Abstract
Background: A subset of myasthenia gravis (MG) patients is refractory to standard therapies. Identifying the characteristics of this population is essential as newer treatment strategies emerge that may be more effective in this group.
Objective: The aim of our study is to describe the clinical features of refractory MG patients and compare them to those of non-refractory patients.
Methods: A retrospective chart review was completed of 128 MG patients referred to a tertiary neuromuscular clinic from 2003 to 2011. Patients were classified as refractory or non-refractory based on predefined criteria, and clinical features were compared.
Results: Nineteen out of 128 patients were classified as refractory (14.8 percent). Compared to the non-refractory patients, the refractory patients were more likely to be younger at onset, female, thymomatous, and MuSK-antibody positive.
Conclusion: Refractory MG patients represent a small but distinct group for whom exploring newer therapeutic approaches and immunopathologic differences is warranted.
Keywords: clinical features, myasthenia gravis, refractory disease
Introduction
Myasthenia gravis (MG) is an autoimmune neuromuscular disorder characterized by fatigable muscle weakness. MG is specifically thought to be an antibody-mediated disease. In approximately 85 percent of patients, antibodies are detected against the nicotinic acetylcholine receptor (nAChR) at the neuromuscular junction [1-3]. The remaining patients have antibodies against other components of the postsynaptic muscle endplate, such as muscle-specific receptor tyrosine kinase (MuSK), or are double seronegative (unidentified or undetected antibody) [2,3].
Current treatment options include acetylcholinesterase inhibitors, short-term immune therapies such as plasmapheresis or intravenous immunoglobulin (IVIG), and long-term immune therapies with immunosuppressive agents such as corticosteroids, azathioprine, and cyclosporine. Thymectomy is also a treatment option [2-4].
In spite of these treatments, a subset of patients remains refractory to conventional therapies [5]. Refractory MG patients experience frequent clinical relapse upon tapering their immunotherapy, are not clinically stable on their immunotherapy regimen, or develop severe side effects from immunosuppressive therapy [6]. Despite research on MG, relatively little is known about these patients. Investigating the unique clinical features of this patient population may help to identify these patients and customize treatment strategies. In our study, we retrospectively categorized MG patients as refractory or non-refractory based on predefined criteria and compared clinical characteristics between the two groups.
Methods
Patients
We conducted a retrospective study of 128 sequential MG patients referred to our neuromuscular clinic from September 2003 to February 2011. All patients had a confirmed diagnosis of MG based on the following criteria: 1) presence of anti-AChR or anti-MuSK antibodies in conjunction with either a positive decremental response on repetitive nerve stimulation testing at 3 Hz or a clinical examination consistent with MG or 2) positive decremental response on repetitive nerve stimulation testing at 3 Hz in conjunction with a clinical examination consistent with MG and absence of other disorders that can produce weakness or fatigue. Refractory patients were defined as those who could not lower their immunotherapy without clinical relapse, were not clinically controlled on their immunotherapy regimen, or had severe side effects from immunosuppressive therapy. The study was approved by the Yale Human Investigation Committee.
Statistical Analysis
Data analyses were performed using Shapiro-Wilk tests, chi-squared tests, Fischer’s exact tests, and Wilcoxon two-sample tests on SAS and GraphPad. Results were considered significant when p < 0.05.
Results
Patients
Nineteen patients were identified as refractory by our definition, and 109 were classified as non-refractory. Table 1 shows for each refractory patient the age of onset, gender, antibody status, previous therapies, and which refractory criteria were met.
Table 1. Characteristics of refractory MG patients.
| Patient | Antibody status | Gender/Age of onset | Refractory Criteriaa | Previous MG therapiesb |
| 1 | MuSK | F/53 | 1, 2, 3 | Az, PPX |
| 2 | MuSK | F/51 | 1, 2, 3 | Az, IVIG, Pyr |
| 3 | MuSK | F/29 | 1, 2, 3 | IVIG, PPX, Thy |
| 4 | MuSK | F/28 | 1, 3 | Pyr, Thy |
| 5 | MuSK | F/36 | 1, 2, 3 | IVIG, Pyr |
| 6 | MuSK | F/17 | 1, 2, 3 | Az, IVIG, Pyr, Thy |
| 7 | MuSK | F/20 | 1, 2, 3 | P, PPX |
| 8 | MuSK | F/43 | 1, 2, 3 | Cs, IVIG, MM, MTX, Pyr, PPX, Ta, Thy |
| 9 | MuSK | M/62 | 1, 2, 3 | IVIG, MM, P |
| 10 | AChR | M/24 | 2 | P, PPX, Pyr, Thy |
| 11 | AChR | M/59 | 1, 2, 3 | Az, IVIG, P |
| 12 | AChR | M/62 | 1, 2, 3 | Az, IVIG, P, PPX, Thy |
| 13 | AChR | M/28 | 1 | Az, MM, P, Pyr, PPX, Thy |
| 14 | AChR | F/17 | 2, 3 | IVIG, P, PPX, Pyr, Thy |
| 15 | AChR | F/35 | 1, 2, 3 | Az, P, PPX, Thy |
| 16 | AChR | F/48 | 1, 2, 3 | Az, IVIG, P, PPX, Pyr, Thy |
| 17 | AChR | F/50 | 1, 2, 3 | Az, MM, P, PPX, Pyr, Thy |
| 18 | AChR | F/35 | 2 | IVIG, P, Pyr, Thy |
| 19 | AChR | F/35 | 1, 3 | Az, P, Thy |
aRefractory Criteria: (1) inability to lower immunotherapy without clinical relapse, (2) not clinically controlled on immunotherapy regimen, (3) severe side effects from immunotherapy.
bAz, azathioprine; Cs, cyclosporine; IVIG, intravenous immunoglobulin; MM, mycophenolate mofetil; MTX, methotrexate; P, prednisone; PPX, plasma exchange; Pyr, pyridostigmine; Ta, tacrolimus; Thy, thymectomy.
Age of Onset
The age of onset for our total patient population was not normally distributed according to the Shapiro-Wilk test (p = 0.01), with a median of 55 years and an interquartile range (IQR) of 38-69 (Table 2). The median age of onset of the refractory group was 36 with an interquartile range of 28-51, whereas the median age of onset of the non-refractory group was 60 with an IQR of 42-72. A comparative histogram of the refractory and non-refractory groups was suggestive of a bimodal distribution for the latter group with a peak below age 40 and a second peak above age 50, as has been previously reported (Figure 1) [7]. Because the age of onset was not normally distributed for the non-refractory group (p = 0.01), we used the Wilcoxon two-sample test to compare the two groups and found the age of onset of the refractory group to be significantly lower than that of the non-refractory group (p < 0.001).
Table 2. Comparison of non-refractory and refractory MG patients.
| Total (n=128) | Non-refractory (n=109) | Refractory (n=19) | p-valueb | |
| Median age of onset, years (IQRa) | 55 (38-69) | 60 (42-72) | 36 (28-51) | <0.001 |
| Female, n (%) | 65 (51) | 51 (47)1 | 14 (74) | 0.03 |
| Antibody status available, n (%) | 115 (90) | 96 (88) | 19 (100) | |
| Anti-AChR+ | 82 (71) | 72 (75) | 10 (53) | 0.05 |
| Anti-MuSK+ | 11 (10) | 2 (2) | 9 (47) | <0.001 |
| Double Seronegative | 22 (19) | 22 (23) | 0 | 0.02 |
| Thymectomy, n (%) | 31 (24) | 18 (17) | 13 (68) | <0.001 |
| Thymoma status available, n (%) | 77 (60) | 66 (61) | 11 (58) | |
| Thymomatous | 14 (18) | 9 (14) | 5 (45) | 0.02 |
| Nonthymomatous | 63 (82) | 57 (86) | 6 (55) | |
aIQR, interquartile range; bFor comparisons of non-refractory vs. refractory
Figure 1.
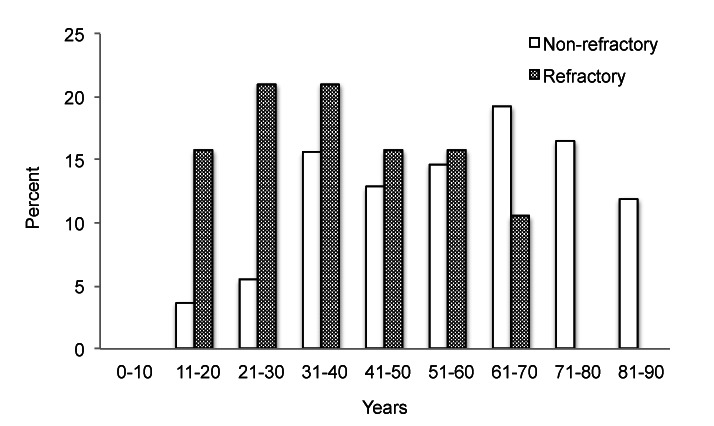
Distribution of age of onset in refractory vs. non-refractory MG patients.
Gender
Out of our total patient population, 51 percent were female. A significantly higher proportion of refractory patients were female in comparison with non-refractory patients (Table 2). Fourteen out of 19 refractory patients and 51 out of 109 non-refractory patients were female, comprising approximately 74 percent and 47 percent of the two groups, respectively (p = 0.03).
Antibody Status
We also determined the percentage of patients with anti-AChR and anti-MuSK antibodies. Antibody status was known for 115/128 (90 percent) patients, and of these, 82 (71 percent) had anti-AChR antibodies, 11 (10 percent) had anti-MuSK antibodies, and 22 (19 percent) were seronegative for both anti-AChR and anti-MuSK antibodies (Table 2). To look for differences in antibody status between the refractory and non-refractory groups, we compared the 19 refractory patients and 96/109 (88 percent) non-refractory patients for whom antibody status was available. We found that 47 percent of refractory patients and only 2 percent of non-refractory patients had anti-MuSK antibodies (p < 0.001). Furthermore, 23 percent of non-refractory patients were seronegative, while no refractory patients were seronegative (p = 0.02). The proportion of refractory patients with anti-AChR antibodies was 53 percent, marginally lower than the 75 percent observed in the non-refractory group (p = 0.05).
Thymectomy
In our patient population, a significantly higher proportion of refractory patients received thymectomy. Thirteen out of 19 refractory patients (68 percent), in contrast to 18 out of 109 non-refractory patients (17 percent), underwent thymectomy (p < 0.001; Table 2). Thymectomy approaches included transsternal, video-assisted thoracoscopic, and robotic thymectomy. Five of the 13 thymectomized refractory patients (39 percent) and nine of the 18 thymectomized non-refractory patients (50 percent) had pathologically confirmed thymomas (p = 0.72). All patients with computed tomography (CT) or pathologically confirmed thymoma received a thymectomy.
Thymoma Status
Information regarding thymoma status was available for 77 (60 percent) out of 128 patients, and of these, 14 (18 percent) patients had thymomas (Table 2). Thymoma status was available for 66/109 (61 percent) non-refractory patients and 11/19 (58 percent) refractory patients. When we compared the patients in the two groups for whom thymoma status was available, 14 percent of non-refractory patients and 45 percent of refractory patients were found to have thymomas (p = 0.02).
Discussion
We have reported on the clinical features of MG patients with refractory disease, defined by their inability to reduce immunotherapy without clinical relapse, inadequate response to immunosuppressive therapy, or the development of severe side effects to immunosuppressive medications. These patients were found to be distinct from the general MG patient population in terms of age of onset, gender proportion, thymoma status, and antibody status, characteristics that may help to identify these patients in the clinic.
The findings of some previous studies, while not specific to refractory disease, are suggestive of our observation that a higher proportion of refractory patients than non-refractory patients have autoantibodies against MuSK. Studies have found that while MuSK-antibody positive MG patients generally respond to conventional immunotherapy, and therefore are not refractory by our definition, they require higher corticosteroid doses to manage symptoms and have lower remission rates than AChR-antibody positive patients [8,9]. Thus, it is conceivable that MuSK-antibody positive MG patients would be more likely to have refractory disease, as our study found.
A literature search did not reveal studies specifically on the clinical features of refractory patients, but mostly case series and short descriptions of refractory patients as part of larger studies aimed at evaluating the effectiveness of nonconventional therapies for the treatment of refractory disease [10-13]. Although the methods for classifying patients as refractory varied slightly among the studies, poor response to conventional immunosuppressive therapies was commonly accepted as one of the defining features of refractory disease. Unfortunately, the findings of our study cannot be compared to these studies because their descriptions of refractory patients were limited to the subset of refractory patients who consented to receive the study-specific treatments and no comparisons were made to non-refractory patients in terms of the clinical features that were examined in our study.
A reason for the lack of information on refractory MG patients as a whole could be that these patients are rare. Out of the 128 patients that were seen in the Yale Neuromuscular Clinic over a span of almost 7.5 years, only 19 (14.8 percent) were refractory by our definition. Even this rate is likely to be an overestimate of the true prevalence, considering that our center is a tertiary referral site.
The small sample size of refractory patients and single center analysis are limitations of our study. A multi-institutional collaboration that examines more clinical features of a greater number of refractory patients may reveal further characteristics of this unique subset of patients. A common set of specific criteria to classify patients as refractory is called for, as they currently differ among research groups.
In conclusion, our results show that refractory MG patients are a subset of MG patients with clinical features that are distinct from those of non-refractory patients. These patients are more likely to be female and have an earlier age of onset, thymomas, and anti-MuSK antibodies. The unique characteristics of refractory patients suggest underlying biological differences between the non-refractory and refractory MG groups. We propose further exploring the immunopathologic mechanisms of disease, as understanding the differences at the molecular level may help to identify these patients earlier and also develop more effective targeted therapy. Studies geared at identifying biomarkers and other predictors of treatment responsiveness are needed at this time.
Acknowledgments
We would like to thank Dr. Valentine Njike at the Yale-Griffin Prevention Research Center for assistance with statistical analysis.
Abbreviations
- MG
myasthenia gravis
- AChR
acetylcholine receptor
- MuSK
muscle-specific receptor tyrosine kinase
- IVIG
intravenous immunoglobulin
- IQR
interquartile range
- Az
azathioprine
- Cs
cyclosporine
- MM
mycophenolate mofetil
- MTX
methotrexate
- P
prednisone
- PPX
plasma exchange
- Pyr
pyridostigmine
- Ta
tacrolimus
- Thy
thymectomy
- CT
computed tomography
Author contributions
JS: Data collection, analysis, drafting and revision of manuscript. JMG: Study conception, data analysis, and revision of manuscript. RJN: Study conception, data analysis, drafting and revision of manuscript.
References
- Drachman DB. Myasthenia gravis. N Eng J Med. 1994;330(25):1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475–490. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116(11):2843–2854. doi: 10.1172/JCI29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32(3):215–226. doi: 10.1055/s-0032-1329200. [DOI] [PubMed] [Google Scholar]
- Zebardast N, Patwa HS, Novella SP, Goldstein JM. Rituximab in the management of refractory myasthenia gravis. Muscle Nerve. 2010;41(3):375–378. doi: 10.1002/mus.21521. [DOI] [PubMed] [Google Scholar]
- Nowak RJ, Dicapua DB, Zebardast N, Goldstein JM. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther Adv Neurol Disord. 2011;4(5):259–266. doi: 10.1177/1756285611411503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somnier FE, Keiding N, Paulson OB. Epidemiology of Myasthenia-Gravis in Denmark — a Longitudinal and Comprehensive Population Survey. Arch Neurol. 1991;48(7):733–739. doi: 10.1001/archneur.1991.00530190081019. [DOI] [PubMed] [Google Scholar]
- Evoli A, Bianchi MR, Riso R, Minicuci GM, Batocchi AP, Servidei S. et al. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Ann NY Acad Sci. 2008;1132:76–83. doi: 10.1196/annals.1405.012. [DOI] [PubMed] [Google Scholar]
- Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23(5):530–535. doi: 10.1097/WCO.0b013e32833c0982. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Adams RN, Hu R, Jones RJ, Brodsky RA. Rebooting the immune system with high-dose cyclophosphamide for treatment of refractory myasthenia gravis. Ann NY Acad Sci. 2008;1132:305–314. doi: 10.1196/annals.1405.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun C, Bourg V, Tieulie N, Thomas P. Successful treatment of refractory generalized myasthenia gravis with rituximab. Eur J Neurol. 2009;16(2):246–250. doi: 10.1111/j.1468-1331.2008.02399.x. [DOI] [PubMed] [Google Scholar]
- Prakash KM, Ratnagopal P, Puvanendran K, Lo YL. Mycophenolate mofetil — as an adjunctive immunosuppressive therapy in refractory myasthenia gravis: The Singapore experience. J Clin Neurosci. 2007;14(3):278–281. doi: 10.1016/j.jocn.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Gladstone DE, Brannagan tH 3rd, Schwartzman RJ, Prestrud AA, Brodsky I. High dose cyclophosphamide for severe refractory myasthenia gravis. J Neurol Neurosurg Psychiatry. 2004;75(5):789–791. doi: 10.1136/jnnp.2003.019232. [DOI] [PMC free article] [PubMed] [Google Scholar]


