Abstract
Background:
The objective of this study is to provide comprehensive overviews of patient-reported urinary symptoms for long-term prostate cancer survivors treated with radiation therapy and for untreated, healthy men.
Methods:
We performed a population-based cross-sectional study using a study-specific postal questionnaire assessing symptoms among 1007 men consecutively treated at the Sahlgrenska University Hospital, Göteborg, Sweden from 1993–2006 (primary or salvage external beam radiation therapy (EBRT) or EBRT and high-dose rate brachytherapy). We also randomly recruited 350 non-pelvic-irradiated matched control men from the Swedish Total Population Register. Symptom prevalence and prevalence ratios were computed.
Results:
Survey participation rate was 89% (874/985) for eligible survivors and 73% (243/332) for eligible controls. Median time from treatment to follow-up was 5 years (range, 1–14 years). Among the 21 investigated symptoms reflecting obstruction, frequency, urgency, pain and incontinence, we found significantly higher prevalence compared with controls for 9 symptoms in the EBRT group, 10 in the EBRT+brachytherapy group and 5 in the salvage EBRT group. The prevalence for a majority of the symptoms was stable over time.
Conclusion:
The presented toxicity profiles provide a thorough understanding of patient-reported urinary symptoms that can assist in developing personalised therapy for prostate cancer.
Keywords: genitourinary toxicity, radiation therapy, prostate cancer
During the past decades, technical developments in radiation therapy have enabled us to decrease the dose in organs surrounding the tumour that in many situations has reduced severe radiation-induced toxicities. This has opened up for treatment situations where a wider spectrum of toxicities can be addressed, including those that previously have been regarded as ‘less severe'. However, there is a lack of comprehensive toxicity profiles based on survivors' own experience after radiation therapy as well as knowledge about how these relate to corresponding symptoms among healthy individuals (background rates).
For prostate cancer, the most frequently described late toxicities after radiation therapy include gastrointestinal symptoms such as rectal bleeding, urgency and incontinence as well as genitourinary symptoms (Bentzen et al, 2010; Budaus et al, 2012; Mohammed et al, 2012; Schmid et al, 2012). Modern treatment techniques, including image-guided intensity modulated radiation therapy and brachytherapy, are expected to reduce the dose to some critical organs in the pelvis (Budaus et al, 2012). For anatomical reasons, however, the dose to the bladder neck and the (prostatic) urethra is hard to reduce. This is also reflected in intensity-modulated radiation therapy not necessarily resulting in less genitourinary symptoms than 3D conformal radiation therapy (Bekelman et al, 2011). Genitourinary symptoms potentially originating from these organs and their background rates are thus important not only to identify in order to better judge which toxicities are to be addressed in the clinic but also to handle those already present.
In this study, we provide comprehensive overviews of late genitourinary toxicity after prostate cancer treatments with primary or salvage external beam radiation therapy (EBRT) or a combination of EBRT and high-dose rate brachytherapy as reported by long-term survivors. Altogether, we included 874 Swedish men who had been treated up to 14 years earlier. A study-specific questionnaire was used to assess 21 atomised symptoms, that is, symptoms that are broken down to specific entities, which likely reflect the underlying radiation-induced pathophysiology c.f. (Bentzen et al, 2010). For comparison, we used information from 243 randomly selected non-pelvic-irradiated control men matched for age and residency from the Swedish Total Population Register.
Materials and methods
Subjects
The 1007 men identified for this study were consecutively treated for prostate cancer at the Sahlgrenska University Hospital in Göteborg, Sweden from 1993 to 2006. Eligibility criteria were age <80 years, no diagnosed distant metastases, sufficient ability to read and write Swedish and being a Swedish resident at the time of follow-up. Altogether 985 men fulfilled the eligibility criteria and agreed to participate in the study. For comparison, 350 population-based control men were selected from the Swedish Total Population Register, which includes all residents of Sweden. For each three survivors of the same age and area of residence, one control man was randomly selected. Of the initially 350 selected men, 332 had no prostate-cancer diagnosis, an additional inclusion criterion for controls, and agreed to participate in the study. The study was approved by the Regional Ethical Review Board in Göteborg and was conducted in 2008.
Treatment
The men were treated with individually planned three-dimensional conformal EBRT with or without high-dose rate brachytherapy or as salvage therapy after surgical prostatectomy.
EBRT was planned based on computed tomography imaging with the patient in a supine position. The planning target volume was defined as the prostate with a 2 cm margin in all directions except posteriorly where the margin was 1.5 cm or no more than half of the rectal area. For men who had undergone prostatectomy, the planning target volume was defined as the post-operative prostatic region with the same margins. An isocentric three-field technique using one anterior and two lateral-wedged fields was employed for all patients. The photon energy was 11 or 15 MV. Total dose was prescribed at the centre of the planning target volume according to the principles of the ICRU (ICRU, 1993).
Brachytherapy was planned based on transrectal ultrasound imaging with the patient in a lithotomy position. The planning target volume was defined as the prostate with a 2 mm margin in all directions except cranially and caudally. The dose distribution was optimised by determining the number of needles, needle positions and dwell times for the source within each needle with the objective of covering the planning target volume with the prescription dose while keeping the urethral absorbed dose <120%. Typically, 11–15 needles were manually inserted through a perineum template and the treatment was delivered using a high-dose rate 192Ir source.
Questionnaire
The questionnaire was in Swedish and was developed to survey symptoms after prostate cancer radiation therapy and has been described in detail previously (Alsadius et al, 2011). It was constructed according to the well-founded method established at the Divisions of Clinical Cancer Epidemiology at the University of Gothenburg in Göteborg and the Karolinska Institutet in Stockholm, Sweden, (Bergmark et al, 1999; Steineck et al, 2002a, Kreicbergs et al, 2004; Steineck et al, 2006). Briefly, symptoms are identified after in-depth interviews with cancer survivors and operationalised into questions that are verified with individuals of the target population to make sure that they are correctly understood. Person-incidence or person-prevalence scales are used to assess symptom occurrence, for example, the presence of urinary leakage or the number of times with pain when urinating, respectively. We also used a person-intensity scale to measure, for example, the amount of urinary leakage.
Following a pilot-study phase, where the questionnaire is tested for logistics, participation rate and rate of missing values and may undergo additional adjustments, the main study is conducted. This one final questionnaire is then sent out to the study participants by mail at one occasion (in this study: survivors, between February and June, 2008; controls, between September and November, 2008). This study design thus results in a cross-sectional study.
The questionnaire contained detailed questions on physical symptoms potentially originating from the gastrointestinal, genitourinary and bony regions of the pelvis as well as questions on quality of life and sexuality, demographics, additional treatments and co-morbidities. Of the 165 questions, 28 were dedicated to urinary symptoms and, in this study, we report on the 21 relating to physical bother (urinary obstruction, including flow, irritative toxicity, including frequency, urgency and pain as well as urinary incontinence). The excluded seven questions concerned wellbeing and quality of life and will be separately reported.
Statistics
All calculations were performed with SAS 9.2 for Windows (SAS Institute Inc., Cary, NC, USA). Each question was dichotomised according to pre-defined cutoffs judged to be clinically relevant and balanced for background noise (Al-Abany et al, 2006).
Symptom prevalence was calculated as the percentage of individuals reporting the symptom within each group. Differences between groups were assessed with a Chi-square test. Prevalence ratios between groups with corresponding 95% confidence intervals (CIs) were calculated using the FREQ Procedure in SAS; age-adjusted ratios were calculated using a log-binomial regression model (GENMOD Procedure with binomial distribution, log link). Time to follow-up was calculated from the date of completed radiation therapy to the date of the midpoint of questionnaire collection (1 April 2008) and was evaluated by plotting the prevalence for each treatment group within selected time intervals. A two-sided P-value⩽0.05 or CIs not including 1.0 were considered statistically significant.
Results
Altogether, 874 out of 985 survivors (89%) and 243 out of 332 control men (73%) answered the questionnaire. Primary EBRT had been given to 302 survivors (35%), the combination of EBRT and brachytherapy to 373 survivors (43%) and salvage EBRT to 199 survivors (23%). The overall treatment time was in most cases 7 weeks.
Demographics and treatment
Demographics and treatment-related characteristics are shown in Table 1. The median age for all men was 71 years and the median time to follow-up for survivors was 5 years (range, 1–14 years). Treatment with anti-androgens was most common in the salvage EBRT group and treatments with gonadotropin-releasing hormone agonists and transurethral resection of the prostate were most common in the EBRT group. The most common fractionation regimen for the EBRT groups was a total dose of 70 Gray (Gy) at 2 Gy per fraction. For survivors treated with the combination of EBRT and brachytherapy, 50 Gy at 2 Gy per fraction and two brachytherapy fractions at 10 Gy per fraction was most common.
Table 1. Demographics and clinical characteristics of the prostate cancer survivors and the non-pelvic-irradiated control men.
| Treatment group characteristic | EBRT, n=302; n (%) | EBRT+BT, n=373; n (%) | POSTOP, n=199; n (%) | CONTROL, n=243; n (%) |
|---|---|---|---|---|
| Age in years (median, range) |
74 (57–80) |
70 (53–80) |
69 (49–80) |
71 (53–80) |
|
Body Mass Index (BMI) in kg m−2 | ||||
| <18.5 (lean) | 1 (<1) | 0 (0) | 0 (0) | 2 (<1) |
| 18.5–24.9 (normal weight) | 97 (32) | 104 (28) | 66 (33) | 93 (38) |
| 25.0–30.0 (overweight) | 144 (48) | 206 (55) | 102 (51) | 119 (49) |
| ⩾30.0 (obese) | 44 (15) | 52 (14) | 25 (13) | 24 (10) |
| Missing |
16 (5) |
11 (3) |
6 (3) |
5 (2) |
|
Comorbiditya | ||||
| Angina pectoris | 26 (9) | 20 (5) | 10 (5) | 21 (9) |
| Diabetes | 35 (12) | 39 (10) | 12 (6) | 39 (16) |
| Hypertension | 133 (44) | 145 (39) | 74 (37) | 91 (37) |
| Pulmonary disease |
23 (8) |
15 (4) |
10 (5) |
17 (7) |
|
Educational level | ||||
| Primary | 149 (49) | 118 (32) | 83 (42) | 104 (43) |
| High school | 62 (21) | 95 (25) | 43 (22) | 75 (31) |
| College/postgraduate | 86 (28) | 156 (42) | 71(36) | 62 (26) |
| Missing |
5 (2) |
4 (1) |
2 (<1) |
2 (<1) |
|
Fractionation regimens | ||||
| 33 × 2.0 Gy | 2 (1) | — | 11 (6) | — |
| 35 × 2.0 Gy | 243 (80) | — | 188 (94) | — |
| 29–30 × 2.0+5 × 3.0 Gy | 38 (13) | — | 0 (0) | — |
| Other EBRT | 15 (5) | — | 0 (0) | — |
| Missing data EBRT | 4 (1) | — | 0 (0) | — |
| 13 × 3.1+1 × 15.0 Gy | — | 6 (2) | — | |
| 25 × 2.0+1 × 15.0 Gy | — | 13 (3) | — | |
| 25 × 2.0+2 × 10.0 Gy | — | 326 (87) | — | |
| 30–32 × 2.0+1 × 10 Gy | — | 24 (6) | — | |
| Other EBRT+BT |
— |
4 (1) |
— |
|
|
Marital status | ||||
| Single | 35 (11) | 50 (14) | 15 (8) | 42 (17) |
| Partner, living alone | 12 (4) | 20 (5) | 11 (6) | 16 (7) |
| Married | 250 (83) | 299 (80) | 171 (86) | 184 (76) |
| Missing |
5 (2) |
4 (1) |
2 (<1) |
1 (<1) |
|
Other treatments | ||||
| Anti-androgen | 34 (12) | 23 (6) | 36 (18) | 0 (0) |
| GnRH | 31 (11) | 20 (5) | 14 (7) | 0 (0) |
| TURP |
32 (11) |
21 (6) |
10 (5) |
10 (4) |
|
Smoking status | ||||
| Current | 34 (11) | 31 (8) | 18 (9) | 24 (10) |
| Former | 147 (49) | 190 (51) | 87 (44) | 115 (47) |
| Never | 118 (39) | 146 (39) | 89 (45) | 99 (41) |
| Missing |
3 (1) |
6 (2) |
5 (2) |
5 (2) |
| Time to follow-up in years (median, range) | 6.5 (1.2–13.9) | 5.1 (1.2–14.4) | 3.3 (1.2–13.9) | — |
Abbreviations: EBRT=external beam radiation therapy; BT=brachytherapy; POSTOP=salvage radiation therapy after surgical prostatectomy; GnRH=gonadotropin-releasing hormone; TURP=transurethral resection of the prostate.
Based on self-reported information.
Physical symptoms
Statistically significantly higher prevalence ratios with respect to controls were found for 9 of the 21 investigated urinary symptoms in the EBRT group, 10 in the EBRT+brachytherapy group and 5 in the salvage EBRT group (Table 2). The prevalence for these symptoms in the control group, that is, the background rates, varied between 1% and 30%. The difference in symptom prevalence between the EBRT and EBRT+brachytherapy groups was not >5% for any studied symptom.
Table 2. Prevalence for 21 urinary symptoms among the prostate cancer survivors treated with external beam radiation therapy (EBRT), EBRT+brachytherapy or salvage EBRT and the non-pelvic-irradiated control men.
| Treatment group | EBRT | EBRT+BT | POSTOP | CONTROL | |
|---|---|---|---|---|---|
|
Symptom |
n/N
(%) PR (95% CI) |
n/N
(%) PR (95% CI) |
n/N
(%) PR (95% CI) |
n/N
(%) PR (95% CI) |
P-value |
| 1. Needs to bear down or push to initiate urination on half or more of the occasions |
26/298 (9) 1.0 (0.6–1.7) |
37/367 (10) 1.1 (0.7–1.9) |
10/196 (5) 0.6 (0.3–1.2) |
21/238 (9) Reference |
0.249 |
| 2. Needs to wait long for urinary flow when feeling the urge to pass urine on half or more of the occasions |
23/297 (8) 0.8 (0.5–1.4) |
29/369 (8) 0.8 (0.5–1.4) |
5/196 (3) 0.3 (0.1–0.7) |
23/238 (10) Reference |
0.032 |
| 3. Sudden involuntary stops when urinating on half or more of the occasions |
26/297 (9) 1.9 (1.0a–3.8) |
27/369 (7) 1.6 (0.8–3.2) |
2/196 (1) 0.2 (0.0–1.0a) |
11/239 (5) Reference |
0.002 |
| 4. Feels that it takes a long time to urinate on half or more of the occasions |
54/297 (18) 1.6 (1.0b–2.4) |
68/369 (18) 1.6 (1.0b–2.4) |
20/196 (10) 0.9 (0.5–1.5) |
28/239 (12) Reference |
0.012 |
| 5. Sensation of bladder being non-empty after urinating on half or more of the occasions |
62/297 (21) 1.4 (1.0a–2.1) |
81/368 (22) 1.5 (1.1–2.2) |
28/196 (14) 1.0 (0.6–1.6) |
35/241 (15) Reference |
0.031 |
| 6. Needs to bear down or push to empty bladder when being at the end of urinating on half or more of the occasions |
53/297 (18) 1.0 (0.7–1.5) |
74/367 (20) 1.2 (0.8–1.7) |
34/195 (17) 1.0 (0.7–1.5) |
41/241 (17) Reference |
0.741 |
| 7. Difficulties in feeling urge to urinate weekly or more often |
12/296 (4) 2.0 (0.7–5.5) |
12/369 (3) 1.6 (0.6–4.4) |
8/196 (4) 2.0 (0.7–5.9) |
5/242 (2) Reference |
0.569 |
| 8. Weak urinary flow when urinating on half or more of the occasions |
96/297 (32) 1.2 (0.9–1.6) |
115/368 (31) 1.2 (0.9–1.5) |
36/196 (18) 0.7 (0.5–1.0a) |
64/242 (26) Reference |
0.003 |
| 9. Needs to urinate more than once nightly |
118/297 (40) 1.8 (1.4–2.3) |
130/367 (35) 1.6 (1.2–2.1) |
40/196 (20) 0.9 (0.6–1.3) |
54/242 (22) Reference |
<0.001 |
| 10. Urinary urgency demanding to quickly get to a toilet more than once weekly |
116/297 (39) 1.3 (1.0b–1.7) |
155/369 (42) 1.4 (1.1–1.8) |
74/197 (38) 1.3 (1.0a–1.6) |
72/241 (30) Reference |
0.024 |
| 11. Cannot wait more than 10 min when feeling urinary urgency |
101/202 (50) 1.2 (0.9–1.4) |
135/271 (50) 1.1 (0.9–1.4) |
67/132 (51) 1.2 (0.9–1.5) |
66/152 (43) Reference |
0.534 |
| 12. Needs to urinate >10 times per day |
21/298 (7) 1.9 (0.9–4.0) |
31/366 (8) 2.3 (1.1–4.7) |
11/197 (6) 1.5 (0.6–3.5) |
9/241 (4) Reference |
0.124 |
| 13. Needs to urinate within 2 h after having urinated on half or more of the occasions |
61/296 (21) 1.6 (1.0b–2.3) |
92/368 (25) 1.9 (1.3–2.7) |
37/197 (19) 1.4 (0.9–2.2) |
32/241 (13) Reference |
0.005 |
| 14. Pain over the bladder when urinating on half or more of the occasions |
5/297 (2) 1.4 (0.3–5.6) |
13/368 (4) 2.8 (0.8–9.9) |
3/197 (2) 1.2 (0.3–6.0) |
3/242 (1) Reference |
0.178 |
| 15. Urethral pain when urinating on half or more of the occasions |
10/298 (3) 1.4 (0.5–3.7) |
23/368 (6) 2.5 (1.0b–6.1) |
8/197 (4) 1.6 (0.6–4.6) |
6/242 (2) Reference |
0.108 |
| 16. Weekly urinary leakage, more than a few drops |
68/298 (23) 2.0 (1.3–3.0) |
67/368 (18) 1.6 (1.0b–2.4) |
66/196 (34) 2.9 (2.0–4.3) |
28/242 (12) Reference |
<0.001 |
| 17. Urgency when leaking urine on half or more than half of the occasions |
41/120 (34) 1.5 (0.9–2.5) |
47/132 (36) 1.5 (0.9–2.5) |
26/112 (23) 1.0 (0.6–1.8) |
15/65 (23) Reference |
0.075 |
| 18. Weekly leakage of urine when not getting to a toilet in time |
50/297 (17) 2.1 (1.3–3.5) |
57/366 (16) 2.0 (1.2–3.2) |
36/194 (19) 2.4 (1.4–4.0) |
19/241 (8) Reference |
0.006 |
| 19. Weekly leakage of urine when coughing, sneezing or laughing |
11/291 (4) 4.6 (1.0b–20.4) |
12/354 (3) 4.1 (0.9–18.1) |
41/194 (21) 25.5 (6.2–104.0) |
2/241 (1) Reference |
<0.001 |
| 20. Weekly leakage of urine associated with physical exercise |
15/295 (5) 3.1 (1.0b–9.1) |
16/370 (4) 2.6 (0.9–7.7) |
55/194 (28) 17.1 (6.3–46.3) |
4/241 (2) Reference |
<0.001 |
| 21. Leakage of more than a few drops of urine when having urinary leakage | 59/282 (21) 2.0 (1.3–3.1) | 74/356 (21) 2.0 (1.3–3.0) | 59/184 (32) 3.1 (2.0–4.7) | 25/240 (10) Reference | <0.001 |
Prevalence ratios for the treated groups with respect to controls.
Abbreviations: EBRT=external beam radiation therapy; BT=brachytherapy; POSTOP=salvage radiation therapy after surgical prostatectomy; PR=prevalence ratio; CI=confidence interval.
Bold-faced numbers indicate a prevalence ratio significantly separated from 1.0 or P<0.05.
Lower CI <1.0.
Lower CI >1.0.
For the salvage EBRT group, only symptoms reflecting incontinence (questions 16 and 18–21) were significantly more prevalent. Also, in this group, the prevalence of symptoms reflecting urinary flow was significantly lower than for the control group (questions 2, 3 and 8). Stress incontinence defined as leakage of urine when coughing, sneezing or laughing was the symptom with the highest prevalence ratio in the EBRT group (question 19; prevalence ratio: 4.6; 95% CI 1.0–20.4). The highest prevalence ratio for the EBRT+brachytherapy group was found for urethral pain when urinating (question 15; prevalence ratio: 2.5; 95% CI: 1.0–6.1). The symptoms reflecting obstruction, frequent urination and urgency (questions 4, 5 and 9–10) were more prevalent in both the EBRT and in the EBRT+brachytherapy groups compared with corresponding symptom prevalence in the control group. Adjusting for age or diabetes mellitus, previously reported to influence the presence of urinary symptoms (Herold et al, 1999; Nilsson et al, 2011), did not change the prevalence ratios notably (data not shown).
Time to follow-up
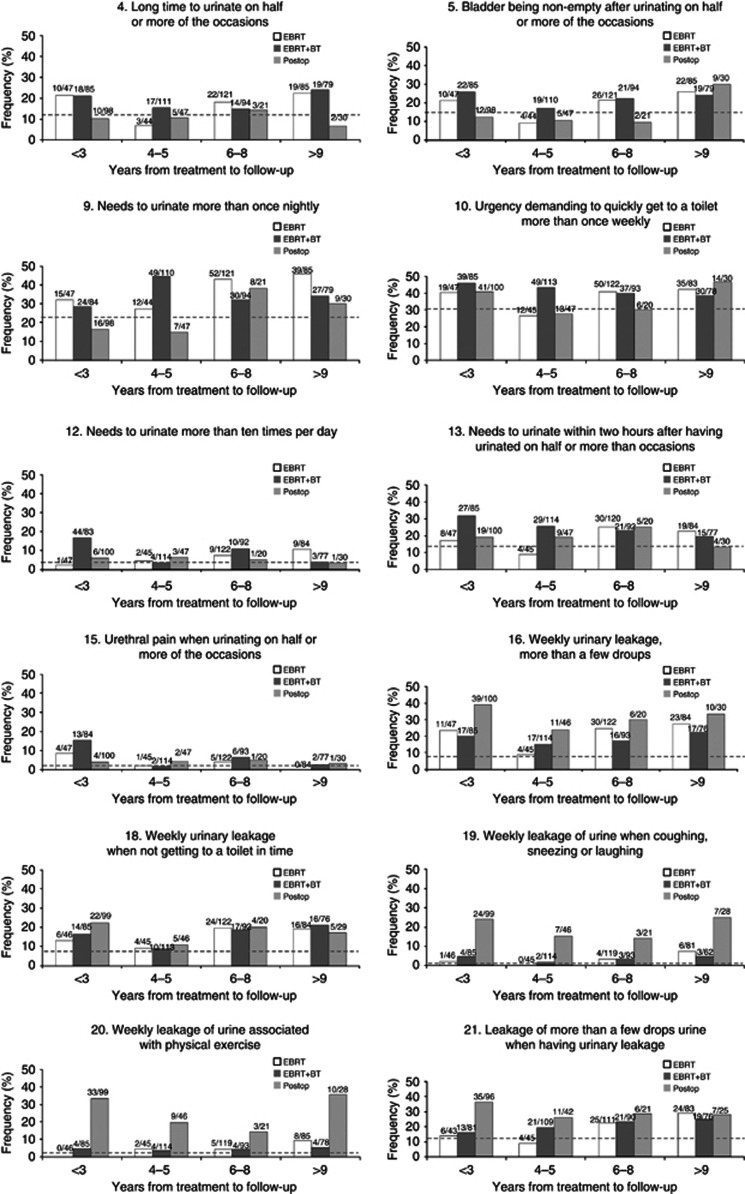
The 12 urinary symptoms with significantly increased prevalence ratios for one or more treatment groups were stratified according to time to follow-up (Figure 1). The temporal pattern of symptom occurrence varied, but the prevalence generally changed moderately over time. For a majority of the symptoms, survivors with time to follow-up <3 years had a higher prevalence than survivors with time to follow-up between 4 and 5 years. This pattern was significant for question 4 in the EBRT group (P=0.049) and questions 12 and 15 in the EBRT+BT group (P<0.001 for both). A statistically lower prevalence was found for frequency (question 9) in the EBRT+BT group (P=0.023). It is also worth noting that few symptoms decreased to their respective control background rate.
Figure 1.
Symptoms with higher prevalence ratios between survivors and controls (c.f. Table 2, EBRT: symptoms 4, 9, 10, 13, 16, 18, 19, 20 and 21; EBRT+BT: symptoms 4, 5, 9, 10, 12, 13, 15, 16, 18 and 21 and POSTOP: symptoms 16, 18, 19, 20 and 21). Survivors are grouped by treatment and by years from treatment to follow-up. The number of individuals in each group is based on the number answering the question under consideration. Dotted lines indicate the symptom prevalence in the control group (n=243).
Discussion
Among the 21 investigated patient-reported urinary symptoms, we found significantly higher prevalence compared with population-based controls for 9 symptoms in the EBRT group, 10 symptoms in the EBRT+brachytherapy group and 5 symptoms in the salvage EBRT group. Survivors in the EBRT and EBRT+brachytherapy groups had similar toxicity profiles, including symptoms reflecting urinary obstruction and irritative toxicity. The prevalence for a majority of the symptoms was stable over the studied time to follow-up.
This study adds to the current knowledge about radiation-treated prostate cancer survivors by providing comprehensive urinary toxicity profiles and relating them to comparable symptoms among untreated, healthy men (PubMed search on June 29, 2012 using various combinations of the search criteria: ‘prostate cancer', ‘late radiation toxicity', ‘patient-reported outcomes', ‘genitourinary toxicity', ‘external beam radiation therapy', ‘brachytherapy', ‘prostatectomy', ‘case-control', ‘cohort study' gave no relevant hits). Symptom background rates, or occurrences of symptoms in a population similar to the one under consideration, become more important to acknowledge when estimating risks for ‘less severe' toxicities. If a substantial background rate is overlooked, the risk attributed to the treatment will be overestimated. Modifying the treatment may then lead to a jeopardised tumour control without actually lowering the symptom risk as much as anticipated.
The symptom prevalence for a majority of the investigated symptoms was similar in the EBRT group and the EBRT+brachytherapy group. This is somewhat in contrast to previous reports, where the combination of EBRT and brachytherapy is reported to increase the risk of adverse genitourinary effects compared with EBRT alone (Mohammed et al, 2012). In the present study, only the symptoms reflecting pain were notably different between these groups suggesting that this is important to acknowledge when considering the long-term treatment effects of adding brachytherapy to prostate cancer patients. The high prevalence of incontinence in the salvage EBRT group was expected and is most likely explained by the effects of prostatectomy rather than the effects of radiation therapy. Men who have undergone prostatectomy are reported to have more problems reflecting urinary incontinence and adding postoperative radiation therapy has been reported to worsen already present symptoms (Parsons et al, 2009; Mirza et al, 2011; Mohammed et al, 2012; Schmid et al, 2012).
Information about the temporal pattern of urinary symptoms is diverging in the literature. Symptoms reflecting obstruction, urgency and frequency are reported to remain at the same level with time (Potosky et al, 2004; Moinpour et al, 2008) while incontinence is typically reported to increase (Fransson, 2008; Parsons et al, 2009). In our study, the symptom prevalence tended to be higher the first years after radiation therapy and then decreased. After 6 years, the prevalence began to increase again, but it often returned to a similar level as during the first years for survivors with >9 years between treatment and follow-up. Typically, there was a slower increase or stability in symptom prevalence for these time intervals compared with the more rapid decrease for the time intervals before 6 years. The reasons for this very late increase can be an effect of aging (Nilsson et al, 2011) or a very late development of radiation-induced injury. This needs to be further investigated in more detailed analyses than what is within the scope of this work. That the symptom prevalence in general stayed above the control background rates regardless of time to follow-up suggests that many of these symptoms can assist in finding tolerance doses for patient-reported late genitourinary toxicity.
Diabetes mellitus has been reported to influence the presence of RTOG grade ⩾2 late genitourinary toxicity (Herold et al, 1999). We therefore recalculated our results excluding the men with diabetes mellitus for both survivors and controls. These results were similar to the results for the whole group making it difficult to draw any conclusions about the effect of diabetes on the symptoms investigated in this work. Further analyses are needed to decide if patients with diabetes mellitus are more prone than patients without diabetes mellitus to experience these kinds of toxicities.
The strengths of this study include a large group of survivors with a long time to follow-up and information from non-pelvic-irradiated men. We used epidemiological methods adapted to the cancer-survivorship field according to the hierarchical step-model for causation of bias (Steineck et al, 2006). The Swedish Total Population Register allows us to follow up all eligible men and, together with a high participation and response rate for survivors and controls, the risk of selection-induced problems can be reduced. The cross-sectional long-term follow-up design allows us to assess symptom occurrence over time without the influence of repeated measurements. The use of a postal questionnaire also minimises the risk of interviewer-related problems. The reported toxicity profiles depended on the used treatment techniques and prescribed doses and may not be directly generalisable to other settings. Although the treatment protocols remained stable over time, there may have been minor adjustments for which we lack data, but we have no reason to believe that this would alter the overall results of this study.
Using an atomised approach and a control population when trying to understand the entire spectrum of radiation-induced long-term symptoms for cancer survivors provide a level of detail and perspective that extends current knowledge. The genitourinary toxicity profiles presented here provide an understanding of which radiation-induced toxicities to be addressed in the clinic and can help to develop personalised therapy for prostate cancer. They can also assist in identifying suitable interventions to alleviate existing symptoms after already given treatments. Together with knowledge about critical dose constrains for relevant organs at risk, our data can be used to guide modern radiation therapy towards genitourinary-sparing techniques to avoid future toxicity.
Acknowledgments
We thank all the men who participated in the Swedish Project on Atomized Symptoms After Cancer Treatments (SPASACT) prostate cancer study. Support from the Swedish Cancer Society, the King Gustav V Jubilee Clinic Cancer Foundation in Göteborg, the Swedish state under the ALF agreement in Göteborg and Stockholm and the Assar Gabrielsson foundation is also gratefully acknowledged. Finally, we would like to thank the anonymous referees for the helpful comments and suggestions.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Al-Abany M, Helgason AR, Adolfsson J, Steineck G. Reliability of assessment of urgency and other symptoms indicating anal sphincter, large bowel or urinary dysfunction. Scand J Urol Nephrol. 2006;40:397–408. doi: 10.1080/00365590600795362. [DOI] [PubMed] [Google Scholar]
- Alsadius D, Hedelin M, Johansson KA, Pettersson N, Wilderäng U, Lundstedt D, Steineck G. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer. Radiother Oncol. 2011;101:495–501. doi: 10.1016/j.radonc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Bekelman JE, Mitra N, Efstathiou J, Liao K, Sunderland R, Yeboa DN, Armstrong K. Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:e325–e334. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, Marks LB, Ten Haken RK, Yorke ED. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–S9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmark K, Åvall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. 1999;340:1383–1389. doi: 10.1056/NEJM199905063401802. [DOI] [PubMed] [Google Scholar]
- Budaus L, Bolla M, Bossi A, Cozzarini C, Crook J, Widmark A, Wiegel T. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:112–127. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Fransson P. Patient-reported lower urinary tract symptoms, urinary incontinence, and quality of life after external beam radiotherapy for localized prostate cancer—15 years' follow-up. A comparison with age-matched controls. Acta Oncol. 2008;47:852–861. doi: 10.1080/02841860701654325. [DOI] [PubMed] [Google Scholar]
- Herold DM, Hanlon AL, Hanks GE. Diabetes mellitus: a predictor for late radiation morbidity. Int J Radiat Oncol Biol Phys. 1999;43:475–479. doi: 10.1016/s0360-3016(98)00460-x. [DOI] [PubMed] [Google Scholar]
- ICRU 1993Prescribing, recording and reporting photon beam therapy. International Commissions on Radiation Units and Measurements: Bethesda, MD, USA. Report 50.
- Kreicbergs U, Valdimarsdottir U, Onelöv E, Henter JI, Steineck G. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351:1175–1186. doi: 10.1056/NEJMoa040366. [DOI] [PubMed] [Google Scholar]
- Mirza M, Griebling TL, Wallace Kazer M. Erectile dysfunction and urinary incontinence after prostate cancer treatment. Semin Oncol Nurs. 2011;27:278–289. doi: 10.1016/j.soncn.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Mohammed N, Kestin L, Ghilezan M, Krauss D, Vicini F, Brabbins D, Gustafson G, Ye H, Martinez A. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:204–212. doi: 10.1016/j.ijrobp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Moinpour CM, Hayden KA, Unger JM, Thompson IM, Jr., Redman MW, Canby-Hagino ED, Higgins BA, Sullivan JW, Lemmon D, Breslin S, Crawford ED, Southwest Oncology G Health-related quality of life results in pathologic stage C prostate cancer from a Southwest Oncology Group trial comparing radical prostatectomy alone with radical prostatectomy plus radiation therapy. J Clin Oncol. 2008;26:112–120. doi: 10.1200/JCO.2006.10.4505. [DOI] [PubMed] [Google Scholar]
- Nilsson AE, Schumacher MC, Johansson E, Carlsson S, Stranne J, Nyberg T, Wiklund NP, Steineck G. Age at surgery, educational level and long-term urinary incontinence after radical prostatectomy. BJU Int. 2011;108:1572–1577. doi: 10.1111/j.1464-410X.2011.10231.x. [DOI] [PubMed] [Google Scholar]
- Parsons BA, Evans S, Wright MP. Prostate cancer and urinary incontinence. Maturitas. 2009;63:323–328. doi: 10.1016/j.maturitas.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Potosky AL, Davis WW, Hoffman RM, Stanford JL, Stephenson RA, Penson DF, Harlan LC. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- Schmid MP, Potter R, Bombosch V, Sljivic S, Kirisits C, Dorr W, Goldner G. Late gastrointestinal and urogenital side-effects after radiotherapy—Incidence and prevalence. Subgroup-analysis within the prospective Austrian-German phase II multicenter trial for localized prostate cancer. Radiother Oncol. 2012;104:114–118. doi: 10.1016/j.radonc.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Steineck G, Bergmark K, Henningsohn L, al-Abany M, Dickman PW, Helgason A. Symptom documentation in cancer survivors as a basis for therapy modifications. Acta Oncol. 2002a;41:244–252. doi: 10.1080/02841860260088782. [DOI] [PubMed] [Google Scholar]
- Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson JE, Norlen BJ, Holmberg L. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002b;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- Steineck G, Hunt H, Adolfsson J. A hierarchical step-model for causation of bias-evaluating cancer treatment with epidemiological methods. Acta Oncol. 2006;45:421–429. doi: 10.1080/02841860600649293. [DOI] [PubMed] [Google Scholar]