Abstract
Motor control is a complex process that requires interplay among the nervous system, muscles and environment. The simple anatomy, well-characterized muscle movements and ample resources for molecular and cellular dissection make the pharynx of the nematode C. elegans an attractive model system for the study of motor control. The C. elegans pharynx shows two clear muscle movements that are essential for food intake, pharyngeal pumping and isthmus peristalsis. Here, we review our recent findings on the mechanism by which food activates the feeding motions. To understand this process, we characterized the behavior of the feeding motions in response to serotonin, an endogenous pharyngeal pumping activator whose action is triggered by food. We found that: (1) the timing of onset and frequencies of the two feeding motions are distinct; (2) isthmus peristalsis is selectively coupled to the preceding pump; (3) like food, serotonin activates isthmus peristalsis as well as pharyngeal pumping. By genetic analysis, we showed that two separate neural pathways activate the two feeding motions explaining the differences between the two feeding motions. We also proposed a model that explains how the two feeding motions are separately controlled, yet coupled by the interaction between the nervous system and the muscles in the pharynx. Finally, we briefly discuss future approaches to further understand the mechanism that couples the two feeding motions in C. elegans and to possibly understand evolution of motor control in the pharynx by expanding findings in C. elegans to other nematode species.
Keywords: motor control, neural circuit, muscle, serotonin, pharynx, feeding activation
Introduction
The motor output of an organism in its natural habitat is determined by interplay among the nervous system, muscles and environment. The nervous system senses extrinsic and intrinsic cues, integrates the information and sends commands to motor neurons that control muscles. The muscles convert neural signals into mechanical outputs. The connectivity within the neural circuitry and the intrinsic properties of the constituent neurons and of the muscles under control of the neural input are critical parameters that determine motor output, and thus, it is of great interest in the study of motor control to understand the mechanism by which the parameters control motor output.
The pharynx, the feeding organ of the nematode C. elegans, offers multiple advantages as a model to study the mechanism of motor control. The C. elegans pharynx is an encapsulated organ that has a simple anatomy, consisting of 20 neurons and 8 gap-junction-connected muscle types constituting three functional parts, the corpus, the isthmus and the terminal bulb (Fig. 1). The pharynx shows two discrete muscle movements for food intake that are amenable to quantitative analysis. Pharyngeal pumping, the first motion of the pharyngeal muscles, is a coupled muscle contraction and subsequent relaxation of the corpus, anterior isthmus and the terminal bulb. Isthmus peristalsis, the second motion, is a peristaltic motion in the posterior isthmus. Pharyngeal pumping takes up bacteria from environment, accumulates the bacteria in the anterior isthmus and crushes the bacteria for further digestion. Isthmus peristalsis transports the accumulated bacteria from the anterior isthmus to the grinder in the terminal bulb that crushes the bacteria. The frequencies of the two feeding motions are controlled by environmental and endogenous cues in worms and the regulation requires interaction between the nervous system and the muscles. Regulatory molecules involved in regulation of the feeding motions are highly conserved in higher organisms,1 which makes the genetically tractable C. elegans a great model system for molecular dissection of the mechanism of motor control. C. elegans also offers an ample resource for dissection of neural circuits. The wiring pattern of the C. elegans nervous system has been reconstructed from serial electron micrographs2 and various approaches to define the functions of particular neurons are available such as ablation of single neurons or combinations3 or regulation of the activity of neurons by expressing optogenetic tools.4
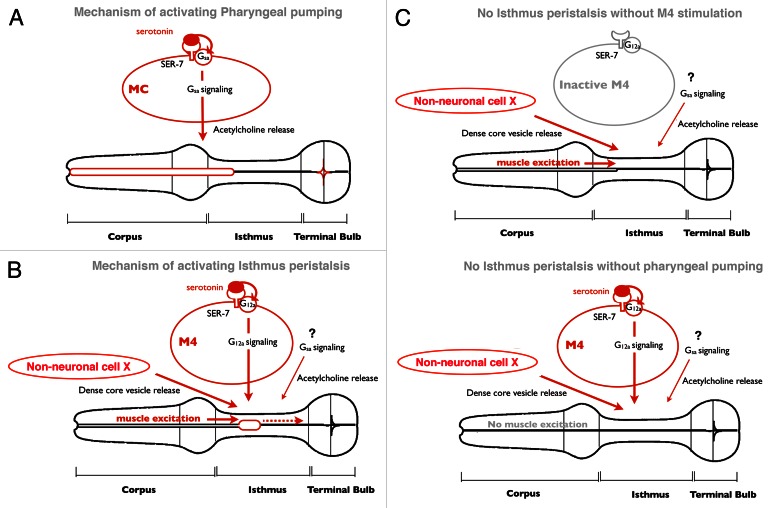
Figure 1. Model of regulation of feeding by serotonin. (A) In response to serotonin, SER-7 in MC cell-autonomously activates its downstream Gsα signaling pathway, which subsequently stimulates pharyngeal pumping by activating cholinergic transmission from MC to the pharyngeal muscles. (B) In response to serotonin, SER-7 in M4 (and possibly in M2) activates its downstream Gα12 pathways in a cell autonomous manner, which subsequently activates M4. The stimulus from active M4, along with dense core vesicle release controlled by an unidentified pathway, activates isthmus peristalsis. The Gsα signaling pathway and downstream cholinergic transmission also contribute to activating isthmus peristalsis but their sites of action have not been characterized. Given that expression of SER-7 in M4 fully restored isthmus peristalsis rate in the ser-7 null mutant, it is plausible that release of dense core vesicles from the unidentified cells is constitutively active rather than triggered by serotonin. (C) Stimuli from an active M4 neuron and from the anterior part of the pharynx, excited by pumping, are both required to activate isthmus peristalsis. In the absence of either stimulus, isthmus peristalsis does not occur.
In this commentary, we will review our recent study that dissects a local neural circuit in the C. elegans pharynx that activates the feeding motions in response to food and discuss future studies. Like other animals, C. elegans activates food intake by activating the feeding muscles in response to food.5,6 Serotonin is an endogenous activator of pharyngeal pumping7 whose action is triggered by food in C. elegans.8 Moreover, based on the expression pattern of serotonin receptors, it seemed probable that serotonin also activates isthmus peristalsis.9 Thus, we studied the mechanism by which serotonin activates the feeding motions.
Serotonin Activates the Two Feeding Motions Via Two Separate Neural Pathways in C. Elegans.
We first examined the feeding motions of wild type worms in the presence of serotonin and made a couple of interesting observations. First, isthmus peristalsis is selectively coupled to the preceding pharyngeal pump. Isthmus peristalsis does not occur in the absence of pumping and every isthmus peristalsis follows a pump after a constant interval of about 150msec, regardless of pharyngeal pumping rate. Second, not every pump is followed by isthmus peristalsis. The ratio of the frequencies of isthmus peristalsis to pharyngeal pumping (IP to PP ratio) was 1:3.4, in average. We also noticed a trend that shows an inverse correlation between the pumping rate and the IP to PP ratio. Overall, slowly pumping worms showed a high IP to PP ratio that frequently reached 1:1 whereas rapidly pumping worms showed a low IP to PP ratio that frequently reached 1:10. Third, as we suspected, serotonin activates isthmus peristalsis as well as pharyngeal pumping.
By genetic analysis and cell specific expression experiments, we found that serotonin activates the two feeding motions via two separate neural pathways. First, a type 7 serotonin receptor SER-7 activates pumping and isthmus peristalsis in response to serotonin mainly by acting in MC and M4, respectively (Figs. 1A and B). MC and M4 are cholinergic motor neurons in the pharynx that are essential for normal fast pumping and for isthmus peristalsis, respectively.10,11 Second, SER-7 activates the two feeding motions mainly by activating two separate downstream G protein signaling pathways (Figs. 1A and B). SER-7 is a serotonin-activated G protein coupled receptor12 and its mammalian homolog was suggested to be coupled to Gαs and Gα12/13 and to activate the G protein signaling cascades.13 Activation of Gαs signaling itself dramatically activated pharyngeal pumping and was also essential for serotonin-induced activation of pumping. In contrast, activation of the Gαs signaling cascade had only moderate effect on isthmus peristalsis. Activation of Gα12/13 signaling pathway dramatically increased isthmus peristalsis, yet had no effect in pharyngeal pumping.
Regulation by two separate neural pathways explains the differences in the timing of onset and the frequencies of the two feeding motions. How then are the feeding motions coupled? The pharyngeal muscles are connected by gap junctions, which electrically couple the muscles. Previous studies suggest that the electrical signal that elicits pumping travels from the corpus to the terminal bulb.14,15 Since the isthmus is inbetween, the excitation must pass through it. Based on the observations that both pharyngeal pumping and M4 activity are essential for isthmus peristalsis,10,16 we propose that triggering isthmus peristalsis requires both muscle excitation during pumping and stimulation by M4. Because of the contribution of muscle excitation during the pump, only M4 firing that occurs within a certain time window from the preceding pump can trigger isthmus peristalsis, resulting in the coupling between the two muscle motions (Fig. 1C). Our model explains how the feeding motions can be separately regulated yet coupled. We speculate that the pharynx evolved this way to support efficient feeding. First, the separate regulation allows worms to adjust the ratio of the frequencies of pharyngeal pumping and isthmus peristalsis (IP to PP ratio) according to the density of food. When food is scarce, worms should take up food as frequently as possible but swallow food only when enough food is accumulated in the anterior isthmus, which decreases the IP to PP ratio. In contrast, when food is abundant, worms do not need to fully activate pharyngeal pumping, but need to swallow frequently to prevent regurgitation, which increases the IP to PP ratio. In fact, our group previously showed that the ratio of IP to PP ratio changes as expected according to the food density.6 Second, coupling of isthmus peristalsis to the preceding pumping allows worms to swallow food with the proper timing. Food accumulated by pumping is transported from the anterior isthmus to the grinder in the terminal bulb by isthmus peristalsis. To prevent futile isthmus peristalsis or an overload of food in the anterior isthmus, it would be the best for worms to swallow food as soon as a pharyngeal pump takes in enough food into the anterior isthmus.
Future Research
In summary, our model suggests that (1) induction of isthmus peristalsis requires both muscle excitation during pumping and stimulation by M4. (2) The relative timing of M4 firing to pumping is a determinant of induction of isthmus peristalsis. The model that we proposed can be tested in transgenic animals expressing channelrhodopsin or halorhodopsin in M4 by asking the following questions: does activation or suppression of M4 activity control isthmus peristalsis as expected? If it does, does induction of isthmus peristalsis require both M4 activity and pharyngeal pumping? If so, is relative timing of M4 firing to pumping important to trigger isthmus peristalsis?
If our model is correct, it would be informative to know how stimulation by M4 and muscle excitation during pumping converge to generate isthmus peristalsis. A previous calcium imaging study on the pharyngeal muscles reported that an anterior-to-posterior calcium wave travels in the posterior isthmus only during isthmus peristalsis,15 suggesting that stimulation by M4 and muscle excitation during pumping contributes to triggering isthmus peristalsis by eliciting the calcium wave. The hypothesis can be tested by testing whether injecting a calcium chelator into the isthmus muscle blocks serotonin-induced isthmus peristalsis. If the calcium wave is indeed essential, it would be also informative to study which pharyngeal muscles during a pump contribute to triggering the calcium wave in the posterior isthmus, assuming that isthmus peristalsis requires pharyngeal pumping.
The study of motor control in the pharynx of C. elegans may also be useful to understand evolution of motor control in animals in different environments. A comparative study of the feeding motions in the pharynx between C. elegans and other nematodes reported that the pumping and peristaltic motions are conserved in other nematodes with differences in the spatial and coupling patterns.6 Given that anatomy of the pharyngeal nervous system is generally conserved in the nematodes,6 further characterization of the feeding motions and cellular dissection of the motor control in the nematodes may provide interesting insight into the different mechanisms of motor control that the nematodes have developed during evolution.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/21833
References
- 1.Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 3.Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 1995;48:225–50. doi: 10.1016/S0091-679X(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leifer AM, Fang-Yen C, Gershow M, Alkema MJ, Samuel AD. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods. 2011;8:147–52. doi: 10.1038/nmeth.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croll NA. Integrated behavior in the feeding phase of Caenohrabditis elegans. J Zool. 1978;184:507–17. doi: 10.1111/j.1469-7998.1978.tb03304.x. [DOI] [Google Scholar]
- 6.Chiang JT, Steciuk M, Shtonda B, Avery L. Evolution of pharyngeal behaviors and neuronal functions in free-living soil nematodes. J Exp Biol. 2006;209:1859–73. doi: 10.1242/jeb.02165. [DOI] [PubMed] [Google Scholar]
- 7.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–4. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 8.Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–4. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- 9.Hobson RJ, Hapiak VM, Xiao H, Buehrer KL, Komuniecki PR, Komuniecki RW. SER-7, a Caenorhabditis elegans 5-HT7-like receptor, is essential for the 5-HT stimulation of pharyngeal pumping and egg laying. Genetics. 2006;172:159–69. doi: 10.1534/genetics.105.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avery L, Horvitz HR. A cell that dies during wild-type C. elegans development can function as a neuron in a ced-3 mutant. Cell. 1987;51:1071–8. doi: 10.1016/0092-8674(87)90593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–85. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- 12.Hobson RJ, Geng J, Gray AD, Komuniecki RW. SER-7b, a constitutively active Galphas coupled 5-HT7-like receptor expressed in the Caenorhabditis elegans M4 pharyngeal motorneuron. J Neurochem. 2003;87:22–9. doi: 10.1046/j.1471-4159.2003.01967.x. [DOI] [PubMed] [Google Scholar]
- 13.Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, et al. 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. 2005;25:7821–30. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raizen DM, Avery L. Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron. 1994;12:483–95. doi: 10.1016/0896-6273(94)90207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimozono S, Fukano T, Kimura KD, Mori I, Kirino Y, Miyawaki A. Slow Ca2+ dynamics in pharyngeal muscles in Caenorhabditis elegans during fast pumping. EMBO Rep. 2004;5:521–6. doi: 10.1038/sj.embor.7400142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song BM, Avery L. Serotonin activates overall feeding by activating two separate neural pathways in Caenorhabditis elegans. J Neurosci. 2012;32:1920–31. doi: 10.1523/JNEUROSCI.2064-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]