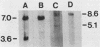Abstract
Avian leukosis virus-induced erythroblastosis results from the specific interruption of the host oncogene, c-erbB, by the insertion of an intact provirus. This insertion results in the expression of two size classes (3.6 and 7.0 kilobases [kb]) of truncated c-erbB transcripts which are initiated in the 5' long terminal repeat of the integrated provirus. Through sequence analysis of erbB cDNA clones we have previously shown that the 3.6-kb activated erbB mRNA contains portions of viral gag and env genes fused to c-erbB sequences (T.W. Nilsen, P.A. Maroney, R.G. Goodwin, F.M. Rottman, L.B. Crittenden, M.A. Raines, and H.-J. Kung, Cell 41:719-726, 1985). In this report we show that the 7-kb mRNA differs from the shorter activated c-erbB mRNA in the length of its 3' untranslated sequence such that the longer mRNA has an extremely long (4.3 kb) 3' untranslated sequence. Additionally, we demonstrate that activated c-erbB mRNA precursors can be processed by alternative splicing to yield mRNAs with viral gag sequences fused directly to c-erbB sequences. Finally, blot hybridization evidence suggests that the two size classes of activated c-erbB mRNA in erythroblastic tissue represent truncated versions of the two c-erbB mRNAs present in normal tissue.
Full text
PDF

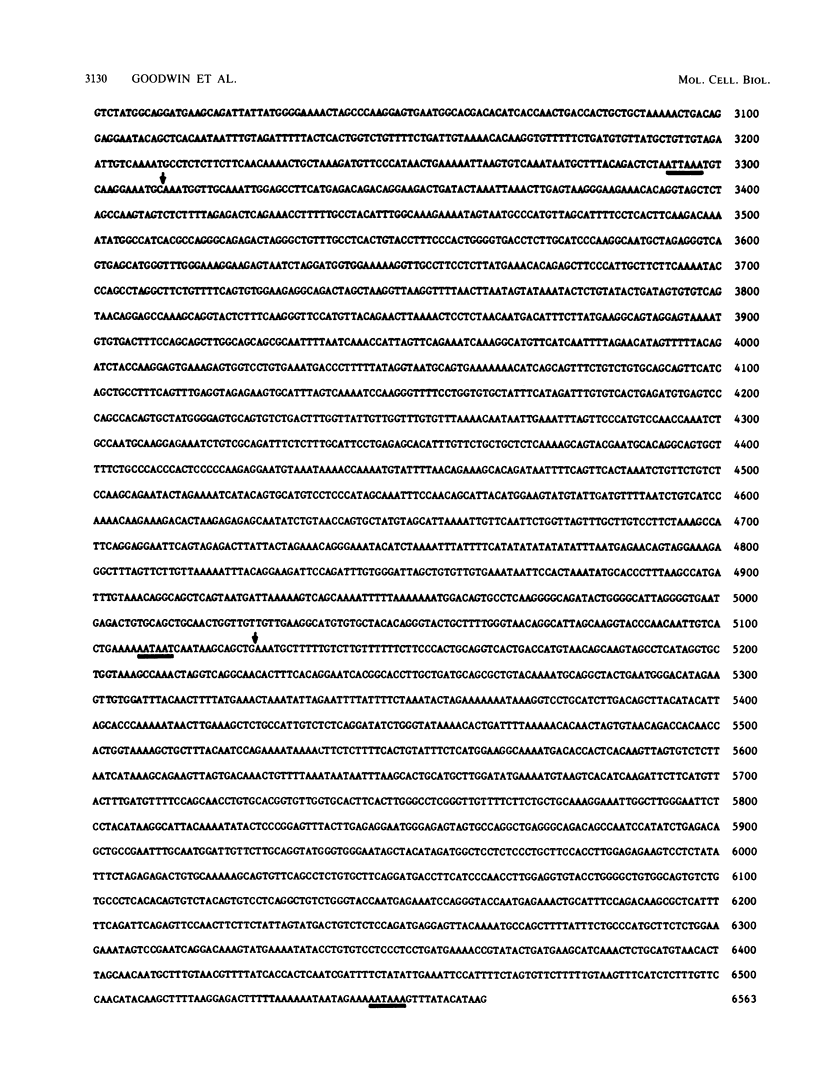
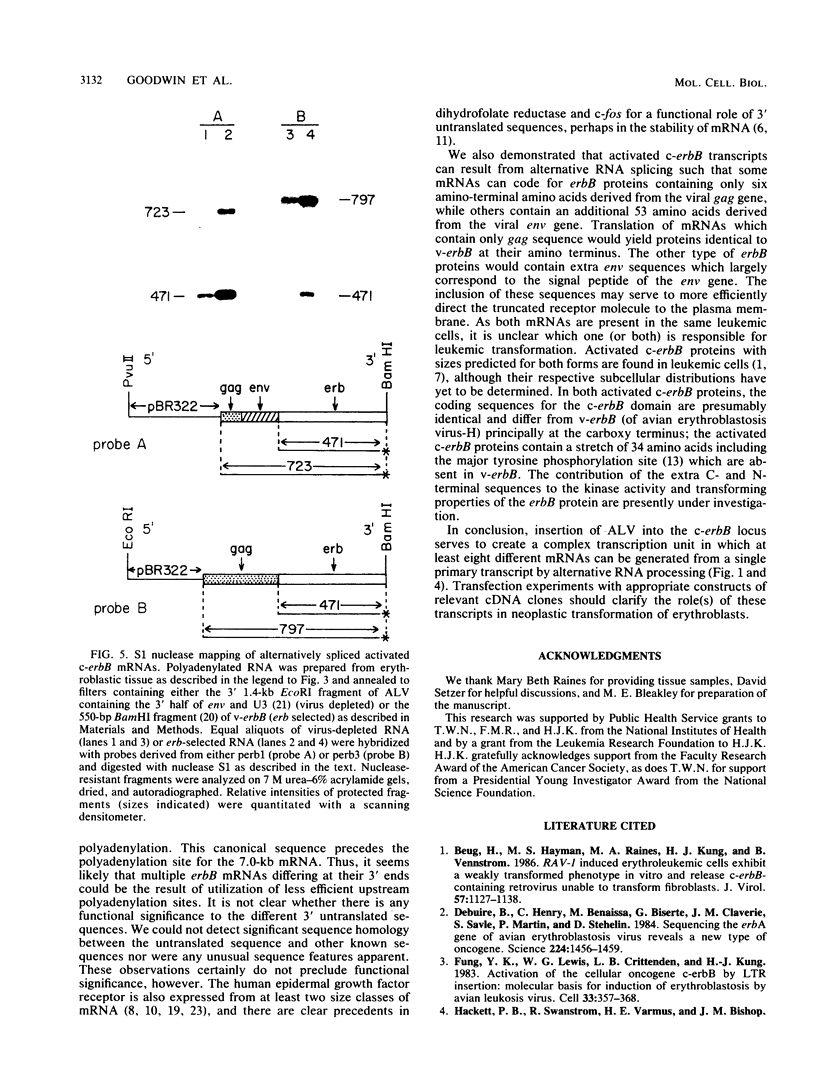

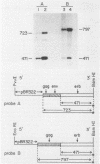
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Hayman M. J., Raines M. B., Kung H. J., Vennström B. Rous-associated virus 1-induced erythroleukemic cells exhibit a weakly transformed phenotype in vitro and release c-erbB-containing retroviruses unable to transform fibroblasts. J Virol. 1986 Mar;57(3):1127–1138. doi: 10.1128/jvi.57.3.1127-1138.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debuire B., Henry C., Bernissa M., Biserte G., Claverie J. M., Saule S., Martin P., Stehelin D. Sequencing the erbA gene of avian erythroblastosis virus reveals a new type of oncogene. Science. 1984 Jun 29;224(4656):1456–1459. doi: 10.1126/science.6328658. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Hunter T. The epidermal growth factor receptor gene and its product. Nature. 1984 Oct 4;311(5985):414–416. doi: 10.1038/311414a0. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Growth-dependent expression of dihydrofolate reductase mRNA from modular cDNA genes. Mol Cell Biol. 1983 Sep;3(9):1598–1608. doi: 10.1128/mcb.3.9.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I., Kris R., Sasson I., Ullrich A., Hayman M. J., Beug H., Schlessinger J. Activation of c-erbB in avian leukosis virus-induced erythroblastosis leads to the expression of a truncated EGF receptor kinase. EMBO J. 1985 Dec 1;4(12):3179–3182. doi: 10.1002/j.1460-2075.1985.tb04062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Kruiger W., Stolarsky L. S., Weber W., Evans R. M., Verma I. M., Gill G. N., Rosenfeld M. G. Expression cloning of human EGF receptor complementary DNA: gene amplification and three related messenger RNA products in A431 cells. Science. 1984 May 25;224(4651):843–848. doi: 10.1126/science.6326261. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Xu Y. H., Ishii S., Clark A. J., Semba K., Toyoshima K., Yamamoto T., Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984 Apr 27;224(4647):417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A. Translational efficiency of cMyc mRNA in Burkitt lymphoma cells. Mol Cell Biol. 1984 Oct;4(10):2235–2238. doi: 10.1128/mcb.4.10.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Raines M. A., Lewis W. G., Crittenden L. B., Kung H. J. c-erbB activation in avian leukosis virus-induced erythroblastosis: clustered integration sites and the arrangement of provirus in the c-erbB alleles. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2287–2291. doi: 10.1073/pnas.82.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Xu Y. H., Ishii S., Clark A. J., Sullivan M., Wilson R. K., Ma D. P., Roe B. A., Merlino G. T., Pastan I. Human epidermal growth factor receptor cDNA is homologous to a variety of RNAs overproduced in A431 carcinoma cells. 1984 Jun 28-Jul 4Nature. 309(5971):806–810. doi: 10.1038/309806a0. [DOI] [PubMed] [Google Scholar]