthesia.
Despite these conclusions, it is important not to place too much weight on the findings of fMRI and other imaging techniques. fMRI studies by definition can only show association and not causality. They demonstrate an increase in activity in certain brain regions, but this may be due to a variety of causes. As shown in the Aleman study, there is increased activity in V1, but the cause of this can only be hypothesized.
fMRI studies are often limited to low sample groups, both due to the heterogeneity of synesthetes and the rare nature of the disease. This means one must be prudent in extrapolating conclusions based on fMRI studies beyond the test subjects in question.
Another criticism of fMRI studies is that V1 activation may also be a sign of mental imagery. For example, if one were to hear the words green grass, one instinctively imagines a visual scene that may be the cause of low order cortical activation. Such activation of V1 in these circumstances has been well demonstrated in non-synesthetes [24]. An experiment by Klein and colleagues showed that when subjects imagined a mental image, there was a reproducible activation of the primary visual cortex according to fMRI.
Cross-Modal Perception Processes
Synesthesia can also inform our understanding of the processes of CMP. Synesthesia is a complex condition characterized by heterogeneity. Synesthetes can be associators, see concurrents in the mind’s eye, or projectors, perceive concurrents in the environment transposed on top of the inducer. Another difference is the varied modalities of the inducers and concurrents. This hints at not one but many underlying processes that likely interact through the network of cortical regions mentioned in the previous section.
Evidence for connections between cortical regions comes from injecting retrograde tracers in the primate striate cortex. Falchier et al. (2002) showed lifelong connections between V1 and A1 and connections between the multimodal temporal region and unimodal V1 [25]. This study also provides an anatomical substrate for the aforementioned integration of processes. However, it is worth noting at this point that CMP functioning may not rely on direct connections via cortical regions but may occur via thalamocortical loops, connections from the cortex to the thalamus [26].
What do anatomical connections between cortical regions show us? The hyperconnectivity hypothesis suggests that interaction between sensory cortical regions causes the mixing of the senses. For this to occur, there must be connections between the sensory cortical regions to facilitate their interaction. Therefore, evidence of unimodal to unimodal connections supports the hyperconnectivity hypothesis [27]. However, the disinhibition-unmasking hypothesis would require connections between high order and low order cortical regions to facilitate the proposed inhibition exhibited by areas of the brain such as the temporal lobe [28].
The experiment by Falchier shows evidence for both the connections between lower order cortical regions, V1 to A1, as well as between high and lower order cortical regions, V1 to the temporal region. Falchier’s experiment, therefore, neither supports one theory nor the other but shows evidence for both and further how these processes may co-exist in the brain both to produce synesthetic phenomena and to underlie CMP. It is therefore possible that both direct and indirect connections exist between V1 and A1 and neither connection may function without the input of the other (Figure 3).
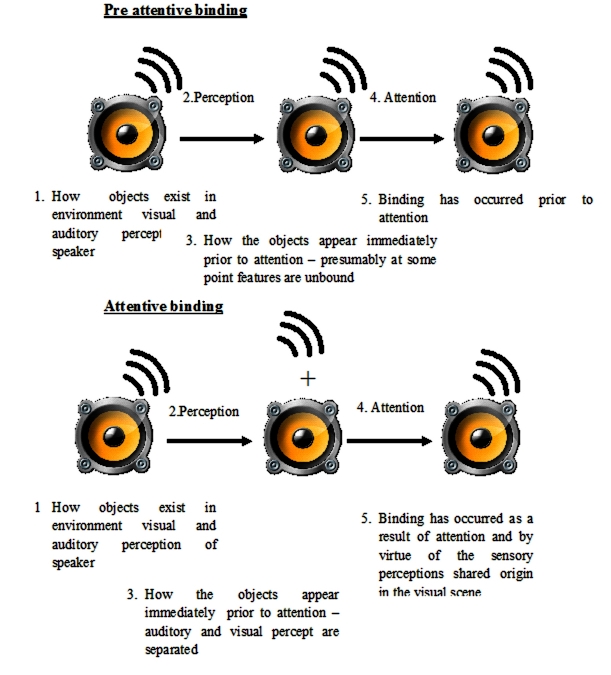
Figure 3.
Preattentive and postattentive binding. This diagram demonstrates the two different forms of sensory binding. In example 1, the sensory percepts, e.g., visual and auditory, are bound after perception and therefore, attention is not required to bind them. In the second example, after perception, the visual and auditory perceptions are unbound and remain so until attention is directed at them. There are differing views as to which schematic better represents our own perception. Synesthesia may help show that attention is not required for binding, and thus example 1 is more accurate.
Thus far, only the cortex has been considered. Are there sub-cortical neurons that could also contribute? Multi-sensory integration (MSI) cells have been found to have maps of sensory space for more than one sense and are possible candidates [28]. These cells have been found in the superior colliculus of guinea pigs, primates, and cats [29,30,31]. More recently, MSI cells were found in the primate cortex [32]. Burnet et al. (2007) showed when MSI cells in the superior colliculus were lesioned, there was a preferential loss of multisensory behavior over unisensory behavior [33]. Additionally, there was a reduction in the receptive field of the remaining neurons.
A possible improvement to Burnet’s experiment could involve a more selective lesion, involving only the bottom layers of the superior colliculus that are more dominated by multimodal input compared with the superficial layers [34]. MSI cells are likely to have a role in synesthesia and CMP. One explanation of MSI cell function could be that the cells in the superior colliculus are characterized by simple reflexive behavior and those in the cortex with complex CMP. A similar experiment to Burnet’s with varying complexities of multimodal behavior could provide evidence for this.
In this essay, the similarities between synesthesia and CMP have been used to inform the latter. However, there are also important differences [35,36]. Two EEG studies have shown important differences between CMP and synesthesia.
The first experiment used EEG recordings to investigate the early sensory processing of synesthetes compared with those or normal individuals while viewing visual stimuli that do not activate a synesthetic perception. If the sensory processing between synesthetes and normal individuals was the same, one would expect that the EEG recordings should show no difference. However, this experiment showed extra brain potentials in the synesthetes that indicate hyperactivation of sensory cortical areas compared with controls [37].
The second experiment also used EEG recording to investigate the difference between synesthetes and controls that were played tones which in the synesthetes were associated with synesthetic concurrents. This group showed EEG differences corresponding to activity in the auditory cortex early on (within 100msecs of tone onset) between the controls and synesthetes. The group therefore predicted differences in the way that synesthetic and normal brains perceived sound [38].
Can synsthesia still inform the understanding of CMP? Yes, but one must proceed cautiously. Many of the studies described in this section have been functional imaging studies and thus are limited in showing associative links between cortical regions rather than causality. There appear to be strong similarities in the underlying process of synesthesia and normal sensory perception. The true extent of the similarities and what they demonstrate is still to be seen.
Studying CMP raises another closely related question: With constant cross-modal interaction, how does the human brain ensure the auditory percept of an object is bound to its visual percept? Synesthesia can again enlighten the situation, this time with regards to the binding problem.
Binding Problem
The computer in front of me consists of features such as shape, sound, and smell. How does this information, transduced in the eyes, ears, and nose, respectively, and then processed in different cortical regions combine to form a uniform perception of the object? The binding problem asks: How can CMP occur without confusing the senses?
What is feature binding? Imagine a child playing with numerous colored blocks. At first, the blocks represent a jumbled selection of different features ― some are square, some are round, and some are triangle. In addition, the blocks are blue, red, yellow, green, and so on. It is only when the child starts grouping the blocks, e.g., all of the red blocks together, associations between the different blocks become evident. Grouping objects by color would seem illogical, as a blue pencil and a blue book share no other characteristics other than their color. However, if features share the same geographical place in our visual scene, it is much more likely that they are in fact the same object. This is how the human brain is thought to bind features [39].
A range of theories have proposed solutions to the binding problem. A seminal work was forwarded in Treisman and Schmidt’s 1980 and 1982 papers regarding feature integration theory (FIT) [39]. They proposed that features are represented in a feature map in the brain that corresponds to the location in the visual field and identity. Therefore, the appearance and the sound of the computer are bound by virtue of their shared geographical location in the visual field. The pair also state that our visual field consists of a variety of objects, and as such, there is activation in our brains caused by all the features of all the objects in our visual field. However, they state that binding only occurs when attention is directed toward a particular location [40,41]. It is this assumption, that binding requires attention, that will be the focus of the following discussion for the following reasons.
The binding problem is a real problem in that it has not been adequately solved, and a solution could help better understand some of the perception deficits seen in patients with common conditions such as stroke [39]. Understanding the binding problem can help better understand how attention influences perception and with that consequences of stroke such as neglect, a rare but highly debilitating condition in which patients can in some cases fail to perceive large parts of their environment [42,43].
Although binding is often very accurate, this is not always the case. It is possible for the brain to make mistakes. How many times have you thought one person said something when in fact it was someone else? These errors in binding (illusory conjunctions) have been used to inform FIT, and researchers have used them to suggest evidence both for and against the requirement of attention for binding [44]. Treisman maintained that binding requires attention, and therefore, like the child and their blocks, before attention the visual scene consists of a collection of jumbled unbound features.
Treisman investigated illusory conjunctions by showing subjects a line of colored shapes or letters, on either side of which were two black digits [41]. The investigators told the subjects to report on the two black digits, thereby diverting their attention from the color shapes. The results showed that the subjects could accurately report on the digits, but not on the shapes; therefore, attention was needed for binding. Treisman suggested that primitive features such as color, orientation, and intensity are available pre-attentively, but such features are unbound prior to attention.
However, there is no consensus as to whether binding can occur prior to attention. Tsal (1989), among others, has criticized FIT, saying binding can occur prior to attention [45]. The evidence demonstrating that binding can occur prior to attention is presented in the remainder of this section.
The study of projector synesthetes can help resolve this issue by showing attention is not required for synesthetic binding and therefore would not necessarily be required for normal binding (Figure 3).
One way to consider projector synesthetes is that they are demonstrating a form of incorrect binding. For instance, the binding of the color red to the number 7. Several pieces of evidence suggest that this type of synesthesia can occur without the awareness of the inducer, the number 7, and thus is pre-attentive.
Smilek et al. (2001) showed that when objectively black shapes were shown on a colored background, their detection was more accurate when the induced color was different from the background [46] (Figure 4).
Figure 4.
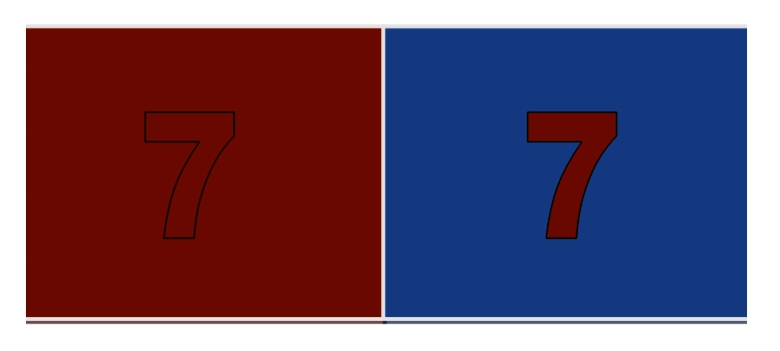
Diagram illustrating Smilek et al. method. In the experiment by Smilek and colleagues, they showed black shapes (that appeared colored to the synesthetes by virtue of their condition) on different colored backgrounds. The detection of the shapes was more accurate when the color that the shapes appeared was different from the background. This figure demonstrates this effect. Here, a black 7 appears red because of the synesthetic perception. On a red background, this 7 is obscured, but on a blue background, the 7 is more visible.
This suggests that the shape was bound to the synesthetic color prior to perception, and thus binding was pre-attentive. This is, therefore, a contradiction to FIT that suggests that binding requires attention. If binding required attention, as stated by Treisman, it would follow that it occurred after attention and therefore perception, which would mean that this form of synesthetic binding should not interfere with perception.
However, Mattingley et al. (2001) suggested that attention is necessary for the binding in synesthesia. His study tested 15 synesthetes by visually presenting them with inducing letters or digits, which were either above or below the detection threshold [47]. If, for example, the letter T was bound to green, then grayscale T would be flashed and either green or red presented. Synesthetes were slower to name the inconsistent rather than the consistent color patches when the letters and digits were detected. This is because when the red color was presented after the T, this interfered with the green ― the synaesthetic concurrent, an example of Stroop interference.
Mattingley concludes that this demonstrates attention is necessary for binding using the logic presented below (Figure 5):
Figure 5.

An example of Stroop interference. Try naming the color of the font of the first row of words and then the second. The color of the font interferes with the naming of the font.
1a) The synesthete shows stroop interference when presented with colors inconsistent with their normal concurrent, e.g., seeing red when seeing the letter T, which they normally associate with green.
1b) The synesthete does not show interference when the inducer is below detection threshold, i.e., they are unable to recognize the letter or digit (also shown by Mattingly in the same experiment).
2a) The synesthete only shows interference after letter and digit recognition is complete.
2b) Interference requires binding, otherwise there would be no confusion if green was not associated T when red is presented.
3. The autonomic binding of color and alphanumeric form therefore arises after letter and digit recognition is complete.
4. Letter and digit recognition requires attention.
5. Thus binding requires attention.
There are three clear criticisms of Mattingley’s experiment.
Firstly, he does not say whether the synesthetes are associators or projectors. If they are the former, then it is not likely that his subject synesthetes are a good model of sensory binding. This is because only projectors bind the concurrent with the inducer in the visual scene.
Secondly, his control was not effective. To show that the lack of Stroop interference in synesthetes was not due to the lack of digit recognition by the visual system, when the inducers were below detection threshold, Mattingley showed the digits caused interference in naming the next digit. However, the control is not robust, as it is likely numbers and colors are processed in different cortical areas, dorsal and ventral stream respectively [48]. It is conceivable that there are two different thresholds for these two types of perception.
Finally, it is known attention can modulate synesthesia and binding at a later stage. This has already been well demonstrated by Navon et al., who used Navon-type local-global stimuli to show the effects of selective attention on synesthetes [49] (Table 2). The table demonstrates Navon type stimuli and interference in reading the final image, the letter T, as it is made up of an interfering character, in this case the letter S. There is no interference in the first two images, but in the last image the letter S interferes with the overall shape of the letter T.
Table 2. Navon stimuli.
| Consistent | Neutral | Conflicting |
| TTTTTTTTTTTTTTTTTTTTTTTTT | +++++++++++++++++++++++++ | SSSSSSSSSSSSSSSSSSSSSSSSS |
| TTTTTTTTTTTTTTTTTTTTTTTTT | +++++++++++++++++++++++++ | SSSSSSSSSSSSSSSSSSSSSSSSS |
| TTTTTTTTTTTTTTTTTTTTTTTTT | +++++++++++++++++++++++++ | SSSSSSSSSSSSSSSSSSSSSSSSS |
| TTTTTTTT | ++++++++ | SSSSSSSS |
| TTTTTTTT | ++++++++ | SSSSSSSS |
| TTTTTTTT | ++++++++ | SSSSSSSS |
| TTTTTTTT | ++++++++ | SSSSSSSS |
| TTTTTTTT | ++++++++ | SSSSSSSS |
| TTTTTTTT | ++++++++ | SSSSSSSS |
| TTTTTTTT | ++++++++ | SSSSSSSS |
Therefore, it is possible the masking did not reduce synesthetic Stroop effects because binding required attention, but rather because of the attentional modification of synesthesia.
One explanation that would reconcile conflicting literature is that there are differences between projector and associator synesthetes. This was indeed shown in Stroop-like tests on both types of synesthetes. In a study by Dixon, it was shown the projectors have a greater level of Stroop interference than associators, which may be accounted for in different forms of binding [50]. This could lead to confusion when reviewing literature as some studies concentrate on projectors, some on associators, some on both, and some, such as Mattingley’s work, don’t state one way or the other.
Another option is that the dichotomy created between pre-attentive and post-attentive binding is artificial. This is a notion forwarded by Anderson who states that the conflicting evidence presented above is not likely to represent two different types of perception but rather different aspects of a single mechanism of synesthesia [51]. What is learned from studying synesthesia is that binding does not always require attention.
This shows that future theories of binding should accommodate the fact that normal binding can, although not always, occur pre-attentively.
What else can synesthesia demonstrate regarding sensory perception? Another controversial subject questions how cross-modal perception develops and the theory of neonatal synesthesia.
Development of Cross-Modal Perception
How does CMP develop? Synesthesia may again help to answer this question. Familial studies have provided strong evidence that there is a genetic component to synesthesia [52]. Additionally, a recent study has used linkage analysis to identify chromosomes 2, 5, 6, and 12 as important in synesthesia [52]. If synesthesia is caused by genes, is it possible that it is present from birth? Maurer (2005) goes further and suggests that all neonates are born with a form of synesthesia [7]. Her neonatal synesthesia (NS) hypothesis suggests that everyone is born with synesthesia, and during development, the modularity of senses appears, causing the synesthesia to disappear; when this development of modularity fails, synesthesia persists. If this hypothesis were correct, it would drastically change the way synesthesia and the development of CMP is considered.
Maurer divides evidence for the NS hypothesis into three areas [7] (Table 3).
Table 3. Mauer's three lines of evidence for neonatal synesthesia.
| Line of reasoning | Explanation | Criticism |
| Anatomical | Evidence of plasticity in the neonatal brain – may underlie synesthesia. | Demonstrates that the neonate brain may be more susceptible to crossmodal cortical connections, but not that this causes synesthesia. |
| Recording | Sensory percepts of one modality are enhanced by those of other modalities. | This is very difficult to demonstrate anything other than cross modal perception rather than synesthesia. |
| Behavioral | Behavioral evidence suggesting neonates can compare sensory information across modality. | Again the studies cited by Mauer cannot demonstrate neonatal synesthesia. |
The first area of evidence is anatomical. Studies have shown the immature cortex is less specialized in the newborn than in the adult. An experiment by Sur showed that when retinal axons are rewired to the auditory cortex in young ferrets, the auditory cortex no longer develops with normal tonotopic stripes but with pinwheels seen in the visual cortex. The neurons in the auditory cortex then become sensitive to visual stimuli. This suggests that the specialization and organization of primary sensory cortical areas are determined by the nature of its input, and thus the modality of the sense is not fixed by virtue of the location of the cortex [53]. Additionally, an experiment in cats showed that enucleation of the eyes at birth, thus depriving the cat of visual stimulus, allowed the auditory cortex to respond to visual stimuli [54]. These experiments are thought to demonstrate the unmasking of normally silent inputs, i.e., visual stimuli to the auditory, which may be present in the newborn and underlie synesthesia [7].
The second line of evidence comes from techniques for recording neural activity. It was shown that stimulation of the wrist elicits a somatosensory evoked response which in newborns is enhanced when accompanied by a white noise [55]. Tzourio-Mazoyer (2002) used a PET scan to show that activation in response to faces compared to illuminated diodes activated areas associated with speech [56]. Both these experiments are taken to show that is enhanced cross-modal interaction in neonates.
The final line of evidence is behavioral. Normally, infants squeeze smooth objects more often than granular objects. An experiment showed when a granular tactile stimulus was coupled with a smooth visual stimulus, the frequency of squeezing increased [57]. When a smooth tactile stimulus was accompanied by granular visual stimulus, the squeeze frequency fell, suggesting newborns can compare sensory information across modalities.
The evidence for the developmental theory of synesthesia remains unconvincing. The aforementioned three branches of experiments do not go beyond that which can be reconciled with the cross-modal transfer (CMT) hypothesis. The CMT hypothesis proposes that objects can be recognized in more than one modality. This is supported by a range of evidence [58]. For instance, one study found that 12-month-olds look longer at an object they had just explored orally; this is presumably because the baby can differentiate between two objects by sight even if they have only felt the shape of the object via the mouth due to an abstract representation of the shape [59,60]. It appears that Maurer’s evidence reflects CMT rather than NS.
Baron-Cohen (1996) proposed an imaging experiment to resolve this issue [58]. He suggested an fMRI study over the course of development to investigate abnormal sense cortical regions’ activation. He predicted that as the infant ages, this abnormal activation will decrease. This experiment is impractical due to the ethical considerations concerned with testing infants for a condition such as synesthesia. Also, the experiment fails to demonstrate that the activation being measured is caused by synesthesia rather than CMT. This study would also fail to prove a conscious percept, which many believe to be necessary for synesthesia [5]. The future of this field may lie in the refinement of animal models of CMP, allowing more invasive electrophysiological and lesion studies in neonatal animals [61,62].
Discounting the NS hypothesis begs the question: What can one learn about CMP from synesthesia? What is immediately apparent is there is not a simple answer to this question. Both main theories of synesthesia, hyperconnectivity and disinhibition-unmasking, implicate environmental influences on synaptic connections in the development of the condition [63]. However, the hereditability of synesthesia suggests an important genetic impact of synaptic wiring. Synesthesia, therefore, gives evidence for CMP being a function of nature and nurture.
Conclusion and Outlook
What can synesthesia demonstrate regarding CMP?
Synesthesia is a form of enhanced CMP. It has been shown from fMRI and TMS disruption studies that the CMP involved in synesthesia is likely to be more localized to higher rather than lower order cortical areas. The etiology of synesthesia is unclear, but it is likely that CMP occurs both as a result of direct connections and feedback connections from higher order cortical structures. Synesthesia also demonstrates the role of attention in binding the features of the visual scene and again suggests that the answer to the binding problem may be somewhere between the two theories of pre-attentive and post attentive models. Finally, consideration of synesthesia suggests CMP is a product of both biological and environmental factors.
As our knowledge has expanded, new questions have arisen: How is crossmodal information integrated in the brain? What role does memory and attention have on CMP [64]? As more questions arise, synesthesia will continue to be a guide to the unpredictable road ahead.
Abbreviations
- CMP
cross-modal perception
- V1
primary visual cortex
- PET
positron emission tomography
- V4
V4 visual cortex
- TMS
transcranial magnetic stimulation
- fMRI
functional magnetic resonance imaging
- PPC
posterior parietal cortex
- A1
primary auditory cortex
- MSI
multi-sensory integration
- EEG
electroencephalography
- FIT
feature integration theory
- CMT
cross-modal transfer
References
- Cytowic RE. Synesthesia: A Union of the Senses. 2nd ed. Cambridge: MIT Press; 2002. [Google Scholar]
- Simner J, Mulvenna C, Sagiv M, Tsakanikos E, Witherby SA, Fraser C. et al. Synaesthesia: the prevalence of atypical cross-modal experiences. Perception. 2006;35(8):1024–1033. doi: 10.1068/p5469. [DOI] [PubMed] [Google Scholar]
- Grossenbacher PG, Lovelace CT. Mechanisms of synesthesia: cognitive and physiological constraints. Trends Cogn Sci. 2005;5(1):36–41. doi: 10.1016/s1364-6613(00)01571-0. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Ramachandran VS. Neurocognitive mechanisms of synesthesia. Neuron. 2005;48(3):509–520. doi: 10.1016/j.neuron.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Calkins MW. A Statistical Study of Pseudo-Chromesthesia and of Mental-Forms. Am J Psychiatry. 1893;5(4):439–464. [Google Scholar]
- Cytowic RE, Eagleman D. Wednesday is Indigo Blue: Discovering the Brain of Synesthesia. 1st ed. Cambridge: MIT Press; 2009. [Google Scholar]
- Maurer D, Mondloch C. In: Synesthesia: Perspectives From Cognitive Neuroscience. 1st ed. Robertson LC, Sagiv N, editors. Oxford: Oxford University Press; 2005. Neonatal Synesthesia: A Re-Evaluation. [Google Scholar]
- Köhler W. Gestalt psychology. 1st ed. New York: Liveright; 1929. [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264(5588):746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- Spence C. Multisensory attention and tactile information-perception. Behav Brain Res. 2002;135:57–64. doi: 10.1016/s0166-4328(02)00155-9. [DOI] [PubMed] [Google Scholar]
- King AJ, Calvert GA. Multisensory integration: perceptual grouping by eye and ear. Curr Biol. 2001;11:322–325. doi: 10.1016/s0960-9822(01)00175-0. [DOI] [PubMed] [Google Scholar]
- Mendez MF. Corticobasal Ganglionic Degeneration With Balint’s Syndrome. J Neuropsychiatry Clin Neurosci. 2000;12:273–275. doi: 10.1176/jnp.12.2.273. [DOI] [PubMed] [Google Scholar]
- Ward J, Huckstep B, Tsakanikos E. Sound-colour synaesthesia: to what extent does it use cross-modal mechanisms common to us all. Cortex. 2006;42(2):264–280. doi: 10.1016/s0010-9452(08)70352-6. [DOI] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal Perception in the Human Brain: Insights from Functional Neuroimaging Studies. Cereb Cortex. 2001;11(12):1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Conway BR, Tsao DY. Color architecture in alert macaque cortex revealed by fMRI. Cereb Cortex. 2006;16(11):1604–1613. doi: 10.1093/cercor/bhj099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavagnier S, Falchier A, Kennedy H. Long-distance feedback projections to area v1: Implications for multisensory integration, spatial awareness, and visual consciousness. Cogn Affect Behav Neurosci. 2004;4(2):117–126. doi: 10.3758/cabn.4.2.117. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: Top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Damasio A. The brain binds entities and events by multiregional activation from convergence zones. Neural Comput. 1989;1:123–132. [Google Scholar]
- Paulesu E, Harrison J, Baron-Cohen S, Watson JD, Goldstein L, Heather J. et al. The physiology of coloured hearing: A PET activation study of colour-word synaesthesia. Brain. 1995;118:661–676. doi: 10.1093/brain/118.3.661. [DOI] [PubMed] [Google Scholar]
- Esterman M, Verstynen T, Ivry RD, Robertson LC. Coming Unbound: Disrupting Autonomatic Integration of Synaesthetic colour and Graphemes by Transcranial Magnetic Stimulation of the Right Parietal Lobe. J Cogn Neurosci. 2006;18(9):1570–1576. doi: 10.1162/jocn.2006.18.9.1570. [DOI] [PubMed] [Google Scholar]
- Aleman A, Rutten GJ, Sitskoorn MM, Dautzenberg G, Ramsey NF. Activation of striate cortex in the absence of visual stimulation: an fMRI study of synaesthesia. Neuroreport. 2001;12(13):2827–2830. doi: 10.1097/00001756-200109170-00015. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hubbard EM. Hearing colors, tasting shapes. Sci Am. 2003;288:52–59. doi: 10.1038/scientificamerican0503-52. [DOI] [PubMed] [Google Scholar]
- Pasalar S, Ro T, Beauchamp MS. TMS of posterior parietal cortex disrupts visual tactile multisensory integration. Eur J Neurosci. 2010;31(10):1783–1790. doi: 10.1111/j.1460-9568.2010.07193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein I, Paradis AL, Poline JB, Kosslyn SM, Le Bihan D. Transient Activity in the Human Calcarine Cortex during Visual-Mental Imagery: An Event-Related fMRI Study. J Cogn Neurosci. 2000;12(2):15–23. doi: 10.1162/089892900564037. [DOI] [PubMed] [Google Scholar]
- Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci. 2002;22:5749–5759. doi: 10.1523/JNEUROSCI.22-13-05749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BK. A thalamic reticular networking model of consciousness. Theor Biol Med Model. 2010;7:10. doi: 10.1186/1742-4682-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy H, Batardiere A, Dehay C, Barone P. In: Synaesthesia: Classic and Contemporary Readings. 1st ed. Baron-Cohen S, Harrison JE, editors. Oxford: Blackwell; 1997. Synaesthesia: implications for developmental neurobiology. [Google Scholar]
- Calvert GA. Crossmodal Perception in the Human Brain: Insights from Functional Neuroimaging Studies. Cereb Cortex. 2001;11(12):1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- King AJ, Palmer AR. Integration of visual and auditory information in bimodal neurones in the guinea-pig superior colliculus. Exp Brain Res. 1985;60:492–500. doi: 10.1007/BF00236934. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Interactions among converging sensory inputs in the superior colliculus. Science. 1983;221:389–391. doi: 10.1126/science.6867718. [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Auditory receptive fields in primate superior colliculus shift with changes in eye position. Nature. 1984;309:345–347. doi: 10.1038/309345a0. [DOI] [PubMed] [Google Scholar]
- Mistlin AJ, Perrett DI. Visual and somatosensory perception in the macaque temporal cortex: the role of ‘expectation’. Exp Brain Res. 1990;82:437–450. doi: 10.1007/BF00231263. [DOI] [PubMed] [Google Scholar]
- Burnet LR, Stein BE, Perrault TJ Jr., Wallace MT. Excitotoxic lesions of the superior colliculus preferentially impact multisensory neurons and multisensory integration. Exp Brain Res. 2007;179:325–338. doi: 10.1007/s00221-006-0789-8. [DOI] [PubMed] [Google Scholar]
- Martino G, Marks LE. Synesthesia: Strong and weak. Curr Dir Psychol Sci. 2001;10:61–65. [Google Scholar]
- Patton P, Belkacem-Boussaid K, Anastasio TJ. Multimodality in the superior colliculus: an information theoretic analysis. Brain Res Cogn Brain Res. 2002;14:10–19. doi: 10.1016/s0926-6410(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Sagiv N, Ward J. Crossmodal interactions: lessons from synesthesia. Prog Brain Res. 2006;155:259–271. doi: 10.1016/S0079-6123(06)55015-0. [DOI] [PubMed] [Google Scholar]
- Barnett KJ, Foxe JJ, Molholm S, Kelly SP, Shalgi S, Mitchell KJ. et al. Differences in early sensory-perceptual perception in synesthesia: a visual evoked potential study. Neuroimage. 2008;43:605–613. doi: 10.1016/j.neuroimage.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Goller AJ, Otten LJ, Ward J. Seeing sounds and hearing colors: an event-related potential study of auditory–visual synesthesia. J Cogn Neurosci. 2009;21:1869–1881. doi: 10.1162/jocn.2009.21134. [DOI] [PubMed] [Google Scholar]
- Holcombe AO. In: The Encyclopaedia of Perception. Goldstein EB, editor. SAGE Publications; 2009. The Binding Problem. [Google Scholar]
- Treisman A, Gelade G. A feature integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Treisman A. Perceptual Grouping and Attention in Visual Search. J Exp Psychol Hum Percept Perform. 1982;812(2):194–214. doi: 10.1037//0096-1523.8.2.194. [DOI] [PubMed] [Google Scholar]
- Robertson LC. Binding, spatial attention and perceptual awareness. Nat Rev Neurosci. 2003;4:93–102. doi: 10.1038/nrn1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Spatial behaviour. Fundamentals of human neuropsychology. 3rd ed. New York: W.H. Freeman & Co.; 2008. [Google Scholar]
- Wolfe JM, Cave KR. The psychophysical evidence for a binding problem in human vision. Neuron. 1999;24:11–17. doi: 10.1016/s0896-6273(00)80818-1. [DOI] [PubMed] [Google Scholar]
- Tsal Y. Do illusory conjunctions support the feature integration theory? A critical review of theory and findings. J Exp Psychol. 1989;15:394–400. [Google Scholar]
- Smilek D, Dixon MJ, Cudahy C, Merikle PM. Synaesthetic photisms influence visual perception. J Cogn Neurosci. 2001;13:930–936. doi: 10.1162/089892901753165845. [DOI] [PubMed] [Google Scholar]
- Mattingley JB, Rich AN, Yelland G, Bradshaw JL. Unconscious priming eliminates automatic binding of color and alphanumeric form in synaesthesia. Nature. 2001;410:580–582. doi: 10.1038/35069062. [DOI] [PubMed] [Google Scholar]
- Ettlinger G. “Object vision” and “spatial vision:” the neuropsychological evidence for the distinction. Cortex. 1990;26(3):319–341. doi: 10.1016/s0010-9452(13)80084-6. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before tress: the precedence of global features in visual perception. Cogn Psychol. 1997;9:353–383. [Google Scholar]
- Dixon MJ, Smilek D, Merikle PM. Not all synaesthetes are created equal: Projector vs. associator synaesthetes. Cogn Affect Behav Neurosci. 2000;4(3):335–343. doi: 10.3758/cabn.4.3.335. [DOI] [PubMed] [Google Scholar]
- Anderson B. There is no such thing as attention. Front Psychol. 2011;2:246. doi: 10.3389/fpsyg.2011.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher JE, Lamb JA, Brocklebank D, Cazier JB, Maestrini E, Addis L. et al. A whole-genome scan and fine-mapping linkage study of auditory-visual synesthesia reveals evidence of linkage to chromosomes 2q24, 5q33, 6p12, and 12p12. Am J Hum Genet. 2005;84:279–285. doi: 10.1016/j.ajhg.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- Yaka R, Yinon U, Wollberg Z. Auditory activation of cortical visual areas in cats after early visual deprivation. Eur J Neurosci. 1999;11:1301–1312. doi: 10.1046/j.1460-9568.1999.00536.x. [DOI] [PubMed] [Google Scholar]
- Wolff PH, Matsumiya Y, Abroms IF, Velzer CV, Lombroso CT. The effect of white noise on the somatosensory evoked responses in sleeping newborn infants. Electroencephalogr Clin Neurophysio. 1974;37:269–274. doi: 10.1016/0013-4694(74)90030-3. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, De Schonen S, Crivello F, Reutter B, Aujard Y, Mazoyer B. Neural correlates of woman face perception by 2-month-old infants. Neuroimage. 2002;15:454–461. doi: 10.1006/nimg.2001.0979. [DOI] [PubMed] [Google Scholar]
- Molina M, Jouen F. Modulation of manual activity by vision in human newborns. Dev Psychobiol. 2001;38:123–132. doi: 10.1002/1098-2302(200103)38:2<123::aid-dev1005>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Is There a Normal Phase of Synaesthesia in Development? Psyche. 1996;227 [Google Scholar]
- Gottfried AW, Rose SA, Bridger WH. Effects of visual, haptic, and manipulatory experiences on infants' visual recognition memory of objects. Dev Psychol. 1978;17:90–98. [Google Scholar]
- Meltzoff AN, Borton RW. Intermodal matching by human neonates. Nature. 1979;282:403–404. doi: 10.1038/282403a0. [DOI] [PubMed] [Google Scholar]
- Neely GG, Hess A, Costigan M, Keene AC, Goulas S, Langeslag M. et al. A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell. 2010;143(4):628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Melchner L, Pallas SL, Sur M. Visual behaviour mediated by retinal projections directed to the auditory pathway. Nature. 2000;404:871–876. doi: 10.1038/35009102. [DOI] [PubMed] [Google Scholar]
- Spector F, Maurer D. Synestheisia: A new approach to understanding the development of perception. Dev Psychol. 2009;45(1):175–189. doi: 10.1037/a0014171. [DOI] [PubMed] [Google Scholar]
- Robinson CW, Sloutsky VM. Development of cross-modal perception. Cogn Sci. 2009;1(1):135–140. doi: 10.1002/wcs.12. [DOI] [PubMed] [Google Scholar]