Abstract
Formation and normal function of neuronal synapses are intimately dependent on the delivery to and removal of biological materials from synapses by the intracellular transport machinery. Indeed, defects in intracellular transport contribute to the development and aggravation of neurodegenerative disorders. Despite its importance, regulatory mechanisms underlying this machinery remain poorly defined. We recently uncovered a phosphorylation-regulated mechanism that controls FEZ1-mediated Kinesin-1-based delivery of Stx1 into neuronal axons. Using C. elegans as a model organism to investigate transport defects, we show that FEZ1 mutations resulted in abnormal Stx1 aggregation in neuronal cell bodies and axons. This phenomenon closely resembles transport defects observed in neurodegenerative disorders. Importantly, diminished transport due to mutations of FEZ1 and Kinesin-1 were concomitant with increased accumulation of autophagosomes. Here, we discuss the significance of our findings in a broader context in relation to regulation of Kinesin-mediated transport and neurodegenerative disorders.
Keywords: axonal transport, transport defects, neurodegeneration, autophagy, FEZ1, Kinesin, Syntaxin, Munc18, SNARE, synapse
Introduction
The neuronal cellular architecture with its numerous extended processes and extensive branching has evolved exquisitely to support its function as an integrator and transducer of inter-neuronal signaling.1 The proper function of this intricate cellular network (and indeed even the viability of the neuron itself) is sustained by a similarly fascinating system of intracellular transport machinery that serves to move organelles and biological raw materials from one part of the neuron to another.2 The Kinesin superfamily of proteins is predominately responsible for anterograde transport of these cargoes in neuronal axons. An unresolved dilemma for this transport machinery is how the limited set of Kinesin members is capable of adapting to the large diversity of intracellular cargo. While Kinesins have been shown to bind to cargo directly, most appear to bind indirectly via a growing array of Kinesin adapters.3 Nevertheless, detailed understanding of mechanisms regulating and coordinating cargo recognition, cargo-motor complex formation and activation of the motor protein remains elusive.4
FEZ1 as a New Motor Adaptor for Presynaptic Cargo
We recently reported that fasciculation and elongation protein zeta 1 (FEZ1/UNC-76) binds the neuronal SNARE protein Syntaxin 1A (Stx1/UNC-64) and Munc18/UNC-18, two presynaptic proteins involved in the control of synaptic vesicle exocytosis.5 FEZ1 was previously shown to function as a Kinesin-1/UNC-116 adaptor and we wondered if it might also function in the axonal delivery of these presynaptic proteins. Since the presence of FEZ2, a close homolog of FEZ1, in mammals might complicate functional analysis of the interactions, we instead opted to use Caenorhabditis elegans, a model organism that is easily amenable to genetic manipulations and in vivo imaging. Satisfyingly, we were able to successfully correlate the biochemical findings with their in vivo function in the worm.
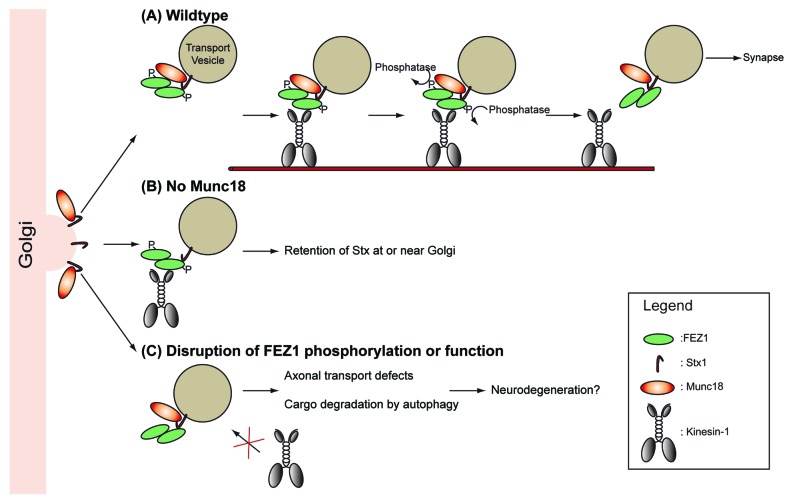
Examination of neurons revealed axonal transport abnormalities in unc-76 and unc-116 mutants as manifested by the appearance of UNC-64 aggregates in cell bodies as well as axons. Importantly, re-expression of FEZ1 or UNC-76 alone in unc-76 mutants was sufficient to restore wild-type axonal distribution of UNC-64 in these animals. We further determined by mass spectrometric analyses that FEZ1 expressed in mammalian cells is phosphorylated at multiple serine sites. Subsequent biochemical analyses demonstrated that FEZ1’s interaction with both Munc18 and Kinesin-1 is tightly regulated by its phosphorylation. Significantly, re-expression of a phosphorylation-defective mutant of FEZ1 (FEZ1 S58A) failed to restore wild-type axonal distribution of UNC-64 in unc-76 mutants. The combined data from the biochemical and functional experiments strongly indicated that FEZ1/UNC-76 functions as a motor adaptor bridging presynaptic cargo and Kinesin-1/UNC-116 (Fig. 1A).
Figure 1. FEZ1/UNC-76 serves as a motor adaptor for presynaptic cargo. (A) Kinesin-1/UNC-116 binds Stx1/UNC-64-containing transport vesicles via the adaptor FEZ1/UNC-76 forming a complex with Stx1/UNC-64 and Munc18/UNC-18. Dephosphorylation of FEZ1/UNC-76 releases the cargo from the motor. (B) In the absence of Munc18/UNC-18, Stx1/UNC-64 is not efficiently transported to the synapse. (C) Defects in adaptor function may result in transport abnormalities leading to cargo degradation and neurodegeneration.
Munc18 a Regulator of Cargo-Motor Complex Formation?
Intriguingly, the intracellular distribution of Munc18/UNC-18 appears unaffected by disruptions to the FEZ1/Kinesin-1 motor. This indicates that the bulk of Munc18 might be transported independently of Stx1. Previous studies have shown that the plasma membrane translocation of Stx1 is tightly dependent on co-expression of Munc18. Indeed, Stx1 is largely confined to ER or Golgi compartments in non-neuronal cell lines overexpressing Stx1 or in PC12 cells where expression of Munc18 has been abrogated (Fig. 1B).6-9 The lack of a UNC-18 transport defective phenotype in both unc-76 and unc-116 mutants raises the intriguing possibility that Munc18/UNC-18 might not be a cargo for the FEZ1/Kinesin-1 motor complex. So why does FEZ1 concurrently bind Munc18?
A possible explanation is that Munc18 regulates the formation of the Stx1/FEZ1/Kinesin-1 transport complex. Indeed, Munc18 was suggested to play a protective function during the synthesis and maturation of Stx1 by preventing export of spurious SNARE complexes from the Golgi.10 Interestingly, binding of Stx1 to FEZ1 appears to be constitutive whereas phosphorylation of FEZ1 is required for Munc18 binding. This suggests that the motor itself is unable to recognize the quality of the cargo in the absence of bound Munc18. The inability to form a functional Stx1/FEZ1/Kinesin-1 transport complex without Munc18 would explain why the cargo becomes trapped at the Golgi.
Supporting this notion, we observe that Stx1 and FEZ1 strongly co-localizes at Golgi sites when a Munc18 mutant unable to bind Stx1 was expressed in mammalian cells. In comparison, both proteins redistribute correctly to the plasma membrane when wild-type Munc18 was used (unpublished observations). Taken together, we postulate that presentation of a preformed Stx1-Munc18 complex to FEZ1/Kinesin-1 and concurrent binding of FEZ1 to both proteins might constitute intermediate checkpoints toward formation of a functional transport complex. Thus, phosphorylation-regulated binding of FEZ1 to Munc18 could serve as a control mechanism to ensure delivery of properly processed Stx1 to synapses. In addition, binding of Munc18 to Stx1 en route to the synapse may allow the former to continue serving its protective function as in the Golgi. Thus, Munc18 could be considered an escort rather than a cargo per se.
Unsurprisingly perhaps, binding of mammalian FEZ1 to Kinesin is also tightly coupled to FEZ1 phosphorylation. Mutation of any of the phospho-serine sites identified prevented FEZ1 binding to Kinesin-1. Thus, unlike its interaction with Munc18, FEZ1 phosphorylation directly impacts its binding to, and consequently binding of the cargo to, the motor and likely functions as a more general mechanism regulating transport complex formation. Using in vitro phosphorylation assays, we recently succeeded in identifying several kinases that recognize these serine residues. The effect of these kinases on influencing FEZ1-Kinesin-1 motor mediated transport in vivo is currently being investigated.
Involvement of FEZ1 in Neurodegenerative Disorders
Defects in intracellular transport and autophagy are increasingly recognized as causative or exacerbating factors leading to the onset or progression of neurodegenerative disorders (ND).11-13 The nematode as a laboratory pet with its easy genetic accessibility has been established as model for human neurodegenerative diseases.14,15 Strikingly, the axonal phenotype observed in unc-76 and unc-116 mutants closely resembles transport abnormalities seen in animal models of tauopathies or Alzheimer disease (Fig. 1C).16-19
FEZ1 is no newcomer as a candidate ND protein. Several previous studies implicate FEZ1 in Schizophrenia, and FEZ1 binds to Disrupted-in-Schizophrenia 1 (DISC1), a gene strongly linked to Schizophrenia.20 Noteworthy, FEZ1 also binds Huntingtin. Mutant Huntingtin is known to inhibit fast axonal transport by activating JNK3 which, in turn, phosphorylates Kinesin-1 and reduces its ability to bind microtubules.21,22 Considering that disrupting FEZ1 function in neurons also affects intracellular transport of other synaptic proteins and mitochondria, it is conceivable that perturbations of FEZ1 function will have a broader impact on intracellular transport defects.20,23,24
Using electron microscopy, we observed the appearance of autophagosomes in axonal processes of unc-76 and unc-116 mutants. This is in excellent agreement with recent reports from animal models of ND where reduced motor function is associated with increased autophagic vesicles and the onset of neuron degeneration.25,26 Conceivably, motor defects generate stranded cargo that is removed by autophagy. These studies suggest that a buildup of such cargo may eventually overwhelm the autophagic apparatus. As FEZ1 is also known to regulate autophagy, it is possible that FEZ1 may be involved in targeting the stranded cargo for autophagic degradation.27
Outlook
A growing amount of studies place FEZ1 as a hub protein connecting several biological processes pertinent to neurodegenerative diseases. It will be interesting to see if FEZ1 functions as a master orchestrator of neuronal growth and homeostasis by balancing kinesin-based delivery of cargo with autophagy.
Acknowledgments
The research leading to these results has received funding from the European Union Sixth and Seventh Framework Programmes under grant agreement no. LSHM-CT-2005–019055 (‘EUSynapse’), no. HEALTH-F2–2009–241498 (‘EUROSPIN’).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/21564
References
- 1.Miller JP, Jacobs GA. Relationships between neuronal structure and function. J Exp Biol. 1984;112:129–45. doi: 10.1242/jeb.112.1.129. [DOI] [PubMed] [Google Scholar]
- 2.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 3.Akhmanova A, Hammer JA., 3rd Linking molecular motors to membrane cargo. Curr Opin Cell Biol. 2010;22:479–87. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev. 2008;88:1089–118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- 5.Chua JJ, Butkevich E, Worseck JM, Kittelmann M, Grønborg M, Behrmann E, et al. Phosphorylation-regulated axonal dependent transport of syntaxin 1 is mediated by a Kinesin-1 adapter. Proc Natl Acad Sci USA. 2012;109:5862–7. doi: 10.1073/pnas.1113819109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe J, Calegari F, Taverna E, Longhi R, Rosa P. Syntaxin 1A is delivered to the apical and basolateral domains of epithelial cells: the role of munc-18 proteins. J Cell Sci. 2001;114:3323–32. doi: 10.1242/jcs.114.18.3323. [DOI] [PubMed] [Google Scholar]
- 7.Rowe J, Corradi N, Malosio ML, Taverna E, Halban P, Meldolesi J, et al. Blockade of membrane transport and disassembly of the Golgi complex by expression of syntaxin 1A in neurosecretion-incompetent cells: prevention by rbSEC1. J Cell Sci. 1999;112:1865–77. doi: 10.1242/jcs.112.12.1865. [DOI] [PubMed] [Google Scholar]
- 8.Han L, Jiang T, Han GA, Malintan NT, Xie L, Wang L, et al. Rescue of Munc18-1 and -2 double knockdown reveals the essential functions of interaction between Munc18 and closed syntaxin in PC12 cells. Mol Biol Cell. 2009;20:4962–75. doi: 10.1091/mbc.E09-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arunachalam L, Han L, Tassew NG, He Y, Wang L, Xie L, et al. Munc18-1 is critical for plasma membrane localization of syntaxin1 but not of SNAP-25 in PC12 cells. Mol Biol Cell. 2008;19:722–34. doi: 10.1091/mbc.E07-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medine CN, Rickman C, Chamberlain LH, Duncan RR. Munc18-1 prevents the formation of ectopic SNARE complexes in living cells. J Cell Sci. 2007;120:4407–15. doi: 10.1242/jcs.020230. [DOI] [PubMed] [Google Scholar]
- 11.De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci. 2008;31:151–73. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein LS. Axonal transport and neurodegenerative disease: Can we see the elephant? Prog Neurobiol. 2012 doi: 10.1016/j.pneurobio.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee R, Beal MF, Thomas B. Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci. 2010;33:541–9. doi: 10.1016/j.tins.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington AJ, Knight AL, Caldwell GA, Caldwell KA. Caenorhabditis elegans as a model system for identifying effectors of α-synuclein misfolding and dopaminergic cell death associated with Parkinson’s disease. Methods. 2011;53:220–5. doi: 10.1016/j.ymeth.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Fatouros C, Pir GJ, Biernat J, Koushika SP, Mandelkow E, Mandelkow EM, et al. Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds190. [DOI] [PubMed] [Google Scholar]
- 16.Falzone TL, Stokin GB, Lillo C, Rodrigues EM, Westerman EL, Williams DS, et al. Axonal stress kinase activation and tau misbehavior induced by kinesin-1 transport defects. J Neurosci. 2009;29:5758–67. doi: 10.1523/JNEUROSCI.0780-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156:1051–63. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/S0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- 19.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–8. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 20.Maturana AD, Fujita T, Kuroda S. Functions of fasciculation and elongation protein zeta-1 (FEZ1) in the brain. ScientificWorldJournal. 2010;10:1646–54. doi: 10.1100/tsw.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goehler H, Lalowski M, Stelzl U, Waelter S, Stroedicke M, Worm U, et al. A protein interaction network links GIT1, an enhancer of huntingtin aggregation, to Huntington’s disease. Mol Cell. 2004;15:853–65. doi: 10.1016/j.molcel.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, et al. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009;12:864–71. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toda H, Mochizuki H, Flores R, 3rd, Josowitz R, Krasieva TB, Lamorte VJ, et al. UNC-51/ATG1 kinase regulates axonal transport by mediating motor-cargo assembly. Genes Dev. 2008;22:3292–307. doi: 10.1101/gad.1734608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gindhart JG, Chen J, Faulkner M, Gandhi R, Doerner K, Wisniewski T, et al. The kinesin-associated protein UNC-76 is required for axonal transport in the Drosophila nervous system. Mol Biol Cell. 2003;14:3356–65. doi: 10.1091/mbc.E02-12-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Varo R, Trujillo-Estrada L, Sanchez-Mejias E, Torres M, Baglietto-Vargas D, Moreno-Gonzalez I, et al. Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. Acta Neuropathol. 2012;123:53–70. doi: 10.1007/s00401-011-0896-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falzone TL, Gunawardena S, McCleary D, Reis GF, Goldstein LS. Kinesin-1 transport reductions enhance human tau hyperphosphorylation, aggregation and neurodegeneration in animal models of tauopathies. Hum Mol Genet. 2010;19:4399–408. doi: 10.1093/hmg/ddq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKnight NC, Jefferies HB, Alemu EA, Saunders RE, Howell M, Johansen T, et al. Genome-wide siRNA screen reveals amino acid starvation-induced autophagy requires SCOC and WAC. EMBO J. 2012;31:1931–46. doi: 10.1038/emboj.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]