Abstract
Background:
There have been controversies in prognostic impact of mucinous histology on colorectal cancer, and its implication in patients treated with adjuvant 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) is unclear.
Methods:
Stage II and III colorectal cancer patients who underwent curative resection followed by adjuvant FOLFOX were included. Patients were grouped according to the mucinous content: >50%, mucinous adenocarcinoma (MAC); <50%, adenocarcinoma with intermediated mucinous component (AIM); and without any mucinous component, non-MAC (NMA). Clinicopathological features and disease-free survival (DFS) were compared.
Results:
Among a total of 521 patients, 27 patients (5.2%) had MAC, 41 patients (7.9%) had AIM, and 453 patients (86.9%) had NMA. Mucinous adenocarcinoma and AIM had higher frequency of proximal location and microsatellite instability, but lower frequency of angiolymphatic invasion. Disease-free survival was significantly worse in the MAC compared with NMA (3-year DFS 57% and 86%, respectively; P<0.001) and AIM (3-year DFS 87%, P=0.01 vs MAC). Multivariate analysis revealed MAC as an independent negative prognostic factor of DFS (adjusted hazard ratio 7.96, 95% confidence interval 3.76–16.8).
Conclusion:
Adenocarcinoma with intermediated mucinous component and MAC have distinct clinicopathological features compared with NMA. Mucinous adenocarcinoma has an adverse prognostic impact on stage II or III colorectal cancer treated with adjuvant FOLFOX.
Keywords: colorectal cancer, mucinous adenocarcinoma, mucinous histology, adjuvant chemotherapy, oxaliplatin
Colorectal mucinous adenocarcinoma (MAC) is a subtype of colorectal adenocarcinoma with prominent mucin (MUC) production. In the World Health Organisation (WHO) classification, MAC is defined as an adenocarcinoma in which >50% of the lesion is composed of pools of extracellular mucin (Bosman, 2010). Tumour with <50% of the lesion composed of mucin is categorised as having mucinous component. Mucinous adenocarcinoma is associated with proximal location of tumour, advanced stage at diagnosis, microsatellite instability (MSI), and BRAF mutation compared with non-MAC (Green et al, 1993; Younes et al, 1993; Kakar et al, 2004; Song et al, 2005; Leopoldo et al, 2008). The frequency of MAC among colorectal cancer varies according to geographic origin, Western studies exhibiting higher frequencies compared with Asian studies, suggesting possible ethnic difference in pathogenesis (Wu et al, 1996; Kanemitsu et al, 2003; Du et al, 2004; Papadopoulos et al, 2004; Kang et al, 2005; Lee et al, 2007; Leopoldo et al, 2008; Xie et al, 2009). Previous studies have reported poor response and survival following palliative chemotherapy in advanced colorectal cancer with mucinous histology (Negri et al, 2005; Catalano et al, 2009). Moreover, a recent meta-analysis showed 2–8% increased hazard of death associated with mucinous histology (Verhulst et al, 2012). However, other studies have failed to demonstrate the adverse prognostic impact of mucinous histology on treatment outcome (Farhat et al, 2008; Catalano et al, 2012; Langner et al, 2012). Different outcomes of MAC according to MSI status have been reported exhibiting better outcome with MSI compared with microsatellite stable (MSS) among MAC (Kakar et al, 2004; Leopoldo et al, 2008).
Adjuvant chemotherapy using 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) is commonly used in stage III colon cancer, as it can reduce recurrence and mortality in these patients (Andre et al, 2009). However, the exact prognostic impact of mucinous histology on colorectal cancer patients treated with adjuvant FOLFOX is unclear. This study aimed at elucidating the prognostic implication of MAC among colorectal cancer in the current era of adjuvant FOLFOX. Furthermore, we investigated clinicopathological features and prognostic effect of mucinous component of <50%, of which only limited data have been reported.
Materials and Methods
Patients and treatment
We retrospectively analysed patients with stage II or III colorectal cancer treated with adjuvant FOLFOX chemotherapy between April 2005 and December 2011. Patients were eligible if they met the following criteria: age over 18, adenocarcinoma histology, stage III or high-risk stage II, complete resection of the tumour with negative margin, and completion of at least six cycle of adjuvant FOLFOX chemotherapy. High-risk stage II was defined if they had any of the following: T4 lesion, obstruction or perforation, lymphovascular invasion, perineural invasion, or poorly differentiated histology. Patients with upper rectal cancer were included if the patient did not receive pre- or post-operative radiation. Exclusion criteria were the following: previous chemotherapy for CRC, previous radiotherapy for CRC, signet ring cell histology, distant metastasis, and history of other malignancy within 5 years. All patients received surgery and chemotherapy at Seoul National University Hospital, and eligible patients were identified from the electronic medical record of Seoul National University Hospital. The study protocol was reviewed and approved by the institutional review board of Seoul National University Hospital.
Patient received FOLFOX chemotherapy as either FOLFOX-4 or modified FOLFOX-6 regimen. Patients who started chemotherapy before July 2009 received FOLFOX-4 and after July 2009 received modified FOLFOX-6. Each cycle of FOLFOX-4 consisted of oxaliplatin (85 mg m−2) on day 1, folinic acid (200 mg m−2), and a bolus of 5-fluorouracil (FU) (400 mg m−2) followed by a 22-h infusion of 5-FU (600 mg m−2) on days 1 and 2, which was repeated every 2 weeks. Modified FOLFOX-6 consisted of oxaliplatin (85 mg m–2), folinic acid (400 mg m–2), and a bolus of 5-FU (400 mg m–2) followed by a 46-h infusion of 5-FU (2400 mg m–2) repeated every 2 weeks. Adjuvant chemotherapy was planned for a total of 12 cycles.
Patients were assessed every 2 weeks during chemotherapy treatment, and then at least every 6 months for 5 years. The post-chemotherapy period assessment included a medical history taking, physical examination, measurement of the carcinoembryonic antigen level, chest computed tomography, and abdominal computed tomography. The diagnosis of recurrence was made on the basis of imaging and, if necessary, biopsy.
Pathological examination
Mucinous adenocarcinoma was defined according to the WHO classification, which is >50% of the tumour lesion composed of pools of extracellular MUC (Bosman, 2010). In the present study, we defined tumours with mucinous area of <50% as adenocarcinoma with intermediate mucinous component (AIM) in order to avoid confusion with MAC. Non-MAC (NMA) was defined as cancer without any mucinous component.
The microsatellite status of each tumour was determined by evaluating five microsatellite markers (D2S123, D5S346, D17S250, BAT25, and BAT26). Either forward or reverse primer for each marker was labelled with fluorescence, and PCR products were electrophoresed and analysed. We classified MSI status as follows: high MSI (MSI-H; instability at ⩾2 microsatellite markers), low MSI (MSI-L; instability at 1 marker), or MSS (no instability). Only MSI-H was regarded as having MSI, and MSI-L was grouped with MSS.
Statistical analysis
The objective of this study was to investigate the effect of mucinous histology (MAC and AIM) on the treatment outcome (disease-free survival (DFS)) of colorectal cancer patients treated with adjuvant FOLFOX chemotherapy. Disease-free survival was calculated from the date of operation to the first date of documented progressive disease or the date of death from any cause. Data from patients who were free of progression were censored at the date of the last follow-up visit for DFS. Categorical variables were compared using χ2-test or Fisher's exact test as appropriate. Disease-free survival was calculated using the Kaplan–Meier method, and comparisons were made using the log-rank tests. Hazard ratios (HRs) were calculated using the Cox proportional hazard model. To adjust for the baseline characteristics, Cox proportional hazard analysis of DFS included age, sex, T stage, N stage, tumour location, angiolymphatic invasion, venous invasion, perineural invasion, and MSI status. Two-sided P-values of <0.05 were considered statistically significant. Statistical analysis was performed with SPSS software for Windows, version 17.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
A total of 521 patients with stage II or III colorectal cancer, treated with adjuvant FOLFOX chemotherapy, were included. Baseline characteristics are summarised in Table 1. Tumour location was caecum in 24, ascending colon in 118, transverse in 39, descending in 33, sigmoid in 275, and rectum in 32 patients. Collectively, 181 patients had tumour in proximal (from caecum to transverse colon) location and 340 patients had tumour in distal location. Tumour stage was stage II in 78 patients (IIA in 53, IIB in 21, and IIC in 4) and stage III in 443 patients (IIIA in 39, IIIB in 289, and IIIC in 115). All stage II patients had high-risk features. Microsatellite instability (MSI-H) was shown in 6.8% of tumours. MSI was more frequently observed in proximal location (13.9% vs 3.0% in distal location, P<0.001). There was no significant difference in MSI incidence according to tumour stage, sex, or age. According to the inclusion criteria, all patients received at least 6 cycles of chemotherapy, and 88.8% of patients completed planned 12 cycles of chemotherapy.
Table 1. Patient characteristics and mucinous histology.
| |
|
|
|
P-values |
|||
|---|---|---|---|---|---|---|---|
| Total (N=521) | NMA (N=453) | AIM (N=41) | MAC (N=27) | AIM vs NMA | MAC vs NMA | AIM vs MAC | |
|
Age | |||||||
| Median (range) | 60 (25–81) | 60 (35–76) | 62 (25–81) | 56 (30–81) | |||
| ⩾65 years |
155 (29.8%) |
128 (28.3%) |
19 (46.3%) |
8 (29.6%) |
0.015 |
0.88 |
0.17 |
|
Sex | |||||||
| Male | 312 (59.9%) | 283 (62.5%) | 13 (31.7%) | 16 (59.3%) | <0.001 | 0.74 | 0.025 |
| Female |
209 (40.1%) |
170 (37.5%) |
28 (68.3%) |
11 (40.7%) |
|
|
|
|
Location | |||||||
| Proximal | 181 (34.7%) | 140 (30.9%) | 25 (61.0%) | 16 (59.3%) | <0.001 | 0.002 | 0.89 |
| Distal |
340 (65.3%) |
313 (69.1%) |
16 (39.0%) |
11 (40.7%) |
|
|
|
|
Stage | |||||||
| II | 78 (15.0%) | 67 (14.8%) | 7 (17.1%) | 4 (14.8%) | 0.70 | 1.0 | 1.0 |
| III |
443 (85.0%) |
386 (85.2%) |
34 (82.9%) |
23 (85.2%) |
|
|
|
|
T stage | |||||||
| T1–3 | 445 (85.4%) | 394 (87.0%) | 35 (85.4%) | 16 (59.3%) | 0.77 | <0.001 | 0.015 |
| T4 |
76 (14.6%) |
59 (13.0%) |
6 (14.6%) |
11 (40.7%) |
|
|
|
|
N stage | |||||||
| N0–1 | 378 (72.6%) | 332 (73.3%) | 29 (70.7%) | 17 (63.0%) | 0.72 | 0.24 | 0.50 |
| N2 |
143 (27.4%) |
121 (26.7%) |
12 (29.3%) |
10 (37.0%) |
|
|
|
|
Angiolymphatic invasion | |||||||
| Present | 223 (42.8%) | 205 (45.3%) | 12 (29.3%) | 6 (22.2%) | 0.048 | 0.019 | 0.52 |
| Absent |
298 (57.2%) |
248 (54.7%) |
29 (70.7%) |
21 (77.8%) |
|
|
|
|
Venous invasion | |||||||
| Present | 57 (10.9%) | 54 (11.9%) | 1 (2.4%) | 2 (7.4%) | 0.070 | 0.76 | 0.56 |
| Absent |
464 (89.1%) |
399 (88.1%) |
40 (97.6%) |
25 (92.6%) |
|
|
|
|
Perineural invasion | |||||||
| Present | 120 (23.0%) | 112 (24.7%) | 5 (12.2%) | 3 (11.1%) | 0.084 | 0.16 | 1.00 |
| Absent |
401 (77.0%) |
341 (75.3%) |
36 (87.8%) |
24 (88.9%) |
|
|
|
|
Microsatellite status | |||||||
| MSS+MSI-L | 483 (93.2%) | 425 (94.4%) | 35 (85.4%) | 23 (85.2%) | 0.022 | 0.073 | 1.00 |
| MSI-H | 35 (6.8%) | 25 (5.6%) | 6 (14.6%) | 4 (14.8%) | |||
Abbreviations: AIM=adenocarcinoma with intermediate mucinous component; MAC=mucinous adenocarcinoma; MSI-H=high microsatellite instability; MSI-L=low microsatellite instability; MSS=microsatellite stable; NMA=non-mucinous adenocarcinoma.
Clinicopathological characteristics of mucinous histology
Twenty-seven patients (5.2%) had MAC and 41 patients (7.9%) had AIM. The remaining 453 patients (86.9%) had NMA. Tumours with mucinous component (AIM and MAC) were more frequently found in proximal location (61.0% in AIM and 59.3% in MAC vs 30.9% in NMA), less likely to show angiolymphatic invasion (29.3% in AIM and 22.2% in MAC vs 45.3% in NMA), and more likely to have microsatellite instability (14.6% in AIM and 14.8% in MAC vs 5.6% in NMA) compared with NMA (Table 1). Venous invasion and perineural invasion tended to be also less frequently observed in AIM and MAC.
Although AIM and MAC showed similar clinicopathological features described above, differences were also found. Adenocarcinoma with intermediated mucinous component was more frequently observed in patients over 65 years old (46.3% in AIM vs 29.6% in MAC and 28.3% in NMA) and in female (68.3% in AIM vs 40.7% in MAC and 37.5% in NMA) patients compared with MAC and NMA. In contrast, higher proportion of MAC had T4 tumour (40.7% in MAC vs 14.6% in AIM and 13.0% in NMA) compared with AIM and NMA.
Prognostic implication of mucinous histology
After a median follow-up duration of 38 months, 72 recurrent events (62 distant metastases and 10 local recurrences) have occurred. The 3-year DFS of the entire cohort was 84.9%.
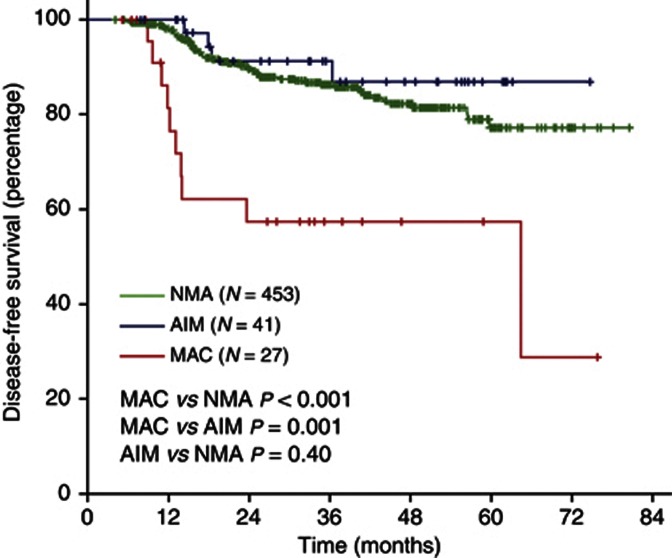
Disease-free survival was significantly worse in the MAC compared with NMA (3-year DFS 57% and 86%, respectively; P<0.001) (Figure 1). Interestingly, DFS of AIM (3-year DFS 87%) was similar to NMA (P=0.40) and significantly better than MAC (P=0.01).
Figure 1.
Kaplan–Meier curves of DFS according to mucinous histology.
At the time of analysis, 10 patients in the MAC group, 4 patients in AIM group, and 58 patients in the NMA group had recurrence. There was no difference in pattern of recurrence (local recurrence vs distant metastasis) between each group (Table 2). Regarding initial sites of distant metastasis, AIM and MAC had higher percentage of peritoneal seeding compared with NMA, whereas lymph node was not involved in MAC. However, we could not make meaningful comparisons as only limited number of recurrent cases, especially for MAC and AIM, are involved in the present analysis. Among the patients with recurrence, we had tissue specimen from metastatic site in seven patients with MAC and two patients with AIM. Review of the pathological specimen from the metastatic site in patients with initial MAC showed that four patients had MAC, two patients had AIM, and one patient had NMA in the metastasis. In cases of initial AIM, one patient had AIM, whereas the other patient had NMA in the metastasis. Collectively, 78% (7 out of 9) of patients with initial tumour with mucinous component (MAC and AIM) retained MUC production in the recurrent metastatic tumour.
Table 2. Pattern of recurrence.
| |
|
|
|
P-values |
|||
|---|---|---|---|---|---|---|---|
| Total (N=72) | NMA (N=58) | AIM (N=4) | MAC (N=10) | AIM vs NMA | MAC vs NMA | AIM vs MAC | |
|
Pattern of recurrencea | |||||||
| Local recurrence | 10 (13.9%) | 8 (13.8%) | 1 (25.0%) | 1 (10.0%) | 0.48 | 1.00 | 0.505 |
| Distant metastasis |
62 (86.1%) |
50 (86.2%) |
3 (75.0%) |
9 (90.0%) |
|
|
|
|
Initial site involved in case of distant metastasisb | |||||||
| Lung | 25 (40.3%) | 22 (44.0%) | 1 (33.3%) | 2 (22.2%) | 1.00 | 0.29 | 1.00 |
| Liver | 19 (30.6%) | 17 (34.0%) | 0 (0%) | 2 (22.2%) | 0.54 | 0.70 | 1.00 |
| Lymph node | 19 (30.6%) | 17 (34.0%) | 2 (66.7%) | 0 (0%) | 0.29 | 0.048 | 0.045 |
| Peritoneum | 20 (32.3%) | 13 (26.0%) | 2 (66.7%) | 5 (55.6%) | 0.19 | 0.12 | 1.00 |
| Bone | 2 (3.2%) | 2 (4.0%) | 0 (0%) | 0 (0%) | 1.00 | 1.00 | — |
Abbreviations: AIM=adenocarcinoma with intermediate mucinous component; MAC=mucinous adenocarcinoma; NMA=non-mucinous adenocarcinoma.
Analysis among patients with recurrence.
Analysis among patients having distal metastasis.
In addition to MAC histology, following clinicopathological features were associated with poor treatment outcome: T4 stage, N2 stage, angiolymphatic invasion, venous invasion, and perineural invasion (Table 3). Other factors including MSI were not significantly associated with DFS. Although MSI-H showed trend towards favourable 3-year DFS compared with MSS/MSI-L (90.0% vs 84.1%), there was no statistically significant difference (P=0.46). There was no significant DFS difference according to MSI in any subgroup of patients (tumour location, stage, or sex). We could not compare the DFS according to MSI because only limited number of patients showed MSI among MAC (four patients) and AIM (six patients).
Table 3. Univariate analysis of disease-free survival.
| Variable | 3-year DFS (%) | P-value |
|---|---|---|
|
Sex | ||
| Male | 85.0 | 0.66 |
| Female |
83.8 |
|
|
Age | ||
| ⩾65 years | 85.7 | 0.82 |
| <65 years |
83.9 |
|
|
Location | ||
| Proximal | 87.0 | 0.25 |
| Distal |
83.1 |
|
|
Stage | ||
| II | 86.8 | 0.35 |
| III |
84.1 |
|
|
T stage | ||
| T1–3 | 86.8 | 0.003 |
| T4 |
73.9 |
|
|
N stage | ||
| N0–1 | 89.4 | <0.001 |
| N2 |
72.6 |
|
|
Angiolymphatic invasion | ||
| Present | 75.4 | <0.001 |
| Absent |
91.2 |
|
|
Venous invasion | ||
| Present | 71.0 | 0.003 |
| Absent |
86.6 |
|
|
Perineural invasion | ||
| Present | 74.3 | <0.001 |
| Absent |
87.8 |
|
|
Histology | ||
| MAC | 57.4 | <0.001 |
| Non-MAC* |
86.3 |
|
|
Microsatellite status | ||
| MSS/MSI-L | 84.1 | 0.46 |
| MSI-H | 90.0 | |
Abbreviations: DFS=disease-free survival; MAC=mucinous adenocarcinoma; MSI-H=high microsatellite instability; MSI-L=low microsatellite instability; MSS=microsatellite stable.
*Non-MAC includes non-mucinous adenocarcinoma and adenocarcinoma with intermediate mucinous component.
To examine whether MAC was independently associated with poor DFS, we used the Cox proportional hazard model in a forward stepwise manner with variables in Table 3 as covariates. Multivariate analysis revealed that MAC was an independent negative prognostic factor (Table 4). Mucinous adenocarcinoma had significantly higher risk of recurrence (adjusted HR 7.96) compared with non-MAC (NMA and AIM). We also conducted multivariate analysis using combined stage (i.e., IIa, IIb, IIc, IIIa, IIIb, and IIIc) instead of individual T stage and N stage as covariates. Similar result was obtained showing MAC as an independent negative prognostic factor (adjusted HR 7.42; P<0.001).
Table 4. Multivariate analysis of disease-free survival.
| Adjusted HR (95% CI) | P-value | |
|---|---|---|
|
Histology | ||
| MAC | 7.96 (3.76–16.8) | <0.001 |
| Non-MACa |
1 |
|
|
T stage | ||
| T4 | 1.94 (1.13–3.34) | 0.017 |
| T1–3 |
1 |
|
|
N stage | ||
| N2 | 2.39 (1.48–3.85) | <0.001 |
| N0–1 |
1 |
|
|
Angiolymphatic invasion | ||
| Present | 2.83 (1.64–4.87) | <0.001 |
| Absent |
1 |
|
|
Perineural invasion | ||
| Present | 2.27 (1.36–3.79) | 0.002 |
| Absent | 1 | |
Abbreviations: CI=confidence interval; HR=hazard ratio; MAC=mucinous adenocarcinoma.
Non-MAC includes non-mucinous adenocarcinoma and adenocarcinoma with intermediate mucinous component.
Discussion
The aim of the present study was to examine whether mucinous histology (MAC and AIM) has prognostic value in stage II and III colorectal cancer patients treated with adjuvant FOLFOX. There have been controversies in prognostic impact of MAC, and some studies showed poorer prognostic role of MAC compared with NMA (Green et al, 1993; Kanemitsu et al, 2003; Negri et al, 2005; Catalano et al, 2009), whereas others did not (Farhat et al, 2008; Catalano et al, 2012; Langner et al, 2012). Most of the previous studies focused only on MAC but not on AIM. Moreover, only limited proportion of patients had been treated with adjuvant FOLFOX, which is the current standard of care in stage III disease. Using a homogeneous cohort of patients treated with adjuvant FOLFOX, we found that MAC was independently associated with a higher risk of recurrence. In contrast, AIM showed similar treatment outcome compared with NMA.
Mucinous adenocarcinoma having poor treatment outcome is in line with previous studies performed with stage IV patients receiving palliative chemotherapy (Negri et al, 2005; Catalano et al, 2009). A recent study including stage II and III colon cancer showed no prognostic role of mucinous histology (Catalano et al, 2012). However, only 16% of patients received oxaliplatin-based adjuvant chemotherapy (Catalano et al, 2012). In contrast, all patients received at least six cycles of adjuvant FOLFOX chemotherapy in the present study. As a result, 88.8% of patients had completed 12 cycles of chemotherapy, and this maybe one explanation for the relatively high 3-year DFS in this study.
Mucins are high-molecular-weight heavily glycosylated proteins that may function as chemical barriers. Over 20 MUC genes are known, which are classified into secreted or transmembrane MUCs (Senapati et al, 2010). It has been shown that loss of MUC expression is an early event in colorectal carcinogenesis in a recent study (Grivennikov et al, 2012). In contrast, a number of secreted MUCs, such as MUC2, have been shown to be preserved in MAC compared with NMA (Byrd and Bresalier, 2004). Taken together, these data suggest that MAC and NMA have different mechanism of carcinogenesis. Distinct expression pattern of MUCs and higher levels of thymidylate synthase and glutathione S-transferase pi (GSTP1) in MAC maybe associated with resistance to FOLFOX (Byrd and Bresalier, 2004; Leteurtre et al, 2004; Glasgow et al, 2005). In the present study, majority of biopsy specimen from distant metastasis retained MUC production, which provides evidence that MUC-producing cells are resistant to adjuvant chemotherapy and responsible for the recurrence. However, it is based on a limited number of patients and should be investigated in a larger series of paired tissues from primary and metastatic tumour. The exact mechanism of poor prognosis of MAC should be elucidated in future studies to improve treatment outcome of these patients with MAC. In the meantime, patients with MAC may benefit from a more stringent follow-up. In addition, future studies analysing the role of mucinous histology as a prognostic marker in non-high-risk stage II CRC may help identify patients that may potentially benefit from adjuvant chemotherapy.
Mucinous adenocarcinoma and MSI show geographical variation in terms of its frequencies. The reported frequencies of MAC are 3–12% in Asian studies compared with 10–39% in Western studies (Wu et al, 1996; Kanemitsu et al, 2003; Du et al, 2004; Papadopoulos et al, 2004; Kang et al, 2005; Lee et al, 2007; Leopoldo et al, 2008; Xie et al, 2009). Microsatellite instability is also more frequently found in Western patients. In stage III colorectal cancer, 11–21% show MSI in Western studies, whereas 5–8% have MSI in Asian studies (Watanabe et al, 2001; Ribic et al, 2003; Kim et al, 2010; Yoon et al, 2011; Zaanan et al, 2011; Hong et al, 2012). Mucinous adenocarcinoma is frequently associated with MSI (Leopoldo et al, 2008; Langner et al, 2012). In terms of prognosis, MSI is generally associated with favourable outcome in colorectal cancer (Popat et al, 2005; Sargent et al, 2010; Zaanan et al, 2011). However, we could not find significant role for MSI. This study was underpowered to detect significant difference of DFS according to MSI status, considering the low frequency of MSI-H in Korean patients (6.8% in this study compared with 10–20% in Western studies) and limited number of events in the entire cohort (3-year DFS of 84.9%). Better outcome of MSI compared with MSS is also observed among patients with MAC (Kakar et al, 2004; Leopoldo et al, 2008). In the present study, MSI was more frequently found in MAC compared with NMA. Moreover, AIM showed similar frequency of MSI with MAC. However, we could not examine the prognostic value of MSI among MAC or AIM, because of the small number of such patients included.
In addition to MSI, MAC is frequently associated with CpG island methylation phenotype (CIMP) and BRAF mutation (Ogino et al, 2006; Kakar et al, 2012). Among the present patient cohort, we had CIMP and BRAF mutation data available in 322 and 269 patients, respectively, which had been reported previously (Han et al, 2013). The frequency of high CIMP in AIM (25%) was similar to MAC (35.3%), which was significantly higher than that in NMA (4.6%). BRAF mutation was found in 5.3% (1/19 patients) of AIM, 6.7% (1/15) of MAC, and 2.1% (5/235) of NMA. The number of BRAF mutations was too small to make statistical comparisons. Even though BRAF mutation has been associated with poor prognosis in colorectal cancer (Pai et al, 2012; Phipps et al, 2012; Safaee Ardekani et al, 2012), it cannot explain the poor prognosis of MAC in the present study, considering its low frequency.
In the present patient cohort, we found that AIM has similar features with MAC in terms of tumour location, angiolymphatic invasion, MSI status, CIMP status, and BRAF mutation. In contrast, AIM showed differences compared with MAC in regard to patient age, sex, and T stage. Most importantly, the prognosis of AIM was significantly better than MAC but comparable to NMA. Although MAC had higher percentage of T4 stage, MAC was independently associated with poor prognosis in the multivariate analysis in which T4 stage was also included as a covariate. Although we could not find differences in the molecular markers tested between AIM and MAC, it is highly likely that AIM and MAC are distinct disease entities, considering the differences in prognosis and clinicopathological characteristics. Only few prior studies have focused on comparing AIM and MAC (Ogino et al, 2006; Langner et al, 2012). Differences were observed in frequencies of MGMT loss, p16 loss, and KRAS mutation according to the degree of mucinous component (Ogino et al, 2006). However, we could not explain the poor DFS of MAC compared with AIM and NMA in the present study with those differences. Genetic or epigenetic mechanism leading to the two mucinous phenotypes, AIM and MAC, and poor prognosis of MAC should be investigated in future studies.
The major limitation of this study is that only patients treated with adjuvant FOLFOX are included. Therefore, we cannot answer whether the higher recurrence of MAC is due to its innate aggressive biology or its resistance to adjuvant chemotherapy. Nevertheless, this is the first study to evaluate the prognostic implication of mucinous histology in colorectal cancer patients treated with adjuvant FOLFOX. The study cohort was relatively homogeneous in terms of stage (only including high-risk stage II and stage III) and treatment (surgery and single chemotherapy regimen at a high-volume single institution). Our findings need further validation in an independent cohort. Another limitation is the relatively short duration of follow-up (median 38 months). We could not evaluate the impact of mucinous histology on overall survival (OS) because only limited number of events (15 deaths) was recorded. However, the median follow-up duration had passed 3 years, DFS at which point has been shown to have good correlation with 5-year OS in colon cancer (Sargent et al, 2005). Further analysis including OS will be performed after longer follow-up in the future.
In conclusion, AIM and MAC have distinct clinicopathological features compared with NMA. Only MAC, but not AIM, has an adverse prognostic impact on stage II or III colorectal cancer treated with adjuvant FOLFOX compared with NMA.
Acknowledgments
This work was supported by a grant from the Korean Healthcare Technology R&D project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A091081).
The authors declared no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- Bosman FT.2010WHO classification of tumours of the digestive system4th edn.IARC: Lyon [Google Scholar]
- Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- Catalano V, Loupakis F, Graziano F, Bisonni R, Torresi U, Vincenzi B, Mari D, Giordani P, Alessandroni P, Salvatore L, Fornaro L, Santini D, Baldelli AM, Rossi D, Giustini L, Silva RR, Falcone A, D'Emidio S, Rocchi M, Luzi Fedeli S. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann Oncol. 2012;23:135–141. doi: 10.1093/annonc/mdr062. [DOI] [PubMed] [Google Scholar]
- Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R, Mari D, Fornaro L, Baldelli AM, Giordani P, Rossi D, Alessandroni P, Giustini L, Silva RR, Falcone A, D'Emidio S, Fedeli SL. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881–887. doi: 10.1038/sj.bjc.6604955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Mah JT, Lee J, Sankila R, Sankaranarayanan R, Chia KS. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum. 2004;47:78–85. doi: 10.1007/s10350-003-0014-9. [DOI] [PubMed] [Google Scholar]
- Farhat MH, Barada KA, Tawil AN, Itani DM, Hatoum HA, Shamseddine AI. Effect of mucin production on survival in colorectal cancer: a case-control study. World J Gastroenterol. 2008;14:6981–6985. doi: 10.3748/wjg.14.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow SC, Yu J, Carvalho LP, Shannon WD, Fleshman JW, McLeod HL. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer. 2005;92:259–264. doi: 10.1038/sj.bjc.6602330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JB, Timmcke AE, Mitchell WT, Hicks TC, Gathright JB, Jr., Ray JE. Mucinous carcinoma--just another colon cancer. Dis Colon Rectum. 1993;36:49–54. doi: 10.1007/BF02050301. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SW, Lee HJ, Bae JM, Cho NY, Lee KH, Kim TY, Oh DY, Im SA, Bang YJ, Jeong SY, Park KJ, Park JG, Kang GH. Methylation and microsatellite status and recurrence following adjuvant FOLFOX in colorectal cancer. Int J Cancer. 2013;132:2209–2216. doi: 10.1002/ijc.27888. [DOI] [PubMed] [Google Scholar]
- Hong SP, Min BS, Kim TI, Cheon JH, Kim NK, Kim H, Kim WH. The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. Eur J Cancer. 2012;48:1235–1243. doi: 10.1016/j.ejca.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kakar S, Aksoy S, Burgart LJ, Smyrk TC. Mucinous carcinoma of the colon: correlation of loss of mismatch repair enzymes with clinicopathologic features and survival. Mod Pathol. 2004;17:696–700. doi: 10.1038/modpathol.3800093. [DOI] [PubMed] [Google Scholar]
- Kakar S, Deng G, Smyrk TC, Cun L, Sahai V, Kim YS. Loss of heterozygosity, aberrant methylation, BRAF mutation and KRAS mutation in colorectal signet ring cell carcinoma. Mod Pathol. 2012;25:1040–1047. doi: 10.1038/modpathol.2012.44. [DOI] [PubMed] [Google Scholar]
- Kanemitsu Y, Kato T, Hirai T, Yasui K, Morimoto T, Shimizu Y, Kodera Y, Yamamura Y. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum. 2003;46:160–167. doi: 10.1007/s10350-004-6518-0. [DOI] [PubMed] [Google Scholar]
- Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161–1168. doi: 10.1007/s10350-004-0932-1. [DOI] [PubMed] [Google Scholar]
- Kim ST, Lee J, Park SH, Park JO, Lim HY, Kang WK, Kim JY, Kim YH, Chang DK, Rhee PL, Kim DS, Yun H, Cho YB, Kim HC, Yun SH, Lee WY, Chun HK, Park YS. Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother Pharmacol. 2010;66:659–667. doi: 10.1007/s00280-009-1206-3. [DOI] [PubMed] [Google Scholar]
- Langner C, Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Schlemmer A, Vieth M, Rehak P. Mucinous differentiation in colorectal cancer--indicator of poor prognosis. Histopathology. 2012;60:1060–1072. doi: 10.1111/j.1365-2559.2011.04155.x. [DOI] [PubMed] [Google Scholar]
- Lee WS, Chun HK, Lee WY, Yun SH, Cho YB, Yun HR, Park SH, Song SY. Treatment outcomes in patients with signet ring cell carcinoma of the colorectum. Am J Surg. 2007;194:294–298. doi: 10.1016/j.amjsurg.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Leopoldo S, Lorena B, Cinzia A, Gabriella DC, Angela Luciana B, Renato C, Antonio M, Carlo S, Cristina P, Stefano C, Maurizio T, Luigi R, Cesare B. Two subtypes of mucinous adenocarcinoma of the colorectum: clinicopathological and genetic features. Ann Surg Oncol. 2008;15:1429–1439. doi: 10.1245/s10434-007-9757-1. [DOI] [PubMed] [Google Scholar]
- Leteurtre E, Gouyer V, Rousseau K, Moreau O, Barbat A, Swallow D, Huet G, Lesuffleur T. Differential mucin expression in colon carcinoma HT-29 clones with variable resistance to 5-fluorouracil and methotrexate. Biol Cell. 2004;96:145–151. doi: 10.1016/j.biolcel.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol. 2005;16:1305–1310. doi: 10.1093/annonc/mdi244. [DOI] [PubMed] [Google Scholar]
- Ogino S, Brahmandam M, Cantor M, Namgyal C, Kawasaki T, Kirkner G, Meyerhardt JA, Loda M, Fuchs CS. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- Pai RK, Jayachandran P, Koong AC, Chang DT, Kwok S, Ma L, Arber DA, Balise RR, Tubbs RR, Shadrach B. BRAF-mutated, microsatellite-stable adenocarcinoma of the proximal colon: an aggressive adenocarcinoma with poor survival, mucinous differentiation, and adverse morphologic features. Am J Surg Pathol. 2012;36:744–752. doi: 10.1097/PAS.0b013e31824430d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos VN, Michalopoulos A, Netta S, Basdanis G, Paramythiotis D, Zatagias A, Berovalis P, Harlaftis N. Prognostic significance of mucinous component in colorectal carcinoma. Tech Coloproctol. 2004;8 (Suppl 1:s123–s125. doi: 10.1007/s10151-004-0131-z. [DOI] [PubMed] [Google Scholar]
- Phipps AI, Buchanan DD, Makar KW, Burnett-Hartman AN, Coghill AE, Passarelli MN, Baron JA, Ahnen DJ, Win AK, Potter JD, Newcomb PA. BRAF mutation status and survival after colorectal cancer diagnosis according to patient and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2012;21:1792–1798. doi: 10.1158/1055-9965.EPI-12-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G. The prognostic value of braf mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One. 2012;7:e47054. doi: 10.1371/journal.pone.0047054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, Ribic C, Grothey A, Moore M, Zaniboni A, Seitz JF, Sinicrope F, Gallinger S. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O'Callaghan CJ, Francini G, Grothey A, O'Connell M, Catalano PJ, Blanke CD, Kerr D, Green E, Wolmark N, Andre T, Goldberg RM, De Gramont A. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci. 2010;35:236–245. doi: 10.1016/j.tibs.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GA, Deng G, Bell I, Kakar S, Sleisenger MH, Kim YS. Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int J Oncol. 2005;26:745–750. [PubMed] [Google Scholar]
- Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381–388. doi: 10.1136/jclinpath-2011-200340. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB, 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Tung SY, Chen PC, Kuo YC. Clinicopathological study of colorectal mucinous carcinoma in Taiwan: a multivariate analysis. J Gastroenterol Hepatol. 1996;11:77–81. doi: 10.1111/j.1440-1746.1996.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Xie L, Villeneuve PJ, Shaw A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int J Oncol. 2009;34:1109–1115. doi: 10.3892/ijo_00000238. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Yu CS, Kim TW, Kim JH, Jang SJ, Cho DH, Roh SA, Kim JC. Mismatch repair status in sporadic colorectal cancer: immunohistochemistry and microsatellite instability analyses. J Gastroenterol Hepatol. 2011;26:1733–1739. doi: 10.1111/j.1440-1746.2011.06784.x. [DOI] [PubMed] [Google Scholar]
- Younes M, Katikaneni PR, Lechago J. The value of the preoperative mucosal biopsy in the diagnosis of colorectal mucinous adenocarcinoma. Cancer. 1993;72:3588–3592. doi: 10.1002/1097-0142(19931215)72:12<3588::aid-cncr2820721207>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Zaanan A, Flejou JF, Emile JF, Des GG, Cuilliere-Dartigues P, Malka D, Lecaille C, Validire P, Louvet C, Rougier P, de Gramont A, Bonnetain F, Praz F, Taieb J. Defective mismatch repair status as a prognostic biomarker of disease-free survival in stage III colon cancer patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer Res. 2011;17:7470–7478. doi: 10.1158/1078-0432.CCR-11-1048. [DOI] [PubMed] [Google Scholar]