Abstract
Background:
With this study, we sought to characterise the impact of pro-inflammatory cytokines on the outcomes of gemcitabine monotherapy (GEM) in patients with pancreatic cancer (PC).
Methods:
Treatment-naive patients with advanced PC and no obvious infections were eligible for enrolment. All of the patients were scheduled to undergo systemic chemotherapy. Serum pro-inflammatory cytokines were measured using an electro-chemiluminescence assay method before chemotherapy. High cytokine levels were defined as values greater than the median. Clinical data were collected prospectively.
Results:
Sixty patients who received GEM were included in the analysis. High IL-6 and IL-1β levels were poor prognostic factors for overall survival in a multivariate analysis (P=0.011 and P=0.048, respectively). Patients with both a high IL-6 level and a high IL-1β level exhibited shortened overall and progression-free survival, a reduction in the tumour control rate, and a high dose intensity of GEM compared with patients with low levels of both IL-6 and IL-1β.
Conclusion:
The serum levels of IL-6 and IL-1β predict the efficacy of GEM in patients with advanced PC.
Keywords: pancreatic cancer, gemcitabine, interleukin-1β, interleukin-6, chemotherapy
An increase in inflammatory markers is associated with poor prognosis in patients receiving systemic chemotherapy for advanced pancreatic cancer (PC) (Tanaka et al, 2008; Morizane et al, 2011). C-reactive protein (CRP) is an index of systemic inflammation that is synthesised in hepatocytes by pro-inflammatory cytokines, including IL-1β (Young et al, 2008), IL-6 (Morrone et al, 1988), IL-8 (Wigmore et al, 1997), and TNF-α (Ganapathi et al, 1998), via the transcription factor nuclear factor-κB (NF-κB) and the activation of the signal transducer and activator of transcription 3 (STAT3) protein (Nishikawa et al, 2008). NF-κB and STAT3 represent major inflammatory pathways for pro-inflammatory cytokines and contribute to the chemoresistance of tumours (Aggarwal et al, 2009). An increase in the effects of pro-inflammatory cytokines is believed to attenuate the benefits of chemotherapy and to result in a poor outcome. Recently, the efficacy of anti-inflammatory therapy has been reported in several diseases: with canakinumab as an IL-1β blocker in the cryopyrin-associated periodic syndrome (Kuemmerle-Deschner et al, 2011), with tocilizumab as an IL-6 receptor blocker in rheumatoid arthritis (Jones et al, 2010), and with siltuximab as an IL-6 blocker in prostate cancer (Dorff et al, 2010). In the blockade of intracellular pathways, ruxolitinib is a Janus kinase inhibitor that suppresses STAT3 phosphorylation and has shown clinical benefits in myelofibrosis (Harrison et al, 2012). The potential for individual pro-inflammatory cytokines to decrease chemotherapeutic efficacy suggests that it may be a candidate for testing anti-inflammatory therapy in advanced PC patients. This study sought to characterise the impact of pro-inflammatory cytokines on the outcomes of systemic chemotherapy in patients with advanced PC.
Materials and methods
Patients
Treatment-naive patients with advanced PC and no obvious infections were eligible for enrolment in this study. Pathological confirmation was obtained from all the patients via either a fine-needle aspiration biopsy or a cytological examination. All the patients were scheduled to undergo chemotherapy at the National Cancer Center Hospital East. A serum sample was obtained on the morning before chemotherapy and was frozen at −70 °C until analysis. Clinical data were prospectively collected before chemotherapy, at 1 month after chemotherapy, and every 3 months after the start of chemotherapy. The tumour stage was evaluated according to the seventh criteria of the International Union Against Cancer (UICC) (Sobin et al, 2009). This study was approved by the National Cancer Center Ethics Committee, and patients who provided written informed consent were examined.
Systemic chemotherapy
Gemcitabine monotherapy (GEM) and GEM-based regimens were conducted according to previous reports (Ioka et al, 2011; Kindler et al, 2011). Most of the patients were scheduled to receive GEM as follows: a dose of 1000 mg m−2 gemcitabine was administered intravenously for 30 min on days 1, 8, and 15 of a 28-day cycle until the occurrence of disease progression, unacceptable toxicity, or patient refusal. The dose intensity of GEM was calculated during the treatment interval between the date of the first administration and the date of the last administration. The planned dose intensity of GEM for a 28-day cycle was 750 mg m−2 per week.
Assessment of the anti-tumour effect
The anti-tumour effect of the systemic chemotherapy was evaluated using contrast computed tomography/magnetic resonance imaging images obtained every 4–8 weeks after treatment. The tumour response was determined as a complete response (CR), partial response (PR), stable disease (SD), progressive disease, or not evaluated according to the Response Evaluation Criteria in Solid Tumors (Therasse et al, 2000). The best overall response for each patient was recorded as the tumour response. The response rate was calculated as CR+PR/all evaluated patients. Disease control was defined as CR, PR, or SD. The disease control rate was calculated as CR+PR+SD/all evaluated patients.
Pro-inflammatory cytokine assays
The serum levels of cytokines were measured using multiplex assays manufactured by Meso Scale Discovery (Gaithersburg, MD, USA). On the bottom of each well of 96-well plate-based assays, antibodies for GM-CSF, IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p40 (IL-12), and TNF-α were spotted by the manufacturer. Following the capture of the cytokines by the spotted antibodies, label detection antibodies were bound to the antigen. The detection antibodies were coupled to electrochemiluminescent labels that emitted light when electrochemically stimulated via carbon-coated electrodes located in the bottom of the array wells. The resulting signal was read using a charge-coupled device. The MSD Multi-Spot Array assay was performed according to the manufacturer's instructions. The raw data were computed as the levels of electrochemiluminescent signals (light) measured using photodetectors and were analysed using Discovery Workbench 3.0 software (Meso Scale Discovery). A four-parameter logistic fit curve was generated for each analyte using the standards and the calculated concentration of each sample.
Statistical analyses
Progression-free survival (PFS) was defined as the time between the start of chemotherapy and either documented disease progression or death. Overall survival (OS) was defined as the interval between the initial administration of chemotherapy and either death or the last follow-up examination. Survival differences in the univariate analyses were calculated using the Cox's proportional hazards regression model. Factors that were strongly associated with a short survival period (P<0.01) were evaluated using a multivariate analysis of the Cox's proportional hazards regression model. Survival curves were drawn using the Kaplan–Meier method, and the difference between two survival curves was evaluated using the log-rank test. The frequency of patients in the two groups was compared using the Fisher's exact test. A comparison of non-categorical data was performed using the Mann–Whitney U test. The significance level was set at P<0.05. All the analyses were performed using the JMP 8 software, Windows version (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Between 2008 and 2009, 110 patients were enrolled in the study. Six patients were excluded from the study analysis because of the presence of inflammation at the start of chemotherapy, as follows: cholecystitis in three patients, cholangitis in two patients, and thrombophlebitis in both lower extremities in one patient. Four patients with rapid systemic weakness because of tumour progression refused to participate in the data collection after registering in the study. One patient with massive ascites who required multiple large-volume paracentesis procedures was judged unable to undergo systemic chemotherapy and was not evaluated in this study. Sixteen patients receiving S-1 monotherapy and 23 patients receiving GEM doublets were excluded because our focus was on the relationship between cytokine levels and the efficacy of GEM. The GEM doublets regimens consisted of GEM plus S-1 in 12 patients, GEM plus a cancer vaccine in 6 patients, and GEM plus axitinib in 5 patients. The remaining 60 patients were treated with GEM alone and were analysed in this study. The starting dose of GEM was 1000 mg m−2 in all the 60 patients. Patient characteristics and the clinical data obtained before chemotherapy are summarised in Table 1.
Table 1. Patient characteristics.
| Variables | N (%) | |
|---|---|---|
| Patients |
|
60 (100) |
| Age (years) |
Median (range) |
66 (35–85) |
| Sex |
Female |
32 (53) |
| ECOG PS |
0 |
32 (53) |
| |
1 |
26 (43) |
| |
2 or 3 |
2 (4) |
| Biliary drainage |
Present |
13 (22) |
| Opioid |
Present |
19 (32) |
| UICC-Stage |
III |
22 (37) |
| |
IV |
38 (63) |
| Liver metastasis |
Present |
29 (48) |
| Ascites |
Present |
21 (35) |
| Primary site |
Head |
19 (32) |
| Size of primary tumour (cm) |
Median (range) |
3.8 (1.8–9.7) |
| Second-line therapy |
Chemotherapy |
21 (35) |
| |
Surgery |
1 (2) |
| |
Best supportive care |
38 (63) |
| C-reactive protein (mg dl−1dl) |
Median (range) |
0.36 (0.01–25.0) |
| GM-CSF (pg ml−1) |
Median (range) |
0.00 (0.00–289) |
| IFN-γ (pg ml−1) |
Median (range) |
0.00 (0.00–16.1) |
| IL-1β (pg ml−1) |
Median (range) |
0.00 (0.00–1.65) |
| IL-2 (pg ml−1) |
Median (range) |
0.00 (0.00–26.7) |
| IL-6 (pg ml−1) |
Median (range) |
1.93 (0.00–34.3) |
| IL-8 (pg ml−1) |
Median (range) |
19.6 (2.31–206) |
| IL-10 (pg ml−1) |
Median (range) |
1.81 (0.00–383) |
| IL-12 (pg ml−1) |
Median (range) |
0.00 (0.00–1700) |
| TNF-α (pg ml−1) | Median (range) | 7.69 (0.00–23.0) |
Abbreviations: CI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group Performance Status; GM-CSF=granulocyte macrophage colony-stimulating factor; HR=hazard ratio; IFN=interferon; IL=interleukin; TNF=tumour necrosis factor; UICC-Stage=stage based on the seventh criteria of the International Union Against Cancer (UICC).
Pro-inflammatory cytokine levels
Each cytokine was studied in the following numbers of patients: GM-CSF (n=58), IFN-γ (n=60), IL-1β (n=60), IL-2 (n=60), IL-6 (n=60), IL-8 (n=60), IL-10 (n=60), IL-12 (n=59), and TNF-α (n=60) (Supplementary Table S1). The number of patients from whom samples were assayed was dependent on the accuracy of the measurement using the diluted sample. The following rates of detectable concentrations were observed: GM-CSF (33.5%), IFN-γ (20.0%), IL-1β (33.4%), IL-2 (20.0%), IL-6 (96.7%), IL-8 (100%), IL-10 (88.3%), IL-12 (37.3%), and TNF-α (98.3%). Undetectable concentrations of any cytokine were recorded as zero. According to the median value of each cytokine in all patients (Table 1), patients with higher concentrations than the median value were defined as the high cytokine group.
Tumour response and survival in patients with GEM alone
The tumour response was evaluated in all the 60 patients. None of the patients (0%) achieved a CR, and two patients (3.3%) had a PR. Twenty-nine patients (48.3%) were characterised as having SD, and one patient was categorised as not evaluated. The disease control rate was 51.6%. One patient was able to receive a pancreaticoduodenectomy after tumour reduction because of a good chemotherapeutic effect. The radiological and symptomatic progression of PC were observed in 48 (80.0%) and 11 patients (18.4%), respectively. Twenty-one patients (35.0%) received second-line chemotherapy for advanced PC: S-1 (n=18) and S-1+oxaliplatin (n=2). Fifty-four patients died from PC before the end of the observation period (August 2011). The median times for OS and PFS were 228 days (95% confidence interval (CI), 138–299 days) and 91 days (95% CI, 49–102 days), respectively.
Univariate and multivariate analyses for OS and PFS using serum levels of cytokines
The univariate and multivariate analysis for OS identified high IL-1β (HR 1.88; P=0.048) and high IL-6 (HR 2.10, P=0.011) levels as independent predictors of a poor OS (Table 2). In the univariate and multivariate analysis for PFS, a high IL-6 level was an independent risk factor for a short PFS (HR 2.32, P=0.003), and a high IL-1β level tended to be an independent risk factor for a poor PFS (HR 1.81, P=0.056).
Table 2. Univariate and multivariate analyses for overall survival and progression-free survival according to cytokine level in patients receiving gemcitabine monotherapy for advanced pancreatic cancer.
| |
|
|
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|
| Tested factor | N | HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
Overall survival | ||||||
| GM-CSF | High | 20 | 1.84 (1.02–3.21) | 0.042 | ||
| IFN-γ | High | 12 | 1.16 (0.53–2.29) | 0.686 | ||
| IL-1β | High | 20 | 2.33 (1.27–4.18) | 0.007 | 1.88 (1.01–3.45) | 0.048 |
| IL-2 | High | 12 | 2.09 (1.01–4.00) | 0.048 | ||
| IL-6 | High | 30 | 2.41 (1.39–4.20) | 0.002 | 2.10 (1.19–3.74) | 0.011 |
| IL-8 | High | 29 | 1.49 (0.87–2.57) | 0.149 | ||
| IL-10 | High | 30 | 1.22 (0.71–2.11) | 0.465 | ||
| IL-12 | High | 22 | 2.06 (1.12–3.72) | 0.020 | ||
| TNF-α |
High |
30 |
0.98 (0.57–1.68) |
0.939 |
|
|
|
Progression-free survival | ||||||
| GM-CSF | High | 20 | 1.61 (0.91–2.76) | 0.098 | ||
| IFN-γ | High | 12 | 1.27 (0.64–2.33) | 0.481 | ||
| IL-1β | High | 20 | 2.33 (1.30–4.08) | 0.005 | 1.81 (0.98–3.27) | 0.056 |
| IL-2 | High | 12 | 2.08 (1.02–3.97) | 0.043 | ||
| IL-6 | High | 30 | 2.67 (1.56–4.56) | <0.001 | 2.32 (1.33–4.07) | 0.003 |
| IL-8 | High | 29 | 1.27 (0.75–2.14) | 0.362 | ||
| IL-10 | High | 30 | 1.46 (0.87–2.45) | 0.148 | ||
| IL-12 | High | 22 | 2.13 (1.21–3.72) | 0.010 | ||
| TNF-α | High | 30 | 1.15 (0.68–1.93) | 0.595 | ||
Abbreviations: CI=confidence interval; GM-CSF=granulocyte macrophage colony-stimulating factor; HR=hazard ratio; IFN=interferon; IL=interleukin; TNF=tumour necrosis factor.
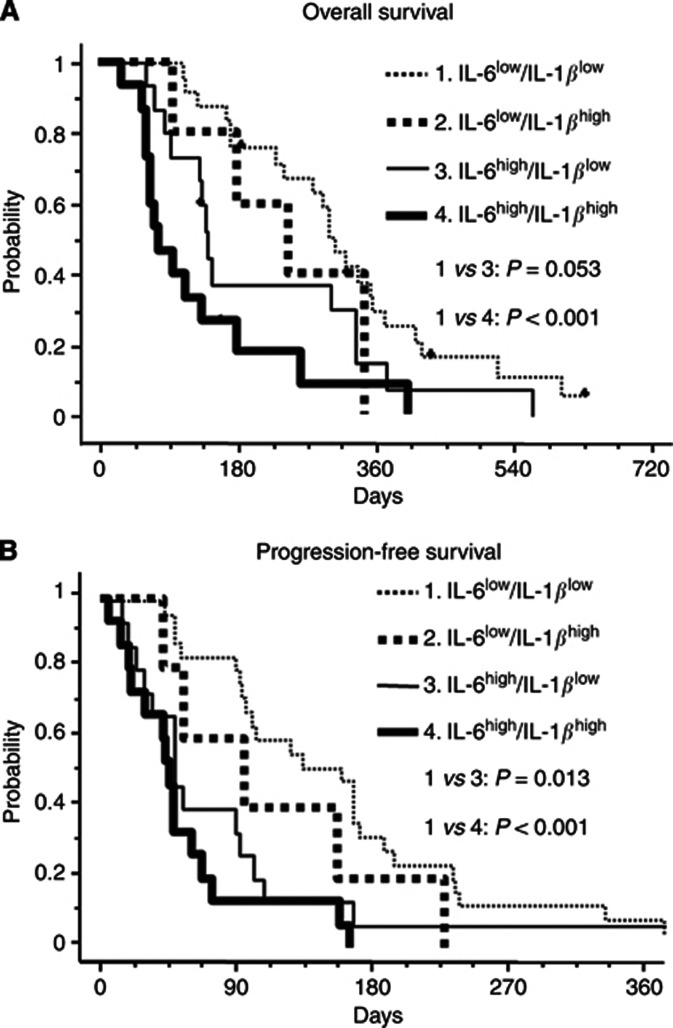
To obtain detailed information regarding the efficacy of chemotherapy and the patient's prognosis according to the IL-6 and IL-1β concentrations, we tested the prognostic values of classifications based on the serum levels of IL-6 and IL-1β using survival curves of OS and PFS as follows: IL-6Low/IL-1βLow (n=21), IL-6Low/IL-1βHigh (n=5), IL-6High/IL-1βLow (n=15), and IL-6High/IL-1βHigh (n=15) (Figure 1). The OS and PFS curves of the IL-6High/IL-1βHigh group revealed higher risks for death and tumour progression than those of the IL-6Low/IL-1βLow group (P<0.001 in OS and P<0.001 in PFS). The difference between the IL-6High/IL-1βLow and the IL-6Low/IL-1βLow groups was obvious for PFS (P=0.013) and tended to be present for OS (P=0.053).
Figure 1.
The OS and PFS curves according to the status of IL-6 and IL-1β.
(A) OS and (B) PFS curves in the IL-6Low/IL-1βLow (dotted line), the IL-6Low/IL-1βHigh (bold dotted line), the IL-6High/IL-1βLow (solid line), and the IL-6High/IL-1βHigh groups (bold line).
Prognosis and disease control classified according to the IL-6 and IL-1 β status in patients with GEM alone
To identify the prognostic values of the IL-6/IL-1β classification, we calculated the risk of death and progression according to the status of the IL-6 and IL-1β levels. The relative risk of death and progression to the IL-6Low/IL-1βLow group was increased in the IL-6High/IL-1βHigh group (HR 4.06; P<0.001, HR 4.26; P<0.001) and in the IL-6High/IL-1βLow group (HR 1.90; P=0.074, HR 2.24; P=0.021) but not in the IL-6Low/IL-1βHigh group (HR 1.48; P=0.497, HR 1.68; P=0.323; Table 3).
Table 3. Impacts of the classification using IL-6 and IL-1β levels on overall survival and progression-free survival in patients with gemicitabine monotherapy for advanced pancreatic cancer.
|
Overall survival | ||||
|---|---|---|---|---|
| IL-6/IL-1β classification | N | Median OS (95%CI) (days) | HR (95% CI) | P-value |
| IL-6Low/IL-1βLow |
25 |
306 (228–355) |
1 |
Ref. |
| IL-6Low/IL-1βHigh |
5 |
246 (97–346) |
1.48 (0.43–3.97) |
0.497 |
| IL-6High/IL-1βLow |
15 |
140 (83–334) |
1.90 (0.94–3.72) |
0.074 |
| IL-6High/IL-1βHigh | 15 | 79 (61–134) | 4.06 (1.96–8.18) | <0.001 |
|
Progression-free survival |
||||
|---|---|---|---|---|
| IL-6/IL-1β classification | N | Median PFS (95%CI) (days) | HR (95% CI) | P-value |
| IL-6Low/IL-1βLow |
25 |
158 (96–187) |
1 |
ref |
| IL-6Low/IL-1βHigh |
5 |
96 (42–229) |
1.68 (0.56–4.11) |
0.323 |
| IL-6High/IL-1βLow |
15 |
48 (23–92) |
2.24 (1.14–4.29) |
0.021 |
| IL-6High/IL-1βHigh | 15 | 46 (19–61) | 4.26 (2.08–8.55) | <0.001 |
Abbreviations: CI=confidence interval; HR=hazard ratio; IL=interleukin; OS=overall survival; PFS=progression-free survival.
Tumour control rates (TCRs) according to the IL-6 and IL-1β classifications were evaluated and are shown in Table 4. The TCRs of the IL-6High/IL-1βHigh and the IL-6High/IL-1βLow groups (20.0% and 40.0%) were lower than that of the IL-6Low/IL-1βLow group (76.0%, P<0.001 and P=0.042). A significant difference in the TCR between the IL-6High/IL-1βHigh group and the IL-6High/IL-1βLow group was not identified, but the actual value of TCR in the IL-6High/IL-1βHigh group was half of that in the IL-6High/IL-1βLow group.
Table 4. Tumour control rates according to serum levels of IL-6 and IL-1β in patients with gemcitabine monotherapy for advanced pancreatic cancer.
| IL-6/IL-1β classification | N | Median (95% CI) (%) | P-value |
|---|---|---|---|
| IL-6Low/IL-1βLow |
25 |
76.0 (56.6–88.5) |
Ref. |
| IL-6Low/IL-1βHigh |
5 |
60.0 (23.1–88.2) |
0.589 |
| IL-6High/IL-1βLow |
15 |
40.0 (19.8–64.3) |
0.042 |
| IL-6High/IL-1βHigh | 15 | 20.0 (7.0–45.2) | <0.001 |
Abbreviations: CI=confidence interval; IL=interleukin.
GEM exposure according to IL-1 β and IL-6 status
The median value of GEM dose intensity within 90 days after the start of chemotherapy (GEM-DI) was 737 mg m−2 per week in patients with GEM alone. GEM-DI was compared among the groups assigned the IL-6/IL-1β classification (Supplementary Table S2). The GEM-DI medians were increased in the IL-6High/IL-1βHigh (814 mg m−2 per week, P=0.003) and the IL-6High/IL-1βLow (781 mg m−2 per week, P=0.012) groups compared with the IL-6Low/IL-1βLow group (698 mg m−2 per week).
CRP levels according to IL-1 β and IL-6 status
IL-6 and IL-1β promote the synthesis of CRP from hepatocyte (Morrone et al, 1988; Young et al, 2008). The serum CRP level is considered to be a good index for the physiological effects of IL-6 and IL-1β. We compared the CRP levels among the groups assigned to the IL-6/IL-1β classifications. The CRP level of the IL-6High/IL-1βHigh group was the highest of the groups with IL-6/IL-1β classifications (Table 5). The IL-6High/IL-1βLow group showed a higher CRP level than the IL-6Low/IL-1βLow group (P=0.001).
Table 5. CRP level according to serum levels of IL-6 and IL-1β in patients with gemcitabine monotherapy for advanced pancreatic cancer.
| IL-6/IL-1β classification | N | Median (95% CI) (mg dl−1) | P-value |
|---|---|---|---|
| IL-6Low/IL-1βLow |
25 |
0.13 (0.06–0.25) |
Ref. |
| IL-6Low/IL-1βHigh |
5 |
0.08 (NA) |
0.140 |
| IL-6High/IL-1βLow |
15 |
1.19 (0.17–2.79) |
0.001 |
| IL-6High/IL-1βHigh | 15 | 5.61 (2.83–10.09) | <0.001 |
Abbreviations: CI=confidence interval; CRP=C-reactive protein; HR=hazard ratio; IL=interleukin; NA=not applicable; OS=overall survival; PFS=progression-free survival.
Discussion
IL-6 is a pleiotropic cytokine with a variety of effects on cells and tissues (Trikha et al, 2003) that is synthesised by many different cell types, including immune cells, fibroblasts, endothelial cells, myocytes, adipocytes, a variety of endocrine cells, and PC cells (Tracey and Cerami, 1993; Van Snick, 1996; Fried et al, 1998; Martignoni et al, 2005). IL-6 mRNA is found in 64% of PC cases, in which the IL-6 mRNA expression ratio in relation to normal pancreatic tissue is strongly upregulated by a median of 62.4-fold (Bellone et al, 2006). The immunohistochemical expression of IL-6 in PC tumours is strong in the cytoplasm of PC cells and weak in inflammatory cells (Martignoni et al, 2005). Furthermore, the serum IL-6 level in patients with PC is higher than in healthy individuals (Okada et al, 1998; Barber et al, 1999; Ebrahimi et al, 2004; Martignoni et al, 2005; Talar-Wojnarowska et al, 2009). A high IL-6 level is correlated with tumour aggressiveness, inflammatory response, and systemic weakness, such as large tumour size, hepatic metastasis, an elevated level of serum CRP, body weight loss, and poorer performance status (Okada et al, 1998; Barber et al, 1999; Ebrahimi et al, 2004; Martignoni et al, 2005; Talar-Wojnarowska et al, 2009). The prognostic impact of the circulating IL-6 level was demonstrated in a study by Ebrahimi et al (2004), in which patients underwent either pancreatic resection or chemotherapy. This study clearly highlights the independent prognostic value of a high IL-6 level on OS in patients receiving GEM for PC. The correlation between high IL-6 levels and a shortened PFS was observed in hepatocellular carcinoma patients receiving sunitinib monotherapy (Zhu et al, 2009) and in diffuse large-cell lymphoma patients receiving chemotherapy (Seymour et al, 1995). To the best of our knowledge, the association between serum IL-6 levels and PFS in patients undergoing systemic chemotherapy for PC has not been previously reported. This study clearly showed the impact of a high IL-6 level on a shortened PFS in patients undergoing GEM for PC.
IL-1β is a pro-inflammatory cytokine that is synthesised by many cell types, including monocytes, tissue macrophages, and PC cells (Bellone et al, 2006; Angst et al, 2008). IL-1β mRNA can be identified in >80% of PC tumour tissues, and the IL-1β mRNA expression ratio in relation to normal pancreatic tissue in resected PC specimens is, on average, strongly upregulated by 28.5-fold (Ebrahimi et al, 2004; Bellone et al, 2006). IL-1β from tumour cells and monocytes contributes to the chemoresistance of PC cells (Arlt et al, 2002; Angst et al, 2008). The serum levels of IL-1β are rarely measured in healthy tissues. In fact, the total daily production of IL-1β was calculated to be approximately 6 ng day−1 in a study using a specific antibody to human IL-1β (Lachmann et al, 2009), whereas in humans injected with an endotoxin, the levels of IL-1β were below the detection limit (<2 pg ml−1) at baseline and were elevated for approximately 2 h, reaching maximal concentrations of 50–60 pg ml−1 (Granowitz et al, 1991). No relationship has been reported between the serum IL-1β level and its clinical significance in PC patients because the serum IL-1β levels are usually below the lower measurable limit of detection (LOD). The LOD for IL-1β was previously found to be 0.3 pg ml−1 using an enzyme-linked immunosorbent assay (Ebrahimi et al, 2004). In this study, the LOD of IL-1β was 0.19 pg ml−1 ml−1, and the detectable rate of serum IL-1β was 33.4%. Our assay for the detection of pro-inflammatory cytokines was based on electrochemiluminescence, which is a superior detection method compared with enzyme-linked immunosorbent assay; hence, our LOD was lower. Recent progress in assay methods has improved the detection of serum IL-1β, enabling the use of the serum IL-1β concentration for predicting the efficacy of chemotherapy and the identification of a patient's prognosis in this study. A high IL-1β serum level was an independent prognostic factor that, in this study, showed a tendency toward an association with a shortened PFS. IL-1β promotes metastasis and angiogenesis because of the upregulation of pro-metastatic genes and molecules, including matrix metalloproteinases and endothelial adhesion molecules, along with vascular endothelial cell growth factor, chemokines, growth factors, and TGFβ (Dinarello, 2010). A high IL-1β level may be related to an aggressive tumour status and may be correlated with a poor prognosis.
The IL-6High/IL-1βHigh group had shortened PFS and OS compared with the IL-6Low/IL-1βLow group. The disease control rate in the IL-6High/IL-1βHigh group was decreased by one-fourth compared with that of the IL-6Low/IL-1βLow group. Interestingly, GEM-DI in the IL-6High/IL-1βHigh was higher than in the IL-6Low/IL-1βLow group. The CRP serum level, a good index of the IL-6 and IL-1β effects via STAT3 and NF-κB, was higher in the IL-6High/IL-1βHigh group. These results may indicate that the resistance of PC tumour cells against GEM was dependent on the effects of IL-6 and IL-1β via STAT3 and NF-κB. GEM leads to DNA damage in PC cells, which results in GEM-induced apoptosis (Arlt et al, 2010). The resistance of PC cells to chemotherapeutic agents is due to an altered balance between pro- and anti-apoptotic proteins, resulting in reduced apoptotic responsiveness (Grivennikov and Karin, 2010). Bcl-2 and Bcl-xL are anti-apoptotic proteins that are activated by STAT3 and NF-κB, whereas Mcl-1, another of the anti-apoptotic proteins, is primarily STAT3-dependent (Arlt et al, 2010). IL-6 and IL-1β can activate STAT3 and NF-κB (Nishikawa et al, 2008), possibly resulting in an increase of anti-apoptotic proteins in PC cells. Based on the above context, the inhibition of STAT3 and NF-κB was expected to resolve the chemoresistance of PC cells.
The IL-6High/IL-1βLow group had poor outcomes for OS and PFS compared with the IL-6Low/IL-1βLow group. The disease control rate in the IL-6High/IL-1βLow group was reduced to half of that in the IL-6Low/IL-1βLow group, though GEM-DI in the IL-6High/IL-1βLow was higher than in the IL-6Low/IL-1βLow group. CRP was able to be synthesised by the effect of IL-6 alone, and the CRP concentration was elevated in the IL-6High/IL-1βLow group compared with the IL-6Low/IL-1βLow group. These results imply that the PC tumour cells were resistant to GEM via IL-6 only. IL-6 binds a nonsignalling α-receptor (IL-6 receptor), and the dimerisation of gp130 (a signalling β-receptor) and the binding of IL-6 to its receptor lead to the activation of receptor-associated kinases within the cell. These lead to the phosphorylation of proximal tyrosine residues within the intracellular portion of gp130 and the subsequent control of STAT1 and STAT3 activity (Jones et al, 2011). Inhibition of the above IL-6 pathway would improve the resistance against GEM in PC tumour cells.
In conclusion, this study demonstrated that the serum levels of IL-6 and IL-1β were predictive of both the efficacy of GEM and the prognosis of patients with advanced PC. Inhibition of the IL-6 and IL-1β pathways may be a candidate target for novel therapies for advanced PC.
Acknowledgments
We thank Ms Kayo Takei, Ms Miho Akimoto, Ms Yuriko Sato, and Ms Youko Yamasaki of the National Cancer Center Hospital East for their secretarial support and Mr Ryuu Kasai of Chugai Pharmaceutical Co., Ltd. for support with the statistical analysis. This work was supported by Grants-in-aid for Cancer Research and for the Third-term Comprehensive 10-year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan; JSPS KAKENHI Grant Number 22790624; the National Cancer Center Research and Development Fund (23-A-2b); and Chugai Pharmacology Co. Ltd.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- Angst E, Reber HA, Hines OJ, Eibl G. Mononuclear cell-derived interleukin-1beta confers chemoresistance in pancreatic cancer cells by upregulation of cyclooxygenase-2. Surgery. 2008;144:57–65. doi: 10.1016/j.surg.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt A, Müerköster SS, Schäfer H.2010Targeting apoptosis pathways in pancreatic cancer Cancer Lettere-pub ahead of print 13 November 2010 [DOI] [PubMed]
- Arlt A, Vorndamm J, Müerköster S, Yu H, Schmidt WE, Fölsch UR, Schäfer H. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res. 2002;62:910–916. [PubMed] [Google Scholar]
- Barber MD, Fearon KC, Ross JA. Relationship of serum levels of interleukin-6, soluble interleukin-6 receptor and tumour necrosis factor receptors to the acute-phase protein response in advanced pancreatic cancer. Clin Sci (Lond) 1999;96:83–87. [PubMed] [Google Scholar]
- Bellone G, Smirne C, Mauri FA, Tonel E, Carbone A, Buffolino A, Dughera L, Robecchi A, Pirisi M, Emanuelli G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother. 2006;55:684–698. doi: 10.1007/s00262-005-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Why not treat human cancer with interleukin-1 blockade. Cancer Metastasis Rev. 2010;29:317–329. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorff TB, Goldman B, Pinski JK, Mack PC, Lara PN, Jr, Van Veldhuizen PJ, Jr, Quinn DI, Vogelzang NJ, Thompson IM, Jr, Hussain MH. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res. 2010;16:3028–3034. doi: 10.1158/1078-0432.CCR-09-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Ganapathi MK, May LT, Schultz D, Brabenec A, Weinstein J, Sehgal PB, Kushner I. Role of interleukin-6 in regulating synthesis of C-reactive protein and serum amyloid A in human hepatoma cell lines. Biochem Biophys Res Commun. 1998;157:271–277. doi: 10.1016/s0006-291x(88)80043-3. [DOI] [PubMed] [Google Scholar]
- Granowitz EV, Santos AA, Poutsiaka DD, Cannon JG, Wilmore DW, Wolff SM, Dinarello CA. Production of interleukin-1-receptor antagonist during experimental endotoxaemia. Lancet. 1991;338:1423–1424. doi: 10.1016/0140-6736(91)92725-h. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin M. Dangerous liaisons: STAT3 and NF-kB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Fukutomi A, Sugimori K, Baba H, Yamao K, Shimamura T, Chen J, Mizumoto K, Furuse J, Funakoshi A, Hatori T, Yamaguchi T, Egawa S, Sato A, Ohashi Y, Cheng A, Okusaka T.2011Randomized phase III study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study J Clin Oncol 29(suppl) abstr 4007 [DOI] [PubMed] [Google Scholar]
- Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, Siri DA, Tomsic M, Alecock E, Woodworth T, Genovese MC. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- Kuemmerle-Deschner JB, Hachulla E, Cartwright R, Hawkins PN, Tran TA, Bader-Meunier B, Hoyer J, Gattorno M, Gul A, Smith J, Leslie KS, Jiménez S, Morell-Dubois S, Davis N, Patel N, Widmer A, Preiss R, Lachmann HJ. Two-year results from an open-label, multicentre, phase III study evaluating the safety and efficacy of canakinumab in patients with cryopyrin-associated periodic syndrome across different severity phenotypes. Ann Rheum Dis. 2011;70:2095–2102. doi: 10.1136/ard.2011.152728. [DOI] [PubMed] [Google Scholar]
- Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, Wittkowski H, Bek S, Hartmann N, Bosset S, Hawkins PN, Jung T. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206:1029–1036. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni ME, Kunze P, Hildebrandt W, Künzli B, Berberat P, Giese T, Klöters O, Hammer J, Büchler MW, Giese NA, Friess H. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin Cancer Res. 2005;11:5802–5808. doi: 10.1158/1078-0432.CCR-05-0185. [DOI] [PubMed] [Google Scholar]
- Morizane C, Okusaka T, Morita S, Tanaka K, Ueno H, Kondo S, Ikeda M, Nakachi K, Mitsunaga S. Construction and validation of a prognostic index for patients with metastatic pancreatic adenocarcinoma. Pancreas. 2011;40:415–421. doi: 10.1097/MPA.0b013e3182021376. [DOI] [PubMed] [Google Scholar]
- Morrone G, Ciliberto G, Oliviero S, Arcone R, Dente L, Content J, Cortese R. Recombinant interleukin 6 regulates the transcriptional activation of a set of human acute phase genes. J Biol Chem. 1988;263:12554–12558. [PubMed] [Google Scholar]
- Nishikawa T, Hagihara K, Serada S, Isobe T, Matsumura A, Song J, Tanaka T, Kawase I, Naka T, Yoshizaki K. Transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1 is essential for cytokine-driven C-reactive protein gene expression. J Immunol. 2008;180:3492–3501. doi: 10.4049/jimmunol.180.5.3492. [DOI] [PubMed] [Google Scholar]
- Okada S, Okusaka T, Ishii H, Kyogoku A, Yoshimori M, Kajimura N, Yamaguchi K, Kakizoe T. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–15. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- Seymour JF, Talpaz M, Cabanillas F, Wetzler M, Kurzrock R. Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol. 1995;13:275–282. doi: 10.1200/JCO.1995.13.3.575. [DOI] [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C.2009TNM Classification of Malignant Tumors7th ednWiley-Blackwell: Oxford, UK [Google Scholar]
- Talar-Wojnarowska R, Gasiorowska A, Smolarz B, Romanowicz-Makowska H, Kulig A, Malecka-Panas E. Clinical significance of interleukin-6 (IL-6) gene polymorphism and IL-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis. Dig Dis Sci. 2009;54:683–689. doi: 10.1007/s10620-008-0390-z. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ikeda M, Okusaka T, Ueno H, Morizane C, Hagihara A, Iwasa S, Kojima Y. Prognostic factors in japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn J Clin Oncol. 2008;38:755–761. doi: 10.1093/jjco/hyn098. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1996;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Wigmore SJ, Fearon KC, Maingay JP, Lai PB, Ross JA. Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes; Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. Am J Physiol. 1997;273:E720–E726. doi: 10.1152/ajpendo.1997.273.4.E720. [DOI] [PubMed] [Google Scholar]
- Young DP, Kushner I, Samols D. Binding of C/EBPbeta to the C-reactive protein (CRP) promoter in Hep3B cells is associated with transcription of CRP mRNA. J Immunol. 2008;181:2420–2427. doi: 10.4049/jimmunol.181.4.2420. [DOI] [PubMed] [Google Scholar]
- Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J, Bhargava P, Meyerhardt J, Clark JW, Kwak EL, Hezel AF, Miksad R, Abrams TA, Enzinger PC, Fuchs CS, Ryan DP, Jain RK. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.