Abstract
Cisplatin is an essential chemotherapeutic drug in the treatment of many cancers. Its use, however, is limited by the development of resistance in many tumors. The ability to re-sensitize resistant tumors could significantly strengthen cisplatin therapy in patients. Caenorhabditis elegans is a suitable model for studying the cytoplasmic role of cisplatin in tumor cells. We have previously shown that the ATPase ASNA-1 has similar roles as a factor governing cisplatin sensitivity in mammalian tumor cells and C. elegans. Here we study the endoplasmic reticulum (ER) resident chaperone ENPL-1/GRP94 and find that its depletion makes worms sensitive to cisplatin. Elevated ER stress levels in enpl-1 mutants is the likely cause of this sensitivity because a correlation can be made between cisplatin sensitivity and the high ER stress levels. We also find that asna-1 mutants have elevated unfolded protein response (UPR) activity and that the intrinsically cisplatin resistant wild-type worms become sensitive when ER stress is high. We conclude that enpl-1 is a cisplatin sensitizing factor and suggest that manipulation of its levels or of UPR activity will enhance the effects of cisplatin based cancer therapy.
Keywords: cisplatin, unfolded protein response, GRP94, asna-1, endoplasmic reticulum stress
Introduction
Cisplatin and other platinum containing drugs have played a crucial role in chemotherapy and anti-cancer treatments for over 30 y. While many tumors initially respond well to these drugs, the emergence of resistant tumors curtails their curative potential. A further limitation of the use of cisplatin is the side effects such as nephrotoxicity and ototoxicity that are undesirable aspects of the anti-cancer treatment.1 Understanding how cisplatin and other platinum-containing drugs exert their cytotoxic effects is important for addressing the problem of drug resistance. It is also important to identify proteins that are able to re-sensitize resistant cells when they are depleted or overexpressed. A more nuanced appreciation of the cell biological effects of cisplatin will also help in designing treatments to ameliorate these effects of drug therapy.
Cisplatin induces cell death by introducing intra- and inter-strand crosslinks in the DNA. These lesions, unless repaired efficiently, will cause cell cycle arrest and apoptosis.2 However, it is clear that cisplatin acts in the cytoplasm as well to exert its killing effect. Only a small fraction of the cisplatin in a cell interacts with the DNA, and levels of cisplatin that do not cause the DNA damage can still result in cell death.3 Furthermore, enucleated cells can also be killed by the drug.4 Cisplatin interacts with several cytoplasmic and mitochondrial proteins and non-nuclear events contribute substantially to the cytotoxic effects of the drug. Endoplasmic reticulum (ER) stress has emerged as one of the important factors that sensitizes cells to cisplatin induced death and treatments that increase severe ER stress increase the efficacy of cisplatin treatment.5,6 In the long-term it will be important to understand the relative contributions of nuclear vs. cytoplasmic events to cisplatin induced death and the likely cross-talk between these compartments.
Identification of genes that modify the response to cisplatin can be effectively performed in genetically amenable model systems and Caenorhabditis elegans is well suited to this effort. Worms have been used to identify the genes that play a role in cisplatin-induced death to model both its effects on DNA and the cytoplasm of the affected cells. Wild-type worms are intrinsically resistant to cisplatin and provide an attractive model for cisplatin resistance tumors. The DNA damaging effects of cisplatin and the worm genes that modify this effect have been studied in embryos and larvae, both of which have dividing cells.7-10 The cytoplasmic roles of cisplatin can be easily studied in isolation from its effects on DNA in adult C. elegans since all somatic cell divisions have been completed in adults. Using this approach, asna-1 mutants were found to be hypersensitive to cisplatin. This finding was consistent with studies of its human homolog in cultured cells. The study also showed that the cisplatin hypersensitivity phenotype of asna-1 mutants could be separated from its role in promotion of insulin signaling.11,15 The sensitivity spectrum to metal salts in C. elegans asna-1 mutants is similar to that seen upon ASNA1 knockdown in human tumor cell lines12-14 and the human homolog can rescue the asna-1 mutant hypersensitivity phenotype. These findings establish that C. elegans can be used as a model system to understand the cytoplasmic effects of cisplatin in human tumors and to identify new genes in the process.15
Endoplasmic reticulum (ER) stress, which leads to elevated levels of unfolded protein response (UPR) has been identified as one of the factors that sensitizes tumor cells to cisplatin. The importance of the UPR is highlighted by studies of the ER resident chaperone GRP78/BiP, a central regulator of UPR. GRP78 confers resistance when overexpressed and sensitivity when knocked down in some tumor types.5,16,17 Another prominent ER chaperone, GRP94/endoplasmin/gp96, which belongs to the HSP90 class of proteins, is coordinately regulated with GRP78.18 GRP94 expression is used as a reporter for induction of UPR in mice19,20 and its depletion leads to elevated UPR in both mice and worms.21,22 Due to these properties it is possible that GRP94 may also play a role in the modulation of chemotherapeutic drug response. However, to date there are no studies that demonstrate a role for mammalian GRP94 in the modulation of cisplatin sensitivity. The single GRP94/endoplasmin homolog in the C. elegans genome called enpl-1 displays good overall structural and sequence conservation with its mammalian counterparts. It is expressed at high levels in the pharynx and intestine and is likely to be an endoplasmic reticulum (ER) resident protein because it has a C-terminal HSEL motif that is important for ER retention (www.wormbase.org). enpl-1 has been identified in a recent screen for new modulators of insulin signaling that was based on similarity to the asna-1 phenotype.23
Given its importance in ER function and the availability of deletion mutants in the gene and relationship to asna-1, we wished to ask whether enpl-1 mutants were sensitive to cisplatin. We show that both enpl-1 and asna-1 mutants have increased ER stress and find that increased ER stress correlates with increased cisplatin sensitivity. Double mutant analysis shows that enpl-1 appears to act in the regulation of UPR in a pathway that is parallel to the ire-1/xbp-1 arm. Finally, evidence is provided to show that increased ER stress can lead to cisplatin sensitivity in intrinsically resistant animals. These findings implicate ER stress in cisplatin-induced cell death in post-mitotic tissues and identify ENPL-1/GRP94 as a new factor which, when downregulated, could resensitize resistant tumor cells to cisplatin.
Results
enpl-1 mutants are sensitive to metal salts
enpl-1 encodes the C. elegans homolog of mammalian GRP94/endoplasmin (cosmid name: T05E11.3). With the availability of two deletion alleles of enpl-1(ok1964 and tm3738), it was possible to investigate whether depletion of ENPL-1 activity affected the sensitivity of worms to the platinum containing drug cisplatin and to other metals. In mammalian cells and in worm asna-1 mutants, sensitivity to cisplatin is associated with sensitivity to sodium arsenite. Adult enpl-1(ok1964) mutants were found to be extremely sensitive to cisplatin and sodium arsenite and moderately sensitivity to zinc (Fig. 1). asna-1 mutants are also sensitive to cisplatin and arsenite but not to zinc (Fig. 1). Thus the two mutants show similarities and differences with respect to their response to metal salts.
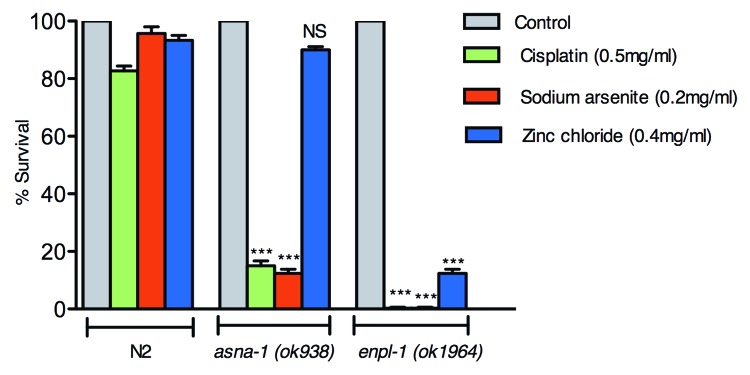
Figure 1.enpl-1 mutants have increased sensitivity to metal salts. Worms of indicated genotypes were exposed to various metals and survival after 24 h was scored. The bars in the graph represent the mean of three experiments. n = 15 per experiment for each strain tested. The error bar represents standard error of mean (Prism 5, GraphPad software). The groups were compared using one way ANOVA with Bonferroni’s multiple comparisons posthoc test. *** denotes p < 0.001 and NS denotes not significant.
The results shown in Figure 1 indicated that enpl-1 (ok1964) mutants appeared to be more sensitive to cisplatin than asna-1 mutants. To ask if this was the case, dose-response studies were conducted in parallel in order to determine the LC50 values for each mutant and for wild-type worms. One day old adults were exposed to cisplatin for 24 h or 48 h and the proportion that were alive was determined (Fig. 2). After a 24 h exposure to cisplatin, the LC50 for enpl-1(ok1964) mutants (215 µM) was significantly lower (p < 0.001) than that for asna-1 mutants (340 µM) (Fig. 2A). By contrast wild-type worms displayed inherent robust resistance to cisplatin after a 24 h exposure. The findings for asna-1 mutants and wild-type animals are consistent with our previous report.15 When animals were exposed to cisplatin for 48 h (Fig. 2B), the resistance of wild-type animals broke down. However, even with this treatment wild-type worms were much more resistant to cisplatin than either mutant. In addition, asna-1 mutants (205 µM) were still less sensitive (p < 0.001) than enpl-1 mutants (152 µM) after 48 h exposure. To rule out effects due to genetic background, enpl-1 (tm3738) mutants were also tested for cisplatin sensitivity and found to be as sensitive as enpl-1(ok1964), thus making it unlikely that the genetic background effects are the reason for the cisplatin hypersensitivity phenotype (Fig. S1).
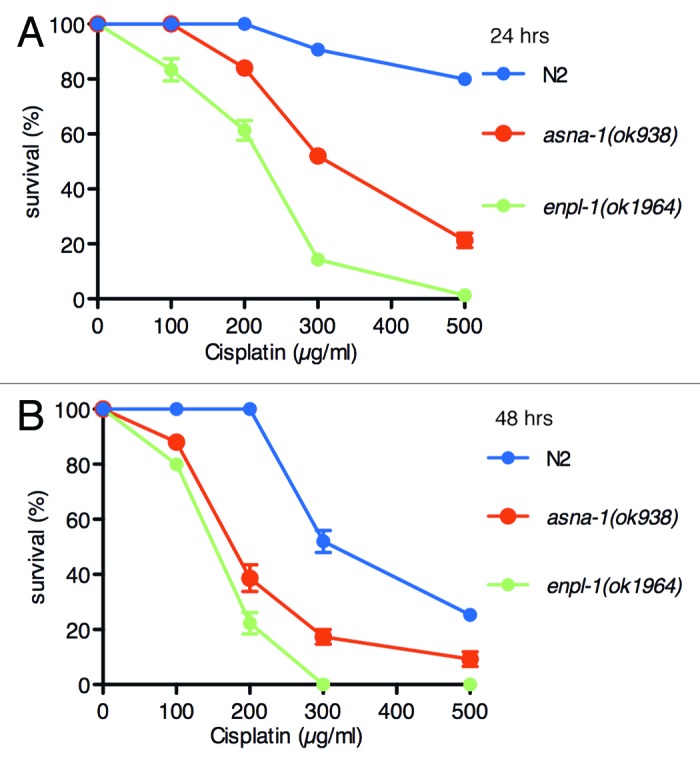
Figure 2.enpl-1 mutants are more sensitive to cisplatin than asna-1 mutants. Survival of wild-type (N2), asna-1 mutants and enpl-1 mutants in various concentrations of cisplatin was scored. Data from three independent experiments (n = 100 for each strain) were plotted as survival curves. LC50 values were determined using binary logistic regression analysis. p < 0.001 for all comparisons between asna-1(ok938) and enpl-1(ok1964) mutants with wild type N2 worms. The error represents mean ± SEM.
enpl-1 mutants and asna-1 mutants have high ER stress levels
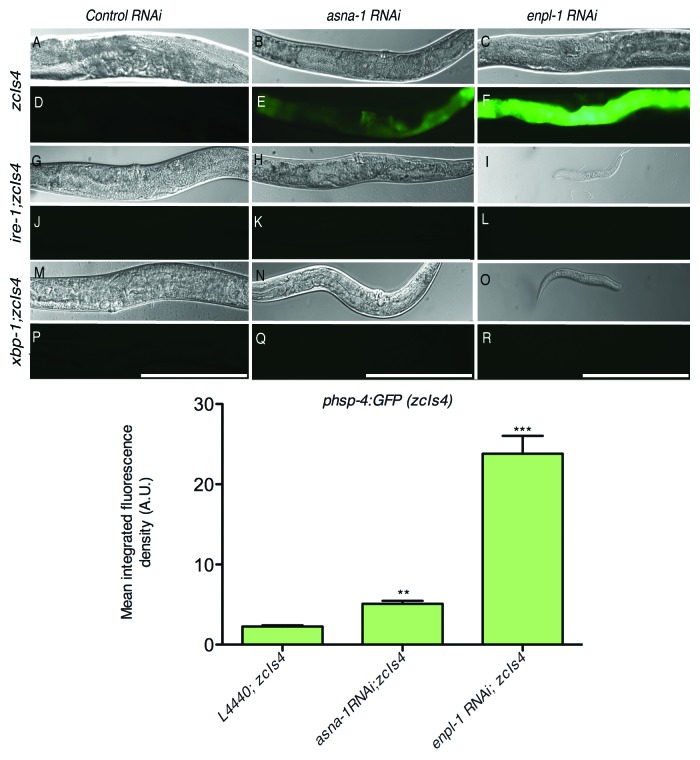
enpl-1 adults have high levels of ER stress as reported on by hsp-4:gfp induction and are extremely sensitive to cisplatin, while wild-type adults which are resistant to cisplatin are not stressed. asna-1 mutants have been previously identified as having an adult cisplatin hypersensitivity phenotype. If the adult cisplatin sensitivity phenotype is associated with elevated ER stress, then asna-1 mutant worms would also be expected to have elevated ER stress. The expression level of hsp-4:GFP is used as a criterion to estimate ER stress levels in worms.24 To examine UPR activity in asna-1 mutants and compare it to that in enpl-1 mutants, RNAi was used to knock down enpl-1 and asna-1 activity in worms carrying a hsp-4:GFP transgene and GFP levels were assessed using fluorescence microscopy. It was clear that GFP levels were much higher in enpl-1(RNAi) animals as compared with asna-1(RNAi) animals (Fig. 3A–F). The quantification of GFP intensity is presented in Figure 3 bottom panel.
Figure 3. Loss of enpl-1 or asna-1 by RNAi induces unfolded protein response. HSP-4:GFP levels were estimated in control, asna-1 or enpl-1 RNAi treated zcIs4, ire-1; zcIs4 or xbp-1; zcIs4 worms. Top panel shows (A)–(F)enpl-1 RNAi or asna-1 RNAi in C. elegans induces phsp-4::GFP and is regulated by ire-1/xbp-1 pathway. (A)–(C) Light micrographs of the worms carrying the phsp-4::GFP(zcIs4) transgene subjected to control RNAi (A), asna-1 RNAi (B) and enpl-1 RNAi (C) (n = 25 for each treatment). (D)–(F) Fluorescence micrographs of the corresponding DIC micrographs of (A)–(C). (G)–(I) Light micrographs of ire-1(zc14) mutants carrying the phsp-4::GFP(zcIs4) transgene subjected t o control RNAi (G), asna-1 RNAi (H) and enpl-1 RNAi (I) (n = 25 for each treatment). (J)–(L) Fluorescence micrographs of the corresponding DIC micrographs of (G)–(I). (M)–(O) Light micrographs of xbp-1 (zc12) mutants carrying the phsp-4::GFP(zcIs4) transgene subjected to control RNAi (M), asna-1 RNAi (N) and enpl-1 RNAi (O) RNAi (n = 25). (P)–(R) Fluorescence micrographs of the corresponding DIC micrographs of (M)–(O). The scale bar is 500 μm. Bottom panel shows quantification of integrated GFP fluorescence density of zcIs4 worms with indicated RNAi treatments. A.U. stands for arbitrary units. ** denotes p < 0.01, *** denotes p < 0.001
We also tested whether depletion of enpl-1 and asna-1 by RNAi could induce heat shock response, using the transgenic strain that expresses GFP driven by hsp16–2 promoter from the dvIs70 transgene.25 RNAi against neither enpl-1 nor asna-1 (n = 50 for each strain) resulted in GFP induction (Fig. S2). The results indicated that the stress response induced by the depletion of either of the genes is ER specific unfolded protein response and not a heat shock response. The possibility of cisplatin interacting with mitochondrial proteins to induce mitochondrial dysfunction was ruled out as the mitochondrial dysfunction reporter phsp-6:GFP was not induced upon exposure to cisplatin (Fig. S4).
Mutants with very high UPR levels can grow and develop normally but certain combinations of double mutants arrest as 2nd larval stage (L2) animals because of the inhibition of regulatory pathways that act in parallel.26,27 enpl-1(RNAi); ire-1(-) worms and enpl-1(RNAi); xbp-1(-) worms arrested as L2 larvae as is the case when double mutants impair two parallel regulatory pathways. This demonstrates that enpl-1 likely acts in parallel to the ire-1/xbp-1 pathway. By contrast, inactivation of asna-1 by RNAi did not produce the strong L2 larval arrest in either ire-1 or xbp-1 mutants (Fig. 3G–R). Taken together, these data show that there is a co-relation between ER stress levels and cisplatin sensitivity in adult worms.
Cisplatin does not induce UPR response in wild-type worms
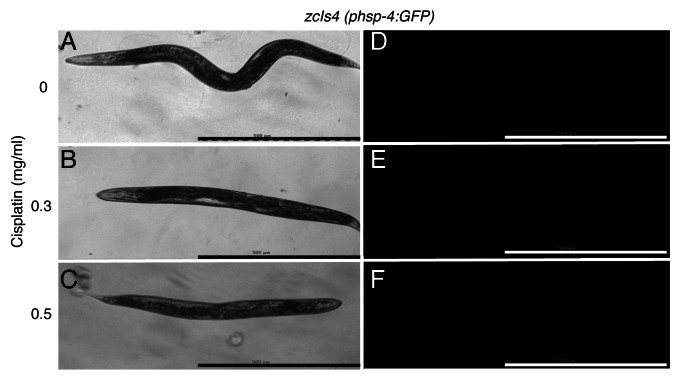
The stressful tumor microenvironment, which includes factors such as hypoxia, acidosis and low nutrient levels, induces low level protective UPR that helps to promote proper ER homeostasis under these conditions and thereby perhaps increase resistance against chemotherapeutics.28 Alternatively, it is possible that in tumors exposed to cisplatin, low-level protective UPR can be induced that could protect against cytotoxicity. Because wild-type worms are intrinsically cisplatin resistant, this phenotype was used to investigate whether the resistance was due to induction of low level UPR by exposure to cisplatin. Wild-type worms bearing a hsp-4:gfp transgene were exposed to cisplatin for 24 h to two doses of cisplatin that killed enpl-1 mutants effectively. Under both conditions no hsp-4:gfp induction was observed (Fig. 4). This indicates that it is likely that that resistance of wild-type animals is due to other mechanisms such as perhaps the action of drug efflux pumps.
Figure 4. Cisplatin does not induce UPR in C. elegans. Effect of various doses of cisplatin on phsp-4:GFP was measured. Light micrographs of worms carrying the phsp-4::GFP(zcIs4) transgene treated with no cisplatin (A), 0.3 mg/ml cisplatin (B) and 0.5 mg/ml cisplatin (C). (D), (E) and (F) are the fluorescence micrographs corresponding to the light micrographs in (A), (B) and (C) respectively. No GFP fluorescence was observed. The scale bar = 500 μm.
Elevated ER stress sensitizes worms to cisplatin
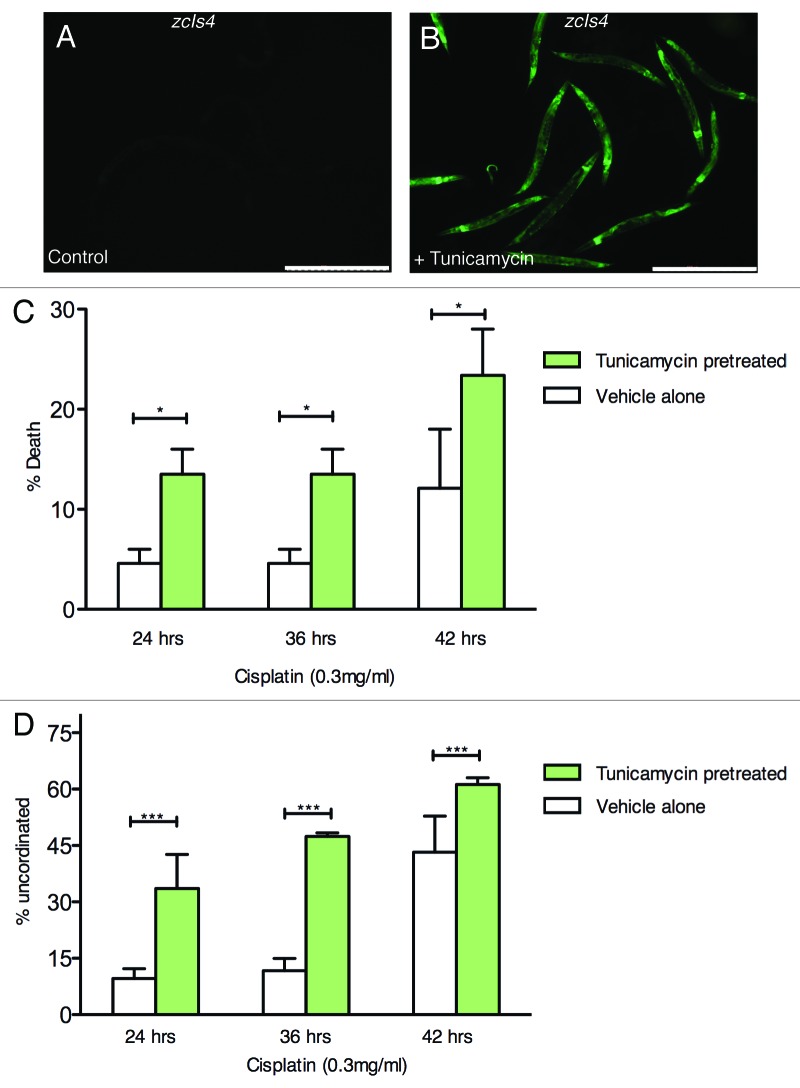
The studies so far demonstrated that there is a strong co-relation between the levels of ER stress and sensitivity to cisplatin. To ask whether increased ER causes cisplatin sensitivity, ER stress was pharmacologically induced in the resistant wild-type animals and the animals were then tested for sensitivity when exposed to cisplatin. Tunicamycin causes ER stress and potently induces UPR in worms.26 To induce high UPR, adult wild-type worms were exposed to tunicamycin at 56 μg/mL for five hours. To ensure that the ER stress level was high, the concentration of tunicamycin used in the experiments was twice that used in a previous study26 in order to induce hsp-4:gfp expression in wild-type worms. (Fig. 5A and B). Tunicamycin treated worms were transferred to cisplatin containing plates for 24, 36 and 42 h and scored for viability and general health as measured by overall appearance and uncoordinated movement. There was a significant reduction in survival of worms at all cisplatin treatment conditions when coupled with tunicamycin pre-treatment compared with cisplatin exposure alone without tunicamycin pre-treatment (Fig. 5C). The tunicamycin/cisplatin combination treatment also made the health status of the animals much worse and effects on general health were detectable at all time-points (Fig. 5D). Tunicamycin treatment alone without subsequent exposure to cisplatin had no effect in viability or health status (n = 100). These findings demonstrate that induction of ER stress can cause the intrinsically resistant wild-type worms to become sensitive to cisplatin. This lends support to the notion that high ER stress leads to cisplatin sensitivity.
Figure 5. Induction of unfolded protein response by tunicamycin increases cisplatin sensitivity in wildtype worms. Worms were pretreated with tunicamycin and then moved to cisplatin containing plates and scored after 24, 36 or 42 h for survival and uncoordinated movement. Fluorescence micrographs of the worms carrying the phsp-4::GFP(zcIs4) transgene subjected for five hours to vehicle only (A) or 56 μg/ml of tunicamycin (B). The scale bar = 500 μm. (C) and (D) N2 worms were exposed to tunicamycin (56 μg/ml) or vehicle only for 5 h with food and then put on cisplatin containing plates (0.3 mg/ml) for 24 h. (C) represents percent death and (D) represents percent uncoordinated phenotype exhibited by the animals Two independent experiments (n = 100 for each time point) were used to obtain the data. Error bars represent the mean ± SEM. The groups were compared using Fisher’s exact test. *** represents p < 0.001, ** represents p < 0.01 and * represents p < 0.05.
In addition to the cisplatin sensitivity, asna-1 mutants have decreased DAF-28/insulin secretion and reduced insulin/IGF signaling (IIS).11 We have previously shown that the cisplatin sensitivity phenotype and the IIS phenotype of asna-1 are genetically separable.15 To address whether cisplatin sensitivity of asna-1 mutants involves the IIS pathway, we induced ER stress by tunicamycin treatment in DAF-28:GFP expressing worms. We find that there is no decrease (n = 25) in DAF-28:GFP secretion in these animals (Fig. S3). Thus, a condition that increases cisplatin sensitivity has no effect on DAF-28:GFP secretion.
Richardson et al. have shown that the IRE-1/XBP-1 pathway acts in parallel with PEK-1 to decrease ER stress in response to exogenous insults, immune challenge and the effects of basal physiology and that xbp-1 mutants have increased ER stress.29 Since XBP-1 activity is depleted in ire-1 mutants these mutants are also expected to have increased ER stress. We find that ire-1(v33) mutants are more sensitive to cisplatin than wild-type animals (Fig. S5). This finding gives further support to our findings that perturbation of ER homeostasis sensitizes worms to cisplatin.
Discussion
Here we demonstrate that loss of ENPL-1/GRP94 sensitizes C. elegans to cisplatin induced death. By comparing wild-type animals, asna-1 mutants and enpl-1 mutants, it is possible to show that there is a positive co-relation between ER stress levels and sensitivity to cisplatin. Severe persistent ER stress has been proposed as an important factor that can sensitize tissues to cisplatin-induced death.30,31 Consistently, we found that the previously identified cisplatin sensitive mutant asna-1 also has elevated ER stress but that it was lower than that seen in enpl-1 mutants. Further, this study shows that resistant wild-type worms became sensitive to cisplatin when UPR was pharmacologically induced. While pretreatment with tunicamycin made wildtype worms sensitive to cisplatin, the worms were not as sensitive to the drug as enpl-1 or asna-1 mutants. This difference might be due to the fact that the wildtype worms were exposed to UPR inducing conditions for five hours while asna-1 and enpl-1 mutants are subjected to it throughout their life.
Due to the availability of hsp-4:gfp as a reporter for UPR, it is possible to obtain an estimate of ER stress in various conditions. With this reporter a correlation could be made between the ER stress levels and cisplatin sensitivity. This finding is in contrast to studies on the effect of cisplatin in mammalian tumor cell lines where UPR is defined as either on or off. Taken together, the four settings for UPR levels (wild-type animals, wild-type pretreated with tunicamycin, asna-1 mutants and enpl-1 mutants) and the associated cisplatin induced death for each setting gives support to the idea that the level of cisplatin sensitivity is reflected in the level of UPR induction.
A caveat of this study is that the different ER stress levels studied here arise due to the use of different genetic mutants or pharmacological treatments. It is possible that cisplatin sensitivity may be caused as a result of other effects on the animal and not only via elevated UPR. However, the effects of tunicamycin are well established to be specific in the pertubation of ER homeostasis and induction of UPR. Work from our lab (B.N, R.G, G.K and P.N; manuscript in preparation) indicates that ENPL-1 and ASNA-1 act in concert to promote larval growth and insulin signaling, and therefore it is likely that the two proteins also act together in modulating the response of worms to cisplatin. It was not possible to test the phenotype of asna-1; enpl-1 double mutants since they appear to die as embryos. Further, enpl-1 is highly expressed in the intestine (www.wormbase.org), which is the organ in which ASNA-1 is required for cisplatin resistance.15 We note that mammalian GRP94 is a chaperone that is specific for secreted and transmembrane proteins and has a role in calcium storage.32 It cannot be ruled out that the effect on cisplatin observed here may be due to the failure of a transmembrane protein to reach the plasma membrane.
The link between high ER stress levels and cisplatin sensitivity is consistent with the work from mammalian cultured tumor cell lines which show that irresolvable ER stress greatly increases the effectiveness of cisplatin and other drugs.28,31,33,34 This concordance between the findings in worms and mammalian cells is important because it further substantiates our conclusions from work on asna-1 mutants that the adult worm, which is post-mitotic for the soma is a useful model to understand the cytoplasmic effects of cisplatin in addition to its effects on DNA damage.
Given the above mentioned parallels between the worm model and the mammalian tumor response, this finding implies that inhibition of the human homolog, GRP94, could re-sensitize cisplatin resistant tumors. It is well established that the ER resident GRP94 is upregulated during ER stress and it has recently been shown that grp94 −/− mouse ES cells have elevated UPR activity.21 However, to the best of our knowledge, there is no evidence in the literature on the mammalian homolog, that GRP94 is a potential target for increasing the effectiveness of cisplatin therapy. The findings presented here indicate that the knockdown of mammalian GRP94 in tumor models will result in increased cisplatin sensitivity and may reverse the cisplatin resistance phenotype of resistant tumor lines. The correlation found here also suggests that in human patients the side-effects of cisplatin such as nephrotoxicity and ototoxicity could be prevented by increasing GRP94 activity or by decreasing ER stress levels and thus remove some of the limits on the use of this essential chemotherapeutic compound.
Materials and Methods
C. elegans strains and maintenance
Worms were maintained under standard conditions35 at 20°C on NGM plates. N2 is the wild type reference for all the strains in the study. The mutants and transgenes used were asna-1(ok938), enpl-1(ok1964), enpl-1(tm3738), zcIs4(phsp-4::GFP), zcIs13(phsp-6:GFP) ire-1(zc14), ire-1(v33), xbp-1(zc12) and dvIs70. All the mutants and transgenic strains are described in Wormbase (www.wormbase.org). The enpl-1(ok1964) mutant was outcrossed eight times to wild-type before use and maintained in trans to the nT1(qIs51) balancer.
Feeding RNAi
A plasmid expressing enpl-1 dsRNA for the RNAi experiments was obtained from the Ahringer lab library36 and the plasmid for asna-1 feeding RNAi experiments has been described.11 Feeding RNAi was performed as described.37
phsp-4::GFP reporter assay
Worms carrying the integrated phsp-4:GFP reporter transgene (zcIs4)24 were treated with feeding RNAi bacteria as described in the feeding RNAi section. Micrographs were taken using in a Leica DMRB microscope and captured with Deltapix DP450 software at 20 × magnification. enpl-1 and asna-1 feeding RNAi were also performed in ire-1(zc14);zcIs4 and xbp-1 (zc12); zcIs4 transgenic strains. For quantification of GFP fluorescence 4th larval stage zcIs4 worms the subjected to various RNAi treatments were mounted on agarose pads in 2mM levamisol and fluorescence images were captured using a Leica DM6000B microscope with 100 msec exposure. Grey scale version of the green channel was extracted using Image J software and integrated GFP fluorescence density was measured between the end of the pharynx and the rectum for n > 20 worms for each treatment.
Chemosensitivity assays
The MYOB agar plates with or without cisplatin, sodium arsenite or zinc chloride were made and the assays performed as described previously.15 In all cases one day old adults were used in the experiments.
Tunicamycin treatment
A 1 mg/ml stock solution of tunicamycin (Sigma, T7765) prepared in dimethyl sulfoxide (DMSO) . This stock was diluted to concentration of 56μg/ml in M9 for all tunicamycin treatments. DMSO alone in M9 served as experimental control. Worms were incubated in a solution containing OP50 bacteria and either tunicamycin, or DMSO only, for five hours using depression slides placed in a humidified chamber. After five hours the worms were moved to plates containing 0.3mg/ml cisplatin and scored after 24 h. In parallel the worms carrying the integrated phsp-4:GFP reporter transgene (zcIs4) were also treated with or without tunicamycin and GFP induction was recorded.
Statistical analysis
Oneway ANOVA with Bonferroni’s multiple comparisons posthoc test was performed when comparing N2, asna-1 (ok938) and enpl-1 (ok1964) worms, which were treated with and without cisplatin, sodium arsenite and zinc chloride (Fig. 1). LC50 values and statistical significance when comparing survival of N2, asna-1 and enpl-1 mutants on cisplatin containing plates (Fig. 2A and B) were determined using a binary logistic regression model. Fisher’s exact test was performed when comparing N2 worms, which were treated with and without tunicamycin and analyzed for viability on cisplatin containing plates (Fig. 5C and D) and to compare cisplatin treated N2 and enpl-1 (tm3738) worms (Fig. S1A and B).
Acknowledgments
This work was supported by the Swedish Cancer Society (CAN2007'901), the Swedish Research Council (K2008–68X-20803–01–3), the Cancer Research Foundation in Northern Sweden and Västerbottens County Council (ALF-means). Some nematode strains used in this work were provided by National Bioresource Project, Japan and the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/worm/article/24059
References
- 1.Hambley TW. Chemistry. Metal-based therapeutics. Science. 2007;318:1392–3. doi: 10.1126/science.1150504. [DOI] [PubMed] [Google Scholar]
- 2.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 3.Yu F, Megyesi J, Price PM. Cytoplasmic initiation of cisplatin cytotoxicity. Am J Physiol Renal Physiol. 2008;295:F44–52. doi: 10.1152/ajprenal.00593.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–6. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- 5.Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–18. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–67. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collis SJ, Barber LJ, Ward JD, Martin JS, Boulton SJ. C. elegans FANCD2 responds to replication stress and functions in interstrand cross-link repair. DNA Repair (Amst) 2006;5:1398–406. doi: 10.1016/j.dnarep.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.van Haaften G, Romeijn R, Pothof J, Koole W, Mullenders LH, Pastink A, et al. Identification of conserved pathways of DNA-damage response and radiation protection by genome-wide RNAi. Curr Biol. 2006;16:1344–50. doi: 10.1016/j.cub.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 9.Muzzini DM, Plevani P, Boulton SJ, Cassata G, Marini F. Caenorhabditis elegans POLQ-1 and HEL-308 function in two distinct DNA interstrand cross-link repair pathways. DNA Repair (Amst) 2008;7:941–50. doi: 10.1016/j.dnarep.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Dittrich CM, Kratz K, Sendoel A, Gruenbaum Y, Jiricny J, Hengartner MO. LEM-3 - A LEM domain containing nuclease involved in the DNA damage response in C. elegans. PLoS One. 2012;7:e24555. doi: 10.1371/journal.pone.0024555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kao G, Nordenson C, Still M, Rönnlund A, Tuck S, Naredi P. ASNA-1 positively regulates insulin secretion in C. elegans and mammalian cells. Cell. 2007;128:577–87. doi: 10.1016/j.cell.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Naredi P, Heath DD, Enns RE, Howell SB. Cross-resistance between cisplatin, antimony potassium tartrate, and arsenite in human tumor cells. J Clin Invest. 1995;95:1193–8. doi: 10.1172/JCI117768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmingsson O, Nöjd M, Kao G, Naredi P. Increased sensitivity to platinating agents and arsenite in human ovarian cancer by downregulation of ASNA1. Oncol Rep. 2009;22:869–75. doi: 10.3892/or_00000511. [DOI] [PubMed] [Google Scholar]
- 14.Hemmingsson O, Zhang Y, Still M, Naredi P. ASNA1, an ATPase targeting tail-anchored proteins, regulates melanoma cell growth and sensitivity to cisplatin and arsenite. Cancer Chemother Pharmacol. 2009;63:491–9. doi: 10.1007/s00280-008-0762-2. [DOI] [PubMed] [Google Scholar]
- 15.Hemmingsson O, Kao G, Still M, Naredi P. ASNA-1 activity modulates sensitivity to cisplatin. Cancer Res. 2010;70:10321–8. doi: 10.1158/0008-5472.CAN-10-1548. [DOI] [PubMed] [Google Scholar]
- 16.Pyrko P, Schönthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–16. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 17.Virrey JJ, Dong D, Stiles C, Patterson JB, Pen L, Ni M, et al. Stress chaperone GRP78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res. 2008;6:1268–75. doi: 10.1158/1541-7786.MCR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 19.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–10. doi: 10.1016/S0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Li Z. Roles of heat shock protein gp96 in the ER quality control: redundant or unique function? Mol Cells. 2005;20:173–82. [PubMed] [Google Scholar]
- 21.Mao C, Wang M, Luo B, Wey S, Dong D, Wesselschmidt R, et al. Targeted mutation of the mouse Grp94 gene disrupts development and perturbs endoplasmic reticulum stress signaling. PLoS One. 2010;5:e10852. doi: 10.1371/journal.pone.0010852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapulkin WJ, Hiester BG, Link CD. Compensatory regulation among ER chaperones in C. elegans. FEBS Lett. 2005;579:3063–8. doi: 10.1016/j.febslet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 23.Billing O, Natarajan B, Mohammed A, Naredi P, Kao G. A directed RNAi screen based on larval growth arrest reveals new modifiers of C. elegans insulin signaling. PLoS One. 2012;7:e34507. doi: 10.1371/journal.pone.0034507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 25.Link CD, Cypser JR, Johnson CJ, Johnson TE. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones. 1999;4:235–42. doi: 10.1379/1466-1268(1999)004<0235:DOOSRI>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/S0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 27.Shen X, Ellis RE, Sakaki K, Kaufman RJ. Genetic interactions due to constitutive and inducible gene regulation mediated by the unfolded protein response in C. elegans. PLoS Genet. 2005;1:e37. doi: 10.1371/journal.pgen.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci. 2007;99:346–53. doi: 10.1093/toxsci/kfm152. [DOI] [PubMed] [Google Scholar]
- 29.Richardson CE, Kinkel S, Kim DH. Physiological IRE-1-XBP-1 and PEK-1 signaling in Caenorhabditis elegans larval development and immunity. PLoS Genet. 2011;7:e1002391. doi: 10.1371/journal.pgen.1002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, et al. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer Res. 2008;68:9323–30. doi: 10.1158/0008-5472.CAN-08-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Raedt T, Walton Z, Yecies JL, Li D, Chen Y, Malone CF, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20:400–13. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eletto D, Dersh D, Argon Y. GRP94 in ER quality control and stress responses. Semin Cell Dev Biol. 2010;21:479–85. doi: 10.1016/j.semcdb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K, Jr., Huang P, et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65:11658–66. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- 34.Rabik CA, Fishel ML, Holleran JL, Kasza K, Kelley MR, Egorin MJ, et al. Enhancement of cisplatin [cis-diammine dichloroplatinum (II)] cytotoxicity by O6-benzylguanine involves endoplasmic reticulum stress. J Pharmacol Exp Ther. 2008;327:442–52. doi: 10.1124/jpet.108.141291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–30. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- 37.Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2:H0002–, h0002, 10. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.