Abstract
C. elegans is a model used to study cholesterol metabolism and the functions of its metabolites. Several studies have reported that, in worms, cholesterol is not a structural component of the membrane as it is in vertebrates. However, as in other animals, it is used for the synthesis of steroid hormones that regulate physiological processes such as dauer formation, molting and defecation. After cholesterol is taken up by the gut, mechanisms of transport of cholesterol between tissues in C. elegans involve lipoproteins, as in mammals. A recent study shows that both cholesterol uptake and lipoprotein metabolism in C. elegans are regulated by molecules whose activities, biosynthesis, and secretion strongly resemble those of mammalian bile acids, which are metabolites of cholesterol that act on metabolism in a variety of ways. Importantly, it was found that oxidative stress upsets the regulation of the synthesis of these molecules. Given the known function of mammalian bile acids as metabolic regulators of lipid and glucose homeostasis, future investigations of the biology of C. elegans bile acid-like molecules could provide information on the etiology of human metabolic disorders that are characterized by elevated oxidative stress.
Keywords: C. elegans, clk-1, bile acids, cholesterol, dafachronic acids, dauer formation, defecation, lipoproteins, metabolic syndrome, oxidative stress, steroid hormones
Cholesterol and Bile Acid Metabolism in Mammals
In mammals, cholesterol is a structural component of the cell plasma membrane that affects permeability and fluidity of the lipid bilayer as well as being a substrate for the biosynthesis of signaling molecules such as steroids hormones, vitamin D, and bile acids (BAs).2 After cholesterol is taken up from food, it is associated with lipoprotein particles and then transported between organs predominantly as cholesteryl esters. Cholesterol is mostly excreted in the bile after having been converted into bile acids in the liver. Bile acids are amphipathic molecules comprising the steroid nucleus of cholesterol with a shortened side chain (Fig. 1). They act as biological detergents that help in the intestinal uptake of lipids, hydrophobic vitamins, and cholesterol itself. They also act as steroid hormone-like substances that integrate several aspects of metabolism, including lipid, glucose, and energy metabolism by regulating gene expression through nuclear hormone receptors such as the farnesoid X receptor (FXR), the pregnane X receptor (PXR), and the vitamin D receptor (VDR) (BA biology is reviewed in detail in refs. 3, 4). As sterols play multiple essential roles in mammals, alterations of their normal metabolism or transport cause diseases. For example, in mammals, BA excretion and recirculation depend on several membrane transporters such as ATP8B1 and ABCB11. Mutations in ATP8B1 cause progressive familial intrahepatic cholestasis type1 (PFIC1) characterized by a pathological retention of bile.5 ATP8B1, a type 4 P-type ATPase, is a predicted phospholipid flippase.6 Studies in mice suggest that ATP8B1 deficiency causes loss of canalicular membrane phospholipid asymmetry and as a result the resistance of the canalicular membrane to hydrophobic BAs is decreased, which causes cholestasis by impairing the activity of ABCB11, the BA export pump.7
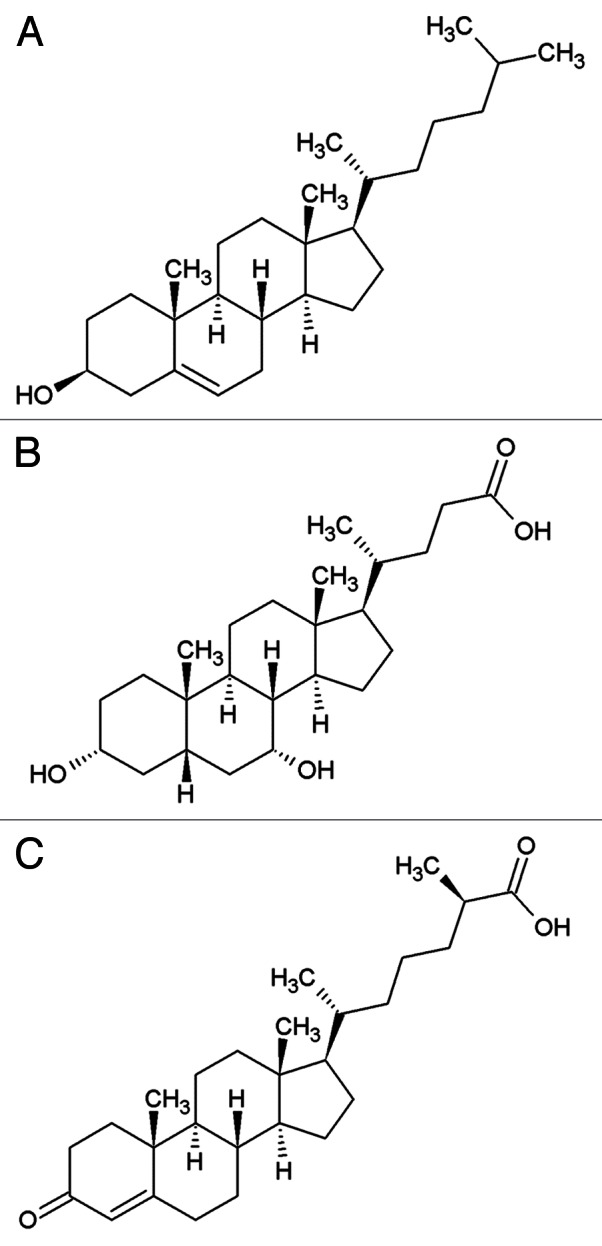
Figure 1. The structures of (A) cholesterol, (B) a mammalian bile acid, chenodeoxycholic acid (CDCA), (C) dafachronic acid.
Cholesterol Metabolism and Functions of Cholesterol Metabolites in C. elegans
Data accumulated in recent years indicate that C. elegans is starting to become a valuable model for studying sterol metabolism and function. In this commentary, we summarize recent advances in our understanding of C. elegans steroid signaling pathways and their regulations of physiological features, in particular dauer formation, molting and defecation. Much about sterols and sterol metabolites in worms, including biosynthesis, transport and targets such as lipoproteins, appears to be evolutionarily conserved, thus providing a model system that can be used to understand metabolic disorders in mammals.
C. elegans and other nematodes, like many other animals such as Drosophila and other insects, are auxotrophic for sterols because they do not possess the enzymes that are required for de novo sterol synthesis but are capable of modifying sterols (reviewed in ref. 8). Although the worm does not synthesize sterols, they are obtained through the diet, and when cultivated in the laboratory cholesterol has to be supplemented in the culture media (generally at 5μg/ml cholesterol). Therefore, by reducing the level of dietary cholesterol or replacing it with labeled or modified sterols, the worm can be used to investigate the metabolism and transport of sterols in a living organism. Worms grown on plates with a reduction in sterol supplementation produce a complex phenotype that includes abnormal molting and inappropriate dauer formation. A complete lack of sterol supplementation leads to lethality. Sterols appear to be required only in very small amounts for normal physiology in worms,9 suggesting that sterols are unlikely to be structural components in worm membranes. However, they are clearly used for the synthesis of signaling molecules, as reviewed below.
Worm signaling molecules derived from cholesterol were described in detail only recently when a number of studies showed that dafachronic acids (DAs), which are molecules that have some of the characters of BAs (Fig. 1), act as hormones that support reproductive development under favorable conditions by binding to a nuclear hormone receptor encoded by daf-12.10 Under unfavorable conditions, such as when worms are starved or overcrowded, DAs are not produced and un-liganded DAF-12 associates with its co-repressor DIN-1, which promotes worms entering into the dauer diapause. Genetic approaches and biochemical studies of the regulation of dauer formation revealed that a crucial step for production of DAs is performed by a cytochrome P450, DAF-9, which hydroxylates cholesterol twice in the C-26 position to produce a carboxyl group.11-14 In addition to DAF-9, two other enzymes required for the synthesis of DAF-12 ligands are DAF-36, a Rieske oxygenase that acts as a cholesterol 7-desaturase converting cholesterol to 7-dehydrocholesterol15 and HSD-1, a 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase.16
Although no ecdysteroids similar to those in insects have been identified in C. elegans, many observations suggest that molting, the process by which a new cuticle is synthesized and the old cuticle is shed, is also likely regulated by a steroid hormone. In insects, ecdysones, which are polyhydroxylated sterols, initiate molting through the activation of the ecdysone receptor, a heterodimeric receptor composed of two nuclear receptors including EcR (the ecdysone receptor) and USP (ultraspiracle) (reviewed in ref. 17). Although no homolog of the ecdysone receptor is known in C. elegans,18 cholesterol deprivation produces a phenotype of incomplete shedding of the old cuticle which is similar to that observed by disruption of two nuclear hormone receptors, nhr-23 and nhr-25, which are C. elegans homologs of Drosophila orphan nuclear receptors that are induced by ecdysone, DHR3 and ΒFTZ-F1, respectively.19,20 Furthermore, mutants displaying molting defects are enhanced by cholesterol deprivation such as mutations in let-767, a steroid modifying enzyme that is a homolog of human 17-estradiol dehydrogenase21 and mutations in lrp-1, the worm homolog of gp330/megalin, a mammalian protein with homology to the LDL receptor.22 These activities might thus be required for the production and function of a steroid hormone that regulates molting.
Cholesterol Transport Through Lipoproteins Distinct from Yolk
In mammals, after BA-facilitated absorption, ingested cholesterol, phospholipids, triglycerides, and lipid-soluble vitamins, are transported from the gut to other tissues that need them via lipoproteins such as chylomicrons. Other lipoproteins such as low density lipoproteins (LDL) serve to re-distribute lipids and cholesterol from the liver to peripheral tissues, and high density lipoproteins (HDL) transport cholesterol from peripheral tissues back to the liver in a process termed reverse cholesterol transport. In humans, defects in cholesterol transport and distribution are associated with several diseases such as atherosclerosis, obesity, type C Niemann-Pick disease and others. The best-known lipoproteins in C. elegans are the yolk particles. The protein moieties of yolk particles are vitellogenins, distant homologs of apolipoprotein B (ApoB), which is the major protein in chylomicrons and LDL.23 In C. elegans there are 5 genes that code for vitellogenins including vit-2 to vit-6.24 To provide nutrients for growth and development of the embryo in C. elegans, cholesterol, fatty acids, and possibly other nutrients are transported from the gut to developing oocytes through the pseudocoelomic cavity by means of yolk particle, indicating that the transport mechanisms of cholesterol in C. elegans are similar to those in mammals.25,26 Yolk is taken up by oocytes by a conserved pathway of receptor-mediated endocytosis via a yolk receptor, RME-2, which is a member of the lipoprotein receptor superfamily.25
Several observations suggest that there are other cholesterol transport systems in worms besides yolk.27 For example, hermaphrodites are capable of transporting cholesterol before the vitellogenins are expressed and males do not express vitellogenins but accumulate cholesterol in developing sperm.26 Furthermore, a mutation in dsc-4/mtp, the worm homolog of the microsomal triglyceride transfer protein (MTP),28 whose activity in mammals is required for production and secretion of ApoB-containing lipoproteins, does not affect yolk production as, in contrast to rme-2 mutants, it produces no defect in oocyte maturation or embryo production. However, disruption of dsc-4/mtp by mutation or RNAi has phenotypic effects on several tissues, shortening the development rate of the germline and the length of the defecation cycle, a rhythmic behavior that is driven by the physiology of the gut (Fig. 2).29 This suggests that DSC-4/MTP is required for the secretion of a type of lipoprotein that is distinct from yolk and that serves to transport lipids between tissues, as do mammalian lipoproteins. Possibly, the core apoprotein for this hypothetical lipoprotein is a vitellogenin that is folded and lipidated by DSC-4/MTP into a particle that is distinct from yolk, as suggested by previous studies that show that human MTP is able to act upon Xenopus egg vitellogenins.30 Furthermore, RNAi knockdowns of dsc-4/mtp and of some vitellogenins, in particular vit-5, have the same phenotypic effects on germline development.28 Another possibility is that DSC-4/MTP might be the apoprotein itself as it is evolutionarily related to vitellogenins and ApoB.23
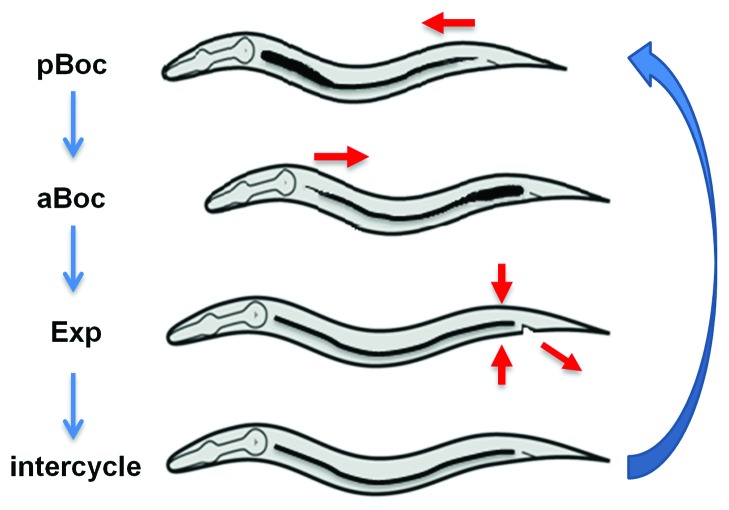
Figure 2. Steps of the defecation motor program (DMP). The first step involves the posterior body muscle contraction (pBoc), which pushes the gut contents anteriorly, followed by the second step, the anterior body muscle contraction (aBoc), which concentrates the gut contents in the pre-anal region. Finally, the enteric muscle contractions result in the expulsion (Exp) of the gut contents. The red arrows indicate the directions in which muscles contract during the various steps of the motor program. The defecation motor program is executed rhythmically, about once a minute.
dsc-4/mtp was identified as a mutation that suppresses the slow defecation and the slow germline development of clk-1 mutants.31 CLK-1 is a conserved mitochondrial enzyme that is necessary for the biosynthesis of the antioxidant and redox cofactor ubiquinone (co-enzyme Q; CoQ). Several observations suggest that dsc-4/mtp suppresses clk-1 by reducing the level of a type of MTP-dependent, LDL-like lipoprotein. For example, reducing the level of dietary cholesterol, which is a major constituent of lipoproteins whose reduction leads to reduce LDL levels in vertebrate, mimics the effects of dsc-4 on the slow germline development and the slow defecation cycle length of clk-1 mutants,28,32 suggesting that those phenotypes of clk-1 mutants are due to high levels of LDL-like lipoproteins biosynthesis and secretion. It is not clear how elevated lipoprotein biosynthesis and secretion slow down the defecation cycle; however, the MTP-dependent lipid transport system appears to be so well conserved between mammals and C. elegans that drugs that have been developed to lower lipid levels in humans can act as suppressors of the slow defecation phenotype of clk-1 mutants.32 For example, the slow defecation is suppressed by drugs that (1) antagonize high LDL levels by increasing HDL levels (e.g., phenylthiourea inhibitors of the HDL receptor SR-BI33), that (1) stimulate reverse cholesterol transport by regulating gene expression through nuclear hormone receptors (e.g., gemfibrozil34), or that (3) inhibit the activity of HMG-CoA reductase to lower cholesterol levels (e.g., fluvastatin and lovastatin). This suggests that the targets of these drugs are conserved to a sufficient degree in worms for these compounds to be active and that C. elegans provides a pharmacological platform that could be used to develop or identify compounds that affect lipid metabolism in mammals as well. In fact, such a compound screen has been performed and a number of molecules that are active on clk-1 mutants have been found to reduce apoB secretion in cultured human cells and to reduce plasma lipoprotein levels in a mouse model of dyslipidemia.32
A Conserved Pathway of Bile Acid Biosynthesis and Secretion in C. elegans
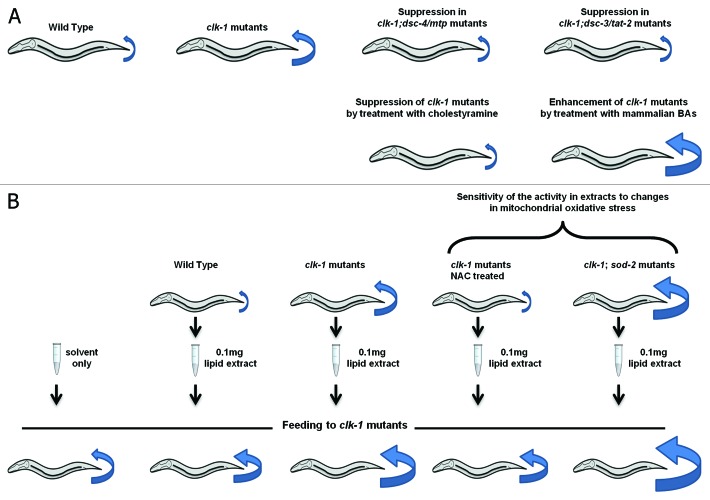
In the same genetic screen in which dsc-4/mtp was identified, we also found another suppressor of the slow defecation phenotype of clk-1 mutants, dsc-3/tat-2 (Fig. 3A). The two mutations appear to function in a common pathway as their effects are not additive.1 DSC-3/TAT-2 (TAT stands for Transbilayer Amphipath Transporters) is a homolog of ATP8B1, which is necessary for normal secretion of BAs in mammals (see above).6,7 Following up this lead we found that feeding clk-1 mutants with cholestyramine, a BA-binding resin that targets BAs and reduces BAs availability, and thus lowers circulating LDL in mammals,35 suppresses the slow defecation of clk-1 mutants, like the dsc-3/tat-2 and the dsc-4/mtp mutations (Fig. 3A). Knockdown of the C. elegans homologs of BA-biosynthetic enzymes similarly suppresses the phenotype (see below). This lead us to propose a model in which C. elegans secretes molecules that are similar to BAs in mammals and in which the altered defecation phenotype of clk-1 mutants is due to enhanced synthesis of such molecules.1 In addition, we found that the regulation of defecation in response to BA availability is deregulated in clk-1 mutants as shown by the observation that treatment with mammalian BAs enhances the phenotype of the mutants but is without effect on the wild type1 (Fig. 3A).
Figure 3. The elevated mitochondrial oxidative stress of clk-1 mutants alters the metabolism bile acid-like molecules, resulting in the slow defecation mutant phenotype. The length of the defecation cycle (defined as the time between pBocs) for each genotype or condition is schematically represented by blue arrows. The decreased or increased size of arrows means that the defecation rates are shortened or lengthened, respectively. (A) The slow defecation phenotype of clk-1 is suppressed by mutations in dsc-4/mtp or dsc-3/tat-2 and treatment with the BA-binding resin cholestyramine. On the other hand, the slow defecation phenotype is enhanced by treatment with mammalian BAs. (B) Lipid extracts from wild type worms contain an activity that mimics the enhancing effect of mammalian BAs on clk-1. Furthermore, extracts from clk-1 mutants contain more of the activity and thus have a stronger enhancing effect. In addition, suppression of clk-1 mutant by treatment with the antioxidant NAC, which suppresses the slow defecation phenotype, reduces the amount of activity in extracts, while enhancement of the phenotype by increase of mitochondrial oxidative stress through loss of SOD-2 increases the amount of activity in extracts.
Although the exact molecular structures of the C. elegans BA-like molecules are not yet known, an activity that can be extracted from C. elegans by ether mimics the effect of mammalian BAs in enhancing the slow defecation cycle of clk-1 mutants. Furthermore, as expected from the genetics-based model, this activity is more abundant in extracts from clk-1 mutants1 (Fig. 3B).
The C. elegans BA-like Molecules are Derived from Cholesterol and Are Distinct from Dafachronic Acids
In mammals, BAs are produced in the liver as oxidized breakdown products of cholesterol through a series of oxidation reactions and a shortening of the side chain.2 Enzymes that catalyze these multistep reactions are located in different cellular compartments, including the endoplasmic reticulum, cytosol, mitochondria, and peroxisomes. For example, the oxidation of the side-chain takes place in the mitochondria, but side-chain shortening takes place in the peroxisomes by β-oxidation.2 To understand whether BA-like molecules in worms are synthesized through pathways similar to those in mammals, we used RNAi on clk-1 mutants to screen homologs of mammalian BA-biosynthetic enzymes. A number of genes were identified, including 13 P450 oxidases and enzymes of peroxisomal β-oxidation.1 In mammals, peroxisomal β-oxidation is involved in cholesterol side-chain shortening. In addition, the clk-1 phenotype is also suppressed by a mutation in daf-36, which encodes a cholesterol 7-desaturase. These data suggest that C. elegans BA-like molecules are indeed cholesterol derivatives and that they might have a shortened side-chain. This is in contrast to DAs, which have some characteristics of BAs, but whose oxidation is not extensive and whose side-chain is not shortened.10 Moreover, neither knockdown of daf-12, the nuclear receptor of DAs nor depletion of daf-9 and hsd-1, two activities that are known to participate in the synthesis of DAs, affect the clk-1 defecation phenotype.1
C. elegans as Model for Studying the Effect of Oxidative Stress on Metabolic Disorders
In both worms and mice, mutations in clk-1 affect mitochondrial function,36,37 with increased mitochondrial oxidative stress,38,39 elevated mitochondrial ROS production,38 elevated oxidative damage,40,41 and increased sensitivity to pro-oxidant drugs.42 To determine whether the elevated mitochondrial oxidative stress was responsible for the altered BA metabolism we tested the effects on clk-1 of treatment with an antioxidant, NAC (N-acetyl-cysteine) and of depletion of SOD-2, the main mitochondrial superoxide dismutases whose reduction should elevate mitochondrial oxidative stress.1 Both treatments were successful in suppressing and enhancing, respectively, the slow defecation cycle. Furthermore, these treatments also consistently altered the level of BA-like activity that could be extracted from treated worms (Fig. 3B).
In mammals, a link of oxidative stress with dyslipidemia and with the other cardiovascular risk factors that constitute the metabolic syndrome has repeatedly been evidenced43-45 but its mechanistic basis has not yet been elucidated. BAs are signaling molecules that regulate sterol, lipid and glucose metabolism. Our findings with worm BAs thus suggest the attractive hypothesis that the mitochondrial oxidative stress that is typical of the aging pattern of a wide variety of animals, including people, could lead to abnormal metabolism of BAs, which could in turn deregulate metabolic pathways and thus lead to the classical pattern of age-dependent metabolic diseases that is prominent in our society. Interestingly, the unexplained variety of phenotypes observed in clk-1 mutants46 might also reflect an effect of abnormal regulation of metabolic processes by BAs.
Perspectives
In this commentary, we have briefly reviewed findings about several processes (such as dauer formation, molting, and defecation) that appear to be regulated by sterol metabolites. However, until now, only DAs have been structurally characterized and the corresponding nuclear hormone receptor identified.10 Likely the BA-like molecules that regulate defecation will likely be found to target at least some of the nearly 260 potential nuclear hormone receptors (NHRs).47 Identifying these receptors might prove particularly useful to understand the pleiotropy of clk-1 and help to provide a more complete model of how the deregulation of BA synthesis by mitochondrial oxidative stress can lead to metabolic disorders.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Robyn Branicky for reading the manuscript. S.H. is Strathcona Chair of Zoology and Robert Archibald and Catherine Louise Campbell Chair of Developmental Biology. The work was supported by a grant from the Canadian Institutes of Health Research to SH (MOP-89761).
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/21457
References
- 1.Liu JL, Desjardins D, Branicky R, Agellon LB, Hekimi S. Mitochondrial oxidative stress alters a pathway in Caenorhabditis elegans strongly resembling that of bile acid biosynthesis and secretion in vertebrates. PLoS Genet. 2012;8:e1002553. doi: 10.1371/journal.pgen.1002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 3.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–66. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 5.Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219–24. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 6.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–7. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 7.Paulusma CC, Groen A, Kunne C, Ho-Mok KS, Spijkerboer AL, Rudi de Waart D, et al. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195–204. doi: 10.1002/hep.21212. [DOI] [PubMed] [Google Scholar]
- 8.Entchev EV, Kurzchalia TV. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin Cell Dev Biol. 2005;16:175–82. doi: 10.1016/j.semcdb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Merris M, Wadsworth WG, Khamrai U, Bittman R, Chitwood DJ, Lenard J. Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: developmental requirement for 4alpha-methyl sterols. J Lipid Res. 2003;44:172–81. doi: 10.1194/jlr.M200323-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–23. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–31. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- 12.Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1:841–51. doi: 10.1016/S1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- 13.Gill MS, Held JM, Fisher AL, Gibson BW, Lithgow GJ. Lipophilic regulator of a developmental switch in Caenorhabditis elegans. Aging Cell. 2004;3:413–21. doi: 10.1111/j.1474-9728.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 14.Matyash V, Entchev EV, Mende F, Wilsch-Bräuninger M, Thiele C, Schmidt AW, et al. Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol. 2004;2:e280. doi: 10.1371/journal.pbio.0020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, Mangelsdorf DJ, et al. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev Cell. 2006;10:473–82. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Patel DS, Fang LL, Svy DK, Ruvkun G, Li W. Genetic identification of HSD-1, a conserved steroidogenic enzyme that directs larval development in Caenorhabditis elegans. Development. 2008;135:2239–49. doi: 10.1242/dev.016972. [DOI] [PubMed] [Google Scholar]
- 17.Thummel CS. Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell. 2001;1:453–65. doi: 10.1016/S1534-5807(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 18.Sluder AE, Maina CV. Nuclear receptors in nematodes: themes and variations. Trends Genet. 2001;17:206–13. doi: 10.1016/S0168-9525(01)02242-9. [DOI] [PubMed] [Google Scholar]
- 19.Kostrouchova M, Krause M, Kostrouch Z, Rall JE. CHR3: a Caenorhabditis elegans orphan nuclear hormone receptor required for proper epidermal development and molting. Development. 1998;125:1617–26. doi: 10.1242/dev.125.9.1617. [DOI] [PubMed] [Google Scholar]
- 20.Kostrouchova M, Krause M, Kostrouch Z, Rall JE. Nuclear hormone receptor CHR3 is a critical regulator of all four larval molts of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2001;98:7360–5. doi: 10.1073/pnas.131171898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuervers LM, Jones CL, O’Neil NJ, Baillie DL. The sterol modifying enzyme LET-767 is essential for growth, reproduction and development in Caenorhabditis elegans. Mol Genet Genomics. 2003;270:121–31. doi: 10.1007/s00438-003-0900-9. [DOI] [PubMed] [Google Scholar]
- 22.Yochem J, Tuck S, Greenwald I, Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126:597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- 23.Smolenaars MM, Madsen O, Rodenburg KW, Van der Horst DJ. Molecular diversity and evolution of the large lipid transfer protein superfamily. J Lipid Res. 2007;48:489–502. doi: 10.1194/jlr.R600028-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Spieth J, Blumenthal T. The Caenorhabditis elegans vitellogenin gene family includes a gene encoding a distantly related protein. Mol Cell Biol. 1985;5:2495–501. doi: 10.1128/mcb.5.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–26. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matyash V, Geier C, Henske A, Mukherjee S, Hirsh D, Thiele C, et al. Distribution and transport of cholesterol in Caenorhabditis elegans. Mol Biol Cell. 2001;12:1725–36. doi: 10.1091/mbc.12.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Branicky R, Desjardins D, Liu JL, Hekimi S. Lipid transport and signaling in Caenorhabditis elegans. Dev Dyn. 2010;239:1365–77. doi: 10.1002/dvdy.22234. [DOI] [PubMed] [Google Scholar]
- 28.Shibata Y, Branicky R, Landaverde IO, Hekimi S. Redox regulation of germline and vulval development in Caenorhabditis elegans. Science. 2003;302:1779–82. doi: 10.1126/science.1087167. [DOI] [PubMed] [Google Scholar]
- 29.Branicky R, Hekimi S. What keeps C. elegans regular: the genetics of defecation. Trends Genet. 2006;22:571–9. doi: 10.1016/j.tig.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Sellers JA, Hou L, Schoenberg DR, Batistuzzo de Medeiros SR, Wahli W, Shelness GS. Microsomal triglyceride transfer protein promotes the secretion of Xenopus laevis vitellogenin A1. J Biol Chem. 2005;280:13902–5. doi: 10.1074/jbc.M500769200. [DOI] [PubMed] [Google Scholar]
- 31.Branicky R, Shibata Y, Feng J, Hekimi S. Phenotypic and suppressor analysis of defecation in clk-1 mutants reveals that reaction to changes in temperature is an active process in Caenorhabditis elegans. Genetics. 2001;159:997–1006. doi: 10.1093/genetics/159.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hihi AK, Beauchamp MC, Branicky R, Desjardins A, Casanova I, Guimond MP, et al. Evolutionary conservation of drug action on lipoprotein metabolism-related targets. J Lipid Res. 2008;49:74–83. doi: 10.1194/jlr.M700167-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Nieland TJ, Shaw JT, Jaipuri FA, Maliga Z, Duffner JL, Koehler AN, et al. Influence of HDL-cholesterol-elevating drugs on the in vitro activity of the HDL receptor SR-BI. J Lipid Res. 2007;48:1832–45. doi: 10.1194/jlr.M700209-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham ML, Collins BJ, Hejtmancik MR, Herbert RA, Travlos GS, Vallant MK, et al. Effects of the PPARα Agonist and Widely Used Antihyperlipidemic Drug Gemfibrozil on Hepatic Toxicity and Lipid Metabolism. PPAR Res. 2010;2010 doi: 10.1155/2010/681963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shepherd J, Packard CJ, Bicker S, Lawrie TD, Morgan HG. Cholestyramine promotes receptor-mediated low-density-lipoprotein catabolism. N Engl J Med. 1980;302:1219–22. doi: 10.1056/NEJM198005293022202. [DOI] [PubMed] [Google Scholar]
- 36.Felkai S, Ewbank JJ, Lemieux J, Labbé JC, Brown GG, Hekimi S. CLK-1 controls respiration, behavior and aging in the nematode Caenorhabditis elegans. EMBO J. 1999;18:1783–92. doi: 10.1093/emboj/18.7.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levavasseur F, Miyadera H, Sirois J, Tremblay ML, Kita K, Shoubridge E, et al. Ubiquinone is necessary for mouse embryonic development but is not essential for mitochondrial respiration. J Biol Chem. 2001;276:46160–4. doi: 10.1074/jbc.M108980200. [DOI] [PubMed] [Google Scholar]
- 38.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/- mice. J Biol Chem. 2008;283:26217–27. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang W, Li J, Hekimi S. A Measurable increase in oxidative damage due to reduction in superoxide detoxification fails to shorten the life span of long-lived mitochondrial mutants of Caenorhabditis elegans. Genetics. 2007;177:2063–74. doi: 10.1534/genetics.107.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Raamsdonk JM, Meng Y, Camp D, Yang W, Jia X, Bénard C, et al. Decreased energy metabolism extends life span in Caenorhabditis elegans without reducing oxidative damage. Genetics. 2010;185:559–71. doi: 10.1534/genetics.110.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ando K, Fujita T. Metabolic syndrome and oxidative stress. Free Radic Biol Med. 2009;47:213–8. doi: 10.1016/j.freeradbiomed.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 44.Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–12. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Wong A, Boutis P, Hekimi S. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics. 1995;139:1247–59. doi: 10.1093/genetics/139.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sluder AE, Mathews SW, Hough D, Yin VP, Maina CV. The nuclear receptor superfamily has undergone extensive proliferation and diversification in nematodes. Genome Res. 1999;9:103–20. [PubMed] [Google Scholar]