Abstract
Objectives
The aims of this piece of work were to: 1) record the background concentrations of blood chromium (Cr) and cobalt (Co) concentrations in a large group of subjects; 2) to compare blood/serum Cr and Co concentrations with retrieved metal-on-metal (MoM) hip resurfacings; 3) to examine the distribution of Co and Cr in the serum and whole blood of patients with MoM hip arthroplasties; and 4) to further understand the partitioning of metal ions between the serum and whole blood fractions.
Methods
A total of 3042 blood samples donated to the local transfusion centre were analysed to record Co and Cr concentrations. Also, 91 hip resurfacing devices from patients who had given pre-revision blood/serum samples for metal ion analysis underwent volumetric wear assessment using a coordinate measuring machine. Linear regression analysis was carried out and receiver operating characteristic curves were constructed to assess the reliability of metal ions to identify abnormally wearing implants. The relationship between serum and whole blood concentrations of Cr and Co in 1048 patients was analysed using Bland-Altman charts. This relationship was further investigated in an in vitro study during which human blood was spiked with trivalent and hexavalent Cr, the serum then separated and the fractions analysed.
Results
Only one patient in the transfusion group was found to have a blood Co > 2 µg/l. Blood/Serum Cr and Co concentrations were reliable indicators of abnormal wear. Blood Co appeared to be the most useful clinical test, with a concentration of 4.5 µg/l showing sensitivity and specificity for the detection of abnormal wear of 94% and 95%, respectively. Generated metal ions tended to fill the serum compartment preferentially in vivo and this was replicated in the in vitro study when blood was spiked with trivalent Cr and bivalent Co.
Conclusions
Blood/serum metal ion concentrations are reliable indicators of abnormal wear processes. Important differences exist however between elements and the blood fraction under study. Future guidelines must take these differences into account.
Keywords: Metal-on-metal, Chromium, Cobalt, Arthroplasty, Hip resurfacing, ARMD, Volumetric wear, Metal ions
Article focus
Documentation of blood metal ion concentrations in a large group of healthy volunteers
The relationship between serum and whole blood concentrations of chromium (Cr) and cobalt (Co) and volumetric wear of hip resurfacing components
A description and investigation of the distribution of Cr and Co ions in the serum and whole blood fractions of patients with metal-on-metal (MoM) hip arthroplasty
Key messages
There is a small variation in metal ion concentrations in the background population relative to the concentrations produced by MoM arthroplasty of the hip
Cr and Co concentrations in the serum and whole blood fractions are closely related to the wear rates of explanted prostheses
Co appears to be a more reliable indicator of abnormally wearing prostheses
Strengths and limitations
Most of the results have been drawn from a single area of the United Kingdom. It is unclear whether the results can be extrapolated to areas further afield
There was a relatively small number of ‘normally wearing’ prostheses that had failed
Introduction
Adverse reaction to metal debris (ARMD) is a term used to describe a range of local pathologies seen in association with metal-on-metal (MoM) hips that include soft-tissue necrosis, large sterile joint effusions, metal staining of tissues, pseudotumours and osteolysis.1,2 Rates of ARMD-related failure of up to 49% have been reported at six years,3 but others have reported failure rates as low as 0.10%.4
Although the extent of soft-tissue lesions does not appear to be dose-related to metal debris exposure,2 there is accumulating evidence to show that such reactions are far more likely to develop if a MoM prosthesis is wearing at a greater rate than expected.2,5 There is currently no convincing evidence in the literature documenting a severe tissue reaction to a well-functioning (in tribological terms) MoM prosthesis.
At present there is no consensus as to what blood concentrations are indicative of an abnormally wearing metal hip. Nor is there consensus as to the interchangeability of serum and whole blood results for the detection of abnormal wear, or even whether chromium (Cr) or cobalt (Co) is the most useful element to measure. This has led to confusion among surgeons and patients. Normal background concentrations of Cr and Co have not even been documented in a large sample size of patients using modern analytical techniques. Furthermore, while the long-term effects of systemic exposure to high concentrations of Cr3+ and Co2+ metal ions are unknown, Cr6+ (hexavalent chromium) is a proven carcinogen,6 and it is also unknown whether or not MoM joints generate this species of Cr.
The purposes of this study were: 1) to record background concentrations of blood Cr and Co concentrations measured using the latest techniques in a large number of healthy subjects; 2) to compare blood metal ion concentrations with the measured volumetric wear of retrieved prostheses in order to identify levels indicative of poorly functioning hip resurfacings; 3) to examine the distribution of Co and Cr in serum and whole blood of patients with MoM hip arthroplasties; and 4) to further understand the partitioning of Cr ions between the serum and whole blood fractions and investigate the potential release of hexavalent Cr.
Materials and Methods
Background concentrations of Co and Cr in a healthy population
A study was begun in 2007 to analyse the background environmental exposure to various heavy metals in the North of England, which has reported to the sponsor but not published data as of yet. We obtained the Cr and Co blood results from this study to use as a reference point to compare with those obtained from patients with MoM joints. Blood samples were taken from a random sample of informed volunteers from the National Blood Service (NBS), which is part of the NHS Blood and Transplant (NHSBT) service. The population under study was from the areas of Northumberland, Tyne & Wear, Cumbria and Durham. The total population is approximately 2.5 million people (2001 census data7), although only a small proportion of the population donate blood (estimated to be around 4% by the NBS8). The study population consisted of subjects aged between 17 and 70 years who had passed the screening health protocols for the NBS. Pregnant women and those with children up to nine months old were excluded, as were transient populations. It was not known whether individuals had metallic implants at the time of venesection. When a blood donation was taken, the first 8 ml to 12 ml were diverted into a sample pouch and then in to two BD Vacutainer Plus Blood Collection Tubes (Beckton Dickson, Franklin Lakes, New Jersey) containing K2EDTA (ethylenediaminetetraacetic acid). Cr and Co analysis was performed using inductively coupled plasma mass spectroscopy (ICPMS) (X-Series II; Thermo Electron Corporation, Bremen, Germany) with Collision Cell Technology (CCT) using 7.5% (v/v) hydrogen (H2) in helium (He) as the collision gas, with in-sample switching between CCT and normal modes. ICPMS is currently accepted to be the preferred mode of blood metal ion measurement.9 Limits of detection (LoD) were 0.63 µg/l and 0.11 µg/l for Cr and Co, respectively. These analyses were carried out at a centre that participates in the Trace Element Quality Assurance Scheme (TEQAS).10 This scheme is a collaboration between seven centres in the United Kingdom that perform trace element analysis using the same techniques and regularly monitor results between units to ensure reproducibility.10 Additionally the laboratory takes part in the Quebec Multielement External Quality Assessment Scheme (QMEQAS).11 Both include Cr and Co and performance in these schemes is consistently within the acceptable ranges quoted. Ethical approval was sought and given through the National Research Ethics Service (NRES), part of the National Patient Safety Agency (ref: 07/Q0901/20).
How do metal ion concentrations relate to volumetric wear of retrieved MoM hip resurfacing prostheses?
In 2008 a prospective study was commenced at the School of Mechanical and Systems Engineering at Newcastle University to analyse failed MoM hip prostheses. The study was conducted after approval from the Local Research Ethics Committee of County Durham and Tees Valley. All mated (head and acetabulum) components retrieved from patients between 2008 and 2012 with failed unilateral hip resurfacings and pre-revision surgery whole blood samples were included in this analysis. Blood samples were taken using an intravenous catheter (Insyte-WTM; Becton Dickinson). After the catheter had been introduced, the metal needle was removed in order to avoid contamination from the needle. A second 5 ml were collected using a Venflon vacuum tube. Blood was placed into EDTA tubes. Concentrations of Cr and Co were determined using ICPMS at the Biochemistry Department, Royal Surrey County Hospital. The quantification limits for both elements were less than 0.2 μg/l and the within assay reproducibility was 2% at a concentration of 8 μg/l. This laboratory demonstrates excellent accuracy in international trace elements external quality assessment schemes and is one of the participants of the United Kingdom TEQAS. Exclusion criteria were: the presence of other metallic implants; loose CoCr backed components; gross immobility at the time of the blood test (University of California, Los Angeles (UCLA) activity score12 of 1) and or abnormal renal function tests (blood urea nitrogen and creatinine).
The bearing surfaces of the retrieved components underwent volumetric wear assessment using a Legex 322 coordinate measuring machine (CMM; Mitutoyo, Tokyo, Japan). The CMM has an accuracy of 0.8 µm. Between 4000 and 7000 points were taken from each component and the total material loss in volumetric terms was calculated using a dedicated Matlab program (Mathworks, Natick, Massachusetts) that has been validated previously.13
The relationship between metal ion concentrations and bearing surface wear rates was examined using linear regression after the data had been log normalised. Receiver operating characteristic (ROC) curves were constructed to assess the sensitivity and specificity of different blood fractions and different metals to detect abnormal wear. ‘Abnormal wear’ was defined for the purposes of this paper after consideration of two factors:
1. Heisel et al14 showed that the volumetric loss during the ‘running in’ phase of hip resurfacings is approximately 1.5 mm3. Thereafter, wear rates are believed to decelerate and it is generally accepted that a well-functioning MoM bearing surface should produce < 1 mm3 of volumetric wear per year once the steady state is achieved.15 Our own analysis of the wear of explanted large-diameter MoM joints showed that this is a reasonable assumption.3
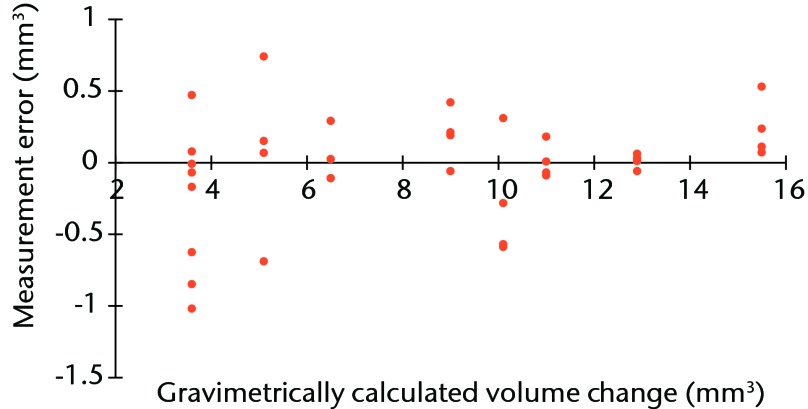
2. In addition to our previous published examination of the accuracy of the method of volumetric wear assessment, we performed a further 30 gravimetric tests on a resurfacing head and acetabular component as we had further enhanced our scanning techniques since the previous publication. This showed that the median error was +0.034 mm3 per component and the limits of the measurement data were +0.530 to -0.283 (Fig. 1). The limits were calculated in a standard way for non-parametric data as follows: Lower limit of data (maximum under measurement) = quartile (Q) 1 – 1.5(Q3 – Q1); Upper limit (maximum over measurement) = Q3 + 1.5(Q3 – Q1).
Fig. 1.
Plot showing the measurement error of our volumetric wear assessment compared with the gold-standard gravimetric test.
Taking into account the above factors, and given the variation in patient mobility (patients with higher activity levels may subject their joints to 6 million cycles per year16), we conducted several ROC analyses defining an ‘abnormal wear’ rate first at a value of 2.0 mm3/year for the steady state phase (comfortably above what is generally considered normal) and then 3.0 mm3/year (a figure based on our previous work13) in an attempt to accommodate patients with high activity levels. We conducted further analyses in order to determine the effects of maximum over and under estimates of wear to give worst outcome sensitivity and specificity values.
The relationship between Cr and Co concentrations in serum and whole blood samples of patients with MoM arthroplasty of the hip
Metal ion analysis of whole blood and serum samples is carried out as part of routine follow-up of patients with MoM hip implants at the University Hospital of North Tees. We collected all available corresponding serum/whole blood samples taken from patients with MoM joints at the hospital up to 2011. Patients had been implanted with Articular Surface Replacement hip resurfacings (ASR; DePuy, Leeds, United Kingdom), Birmingham Hip Resurfacings (BHR; Smith and Nephew, Warwick, United Kingdom), the ASR XL total hip replacement (THR) (DePuy) or the Pinnacle MoM THR system (DePuy). All these devices are manufactured from a similar high-carbon CoCr alloy. Blood samples were collected as previously described. No patients were excluded.
Bland-Altman plots were constructed to assess the agreement between serum and whole blood concentrations of Cr and Co. The calculated difference between serum and whole blood concentrations was plotted for both Co and Cr. The ratio of serum to whole blood Cr concentration was then plotted against the mean of the two values, as the variability between the calculated differences was found to increase as ion concentrations increased. Finally, the ratio of serum to whole blood in Cr was then plotted against whole blood Cr in order to examine the partitioning of Cr between the two fractions as the overall Cr concentration increased. All the above tests were conducted initially with the samples from patients with hip resurfacings separate from those with THRs in order to identify differences in the metal ion generation between the two arthroplasty systems.
In vitro study of Cr species distribution in human blood
Blood was collected from a healthy adult volunteer into a container with EDTA as anticoagulant and also into a plain container. The anti-coagulated blood was divided into a series of portions and each was spiked with solutions of Cr3+, Cr6+ or Co2+, so as to increase the concentration by 0, 2, 5, 10 or 40 μg/l. These samples were further divided into four aliquots that were separated into plasma and washed red blood cells (RBCs) after 45 minutes and four, 24, and 48 hours at room temperature in order to investigate the effect of time of sample collection to sample analysis. The non-anti-coagulated blood was immediately divided into portions, similarly spiked with Cr3+, Cr6+, or Co2+, allowed to clot and the serum separated. The concentrations of Cr and Co were measured in RBCs and serum by ICPMS. The blood was collected as a requirement for the trace elements external quality assessment scheme organised by the Biochemistry Department, Royal Surrey County Hospital,10 and had approval from the Surrey Research Ethics Committee.
Statistical analysis
Windows SPSS v15.0 (SPSS Inc., Chicago, Illinois) was used for statistical analysis throughout and XLSTAT (Addinsoft, New York, New York) was used for graphical representation. A p-value < 0.05 was considered to denote statistical significance.
Results
Background metal ion concentrations
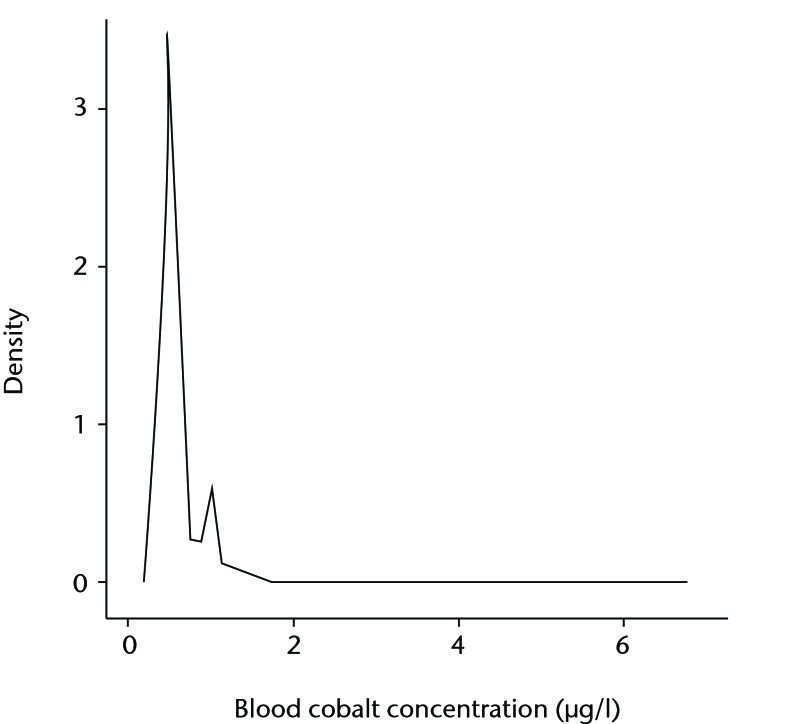
A total of 3042 patients gave samples. There were 1527 males and 1515 females with a mean age of 45 years (16 to 72). The median Cr concentration was 1.5 µg/l (0.6 (below detection limit) to 8.6), and for Co was 0.5 µg/l (0.3 to 6.7). The Shapiro-Wilk test for normality showed that neither Cr nor Co was normally distributed (p = 0.001 and p = 0.002, respectively). A total of 98 patients (3.22%) were found to have blood Cr concentrations > 2 µg/l, whereas only one patient (0.033%) was found to have a blood Co concentration > 2 µg/l. The majority of patients (2831 of 3042, 93.1%) had a Co < 1 µg/l. Figure 2 shows the distribution of Co concentrations. When patients were sub-divided by gender and age, median concentrations of Cr and Co in the various sub groups varied by no more than 0.1 µg/l.
Fig. 2.
Graph showing the distribution of whole blood cobalt concentrations in 3042 healthy volunteers.
How do metal ion concentrations relate to wear of MoM prostheses?
In total, there were 91 retrieved resurfacings with corresponding pre-revision blood Cr and Co values. Of these, 13 patients did not have corresponding serum values, as the patients had been referred from hospitals where the serum fraction was not routinely tested. The indication for revision was ARMD in 82 hips (90.1%), loose titanium-backed acetabular components in two (2.2%), avascular necrosis (AVN) in two (2.2%), pain with an unknown cause in two (2.2%), and one case each of infection, painful impingement and uncomplicated femoral fracture (Table I).
Table I.
Explant patient demographics
| Characteristic | |
|---|---|
| Implants (n) | 91 |
| Mean age (yrs) (range) | 54 (29 to 68) |
| Male:female (n) | 38:53 |
| Mean time to revision (mths) (range) | 54 (8 to 108) |
| Device (n, %)* | |
| ASR | 73 (80.2) |
| BHR | 12 (13.2) |
| Durom | 3 (3.3) |
| Conserve Plus | 2 (2.2) |
| Cormet | 1 (1.1) |
| Reason for revision (n, %) | |
| ARMD† | 82 (90.1) |
| Avascular necrosis | 2 (2.2) |
| Loose acetabular component | 2 (2.2) |
| Unexplained pain | 2 (2.2) |
| Painful impingement | 1 (1.1) |
| Infection | 1 (1.1) |
| Uncomplicated femoral fracture | 1 (1.1) |
| Mean blood concentration (µg/l) (range) | |
| Chromium | 14.7 (1.24 to 123.2) |
| Cobalt | 16.0 (0.63 to 271.0) |
| Mean serum concentration (µg/l) (range)‡ | |
| Chromium | 17.9 (1.9 to 186.7) |
| Cobalt | 15.2 (0.96 to 235.4) |
| Mean volumetric bearing surface wear rate (mm3/yr) (range) | 7.35 (0.50 to 138.1) |
* ASR, Articulating Surface Replacement; BHR, Birmingham Hip Resurfacing † ARMD, adverse reaction to metal debris ‡ serum concentration values were only available for 78 patients
Neither volumetric wear rates nor blood ion concentrations were normally distributed (p < 0.001, Shapiro-Wilk test).
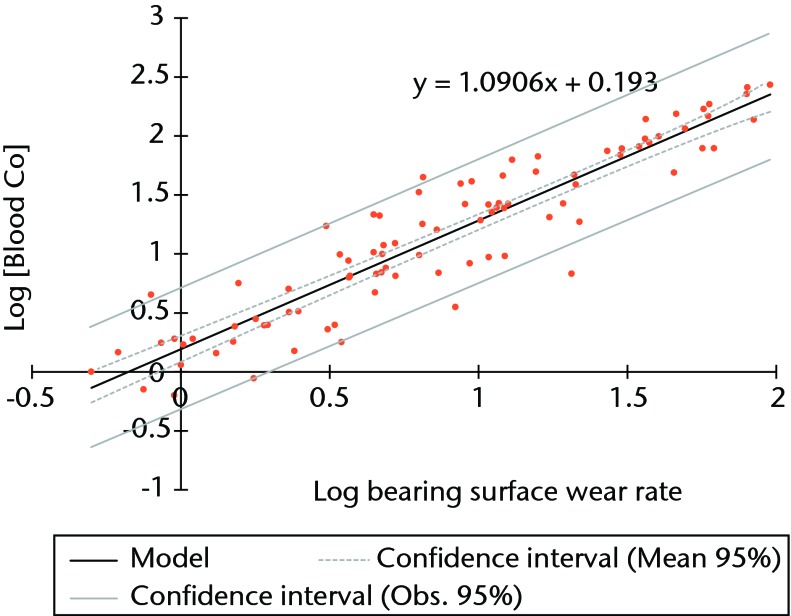
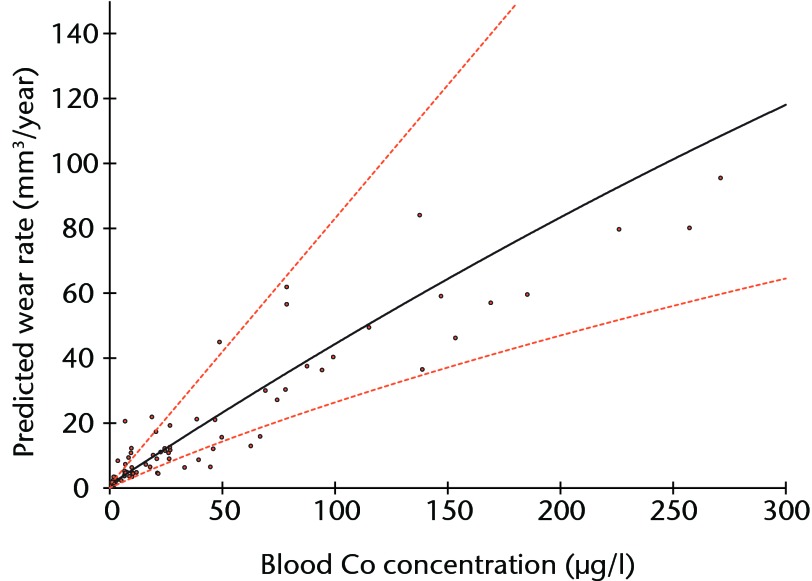
Linear regression using logged values of bearing surface wear rates as the independent variable and logged blood/serum concentrations as the dependent variables returned adjusted R2 values of 0.855 for blood Co (p < 0.001) (Fig. 3), 0.756 for blood Cr (p < 0.001), 0.813 for serum Co (p < 0.001) and 0.785 for serum Cr (p < 0.001). The equation of the best-fit line was used to normalise the logged values in order to translate the results into real clinical values. The results of the Co calculations are illustrated in Figure 4 and reported in Table II.
Fig. 3.
Graph showing the linear regression of blood cobalt (Co) concentration and rates of surface wear (using logged values).
Fig. 4.
Graph showing the prediction of wear rates for given whole blood concentrations of cobalt (Co), using the equation derived from the regression model (Fig. 3) with values normalised to provide clinically relevant figures (Table II). The broken lines represent the 95% confidence interval.
Table II.
Relevant clinical values of blood cobalt (Co) and their relationship to the volumetric bearing surface wear rate of the metal-on-metal joint (CI, confidence interval)
| Whole blood Co (µg/l) | 95% CI rate of wear (mm3/year) |
|---|---|
| 0.5 | 0.47 to 0.64 |
| 1 | 0.77 to 1.16 |
| 2 | 1.26 to 2.10 |
| 3 | 1.68 to 2.96 |
| 4 | 2.07 to 3.79 |
| 5 | 2.43 to 4.58 |
| 10 | 4.00 to 8.28 |
| 15 | 5.35 to 11.7 |
| 20 | 6.58 to 14.9 |
| 30 | 8.80 to 21.1 |
| 40 | 10.8 to 27.01 |
| 50 | 12.7 to 32.7 |
| 100 | 20.9 to 59.0 |
| 150 | 28.0 to 83.4 |
| 200 | 34.4 to 107 |
| 250 | 40.3 to 129 |
| 300 | 46.0 to 151 |
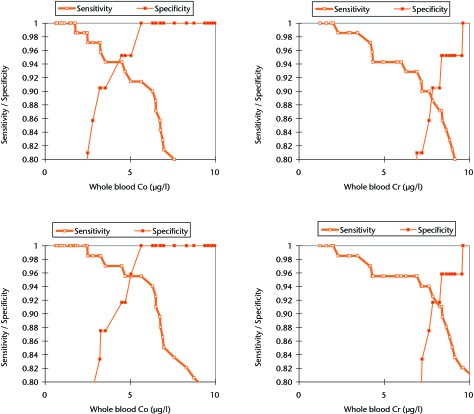
ROC analysis showed whole blood Co to have the most clinically appropriate sensitivity and specificity for the detection of abnormal wear. A whole blood Co concentration of ≥ 4.5 µg/l appeared to be the most reliable clinical threshold value after the consideration of the sensitivity and specificity as well as the confidence limits. Whole blood Co ≥ 4.5 µg/l had a sensitivity of 90.4% (95% confidence interval (CI) 81.1 to 95.5) and specificity of 94.4% (95% CI 72.0 to 100.0) for detecting abnormal wear of ≥ 2.0 mm3 per year (Fig. 5, Table III). When ‘abnormal wear’ was defined as that ≥ 3 mm3 per year, clinically the most useful threshold level appeared to be a blood Co concentration of 5.04 µg/l. This value showed a sensitivity of 92.8% (95% CI 83.7 to 97.2) and specificity of 95.5% (95% CI 76.2 to 100.0) (Table IV).
Fig. 5.
Graphs showing the sensitivity and specificity of whole blood cobalt (Co) and chromium (Cr) to identify abnormal wear defined as ≥ 2 mm3/year (top row) and ≥ 3 mm3/year (bottom row).
Table III.
Sensitivity and specificity of whole blood cobalt (Co) and chromium (Cr) for identifying abnormal wear defined as a wear rate ≥ 2 mm3 per year. The area-under-curve (AUC) value for Co was 0.971 (95% confidence interval (CI) 0.965 to 0.977) and 0.959 (95% CI 0.935 to 0.982) for Cr. The most clinically useful values after consideration of sensitivity and specificity are bolded (PPV, positive predictive value; NPV, negative predictive value)
| Whole blood (µg/l) | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV |
|---|---|---|---|---|
| COBALT | ||||
| 1.900 | 0.973 (0.898 to 0.998) | 0.667 (0.435 to 0.837) | 0.922 | 0.857 |
| 2.290 | 0.959 (0.880 to 0.990) | 0.667 (0.435 to 0.837) | 0.921 | 0.800 |
| 2.420 | 0.959 (0.880 to 0.990) | 0.722 (0.487 to 0.876) | 0.933 | 0.813 |
| 2.470 | 0.959 (0.880 to 0.990) | 0.778 (0.541 to 0.913) | 0.946 | 0.824 |
| 2.500 | 0.945 (0.862 to 0.982) | 0.833 (0.598 to 0.948) | 0.958 | 0.789 |
| 2.800 | 0.945 (0.862 to 0.982) | 0.889 (0.657 to 0.979) | 0.972 | 0.800 |
| 3.210 | 0.932 (0.845 to 0.973) | 0.889 (0.657 to 0.979) | 0.971 | 0.762 |
| 3.260 | 0.918 (0.828 to 0.964) | 0.889 (0.657 to 0.979) | 0.971 | 0.727 |
| 3.540 | 0.904 (0.811 to 0.955) | 0.889 (0.657 to 0.979) | 0.971 | 0.696 |
| 4.500 | 0.904 (0.811 to 0.955) | 0.944 (0.720 to 1.000) | 0.985 | 0.708 |
| 4.700 | 0.890 (0.795 to 0.945) | 0.944 (0.720 to 1.000) | 0.985 | 0.680 |
| CHROMIUM | ||||
| 5.720 | 0.945 (0.862 to 0.982) | 0.722 (0.487 to 0.876) | 0.932 | 0.765 |
| 5.800 | 0.945 (0.862 to 0.982) | 0.778 (0.541 to 0.913) | 0.945 | 0.778 |
| 6.000 | 0.945 (0.862 to 0.982) | 0.833 (0.598 to 0.948) | 0.958 | 0.789 |
| 6.280 | 0.932 (0.845 to 0.973) | 0.833 (0.598 to 0.948) | 0.958 | 0.750 |
| 6.860 | 0.932 (0.845 to 0.973) | 0.889 (0.657 to 0.979) | 0.971 | 0.762 |
| 6.920 | 0.918 (0.828 to 0.964) | 0.889 (0.657 to 0.979) | 0.971 | 0.727 |
| 7.180 | 0.904 (0.811 to 0.955) | 0.889 (0.657 to 0.979) | 0.971 | 0.696 |
| 7.220 | 0.890 (0.795 to 0.945) | 0.889 (0.657 to 0.979) | 0.970 | 0.667 |
| 7.630 | 0.877 (0.779 to 0.935) | 0.889 (0.657 to 0.979) | 0.970 | 0.640 |
| 7.850 | 0.863 (0.763 to 0.925) | 0.944 (0.720 to 1.000) | 0.984 | 0.630 |
| 8.250 | 0.849 (0.747 to 0.915) | 0.944 (0.720 to 1.000) | 0.984 | 0.607 |
| 8.370 | 0.849 (0.747 to 0.915) | 1.000 (0.789 to 1.000) | 1.000 | 0.621 |
| 8.420 | 0.836 (0.732 to 0.904) | 1.000 (0.789 to 1.000) | 1.000 | 0.600 |
Table IV.
Sensitivity and specificity of whole blood cobalt (Co) and chromium (Cr) for identifying abnormal wear defined as a wear rate ≥ 3 mm3 per year. The area-under-curve (AUC) value for Co was 0.979 (95% confidence interval (CI) 0.968 to 0.989) and 0.958 (95% CI 0.930 to 0.980) for Cr. The most clinically useful values after consideration of sensitivity and specificity are bolded (PPV, positive predictive value; NPV, negative predictive value)
| Whole blood (µg/l) | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV |
|---|---|---|---|---|
| COBALT | ||||
| 2.500 | 0.957 (0.874 to 0.989) | 0.727 (0.515 to 0.870) | 0.917 | 0.842 |
| 2.800 | 0.957 (0.874 to 0.989) | 0.773 (0.560 to 0.901) | 0.930 | 0.850 |
| 3.210 | 0.957 (0.874 to 0.989) | 0.818 (0.607 to 0.931) | 0.943 | 0.857 |
| 3.260 | 0.957 (0.874 to 0.989) | 0.684 (0.656 to 0.959) | 0.957 | 0.964 |
| 3.540 | 0.942 (0.855 to 0.981) | 0.684 (0.656 to 0.959) | 0.956 | 0.826 |
| 4.500 | 0.942 (0.855 to 0.981) | 0.909 (0.707 to 0.985) | 0.970 | 0.833 |
| 4.700 | 0.928 (0.837 to 0.972) | 0.909 (0.707 to 0.985) | 0.970 | 0.800 |
| 5.040 | 0.928 (0.837 to 0.972) | 0.955 (0.762 to 1.000) | 0.985 | 0.808 |
| 5.640 | 0.928 (0.837 to 0.972) | 1.000 (0.821 to 1.000) | 1.000 | 0.815 |
| 6.310 | 0.913 (0.819 to 0.962) | 1.000 (0.821 to 1.000) | 1.000 | 0.786 |
| CHROMIUM | ||||
| 6.000 | 0.957 (0.874 to 0.989) | 0.727 (0.515 to 0.870) | 0.917 | 0.842 |
| 6.280 | 0.957 (0.874 to 0.989) | 0.773 (0.560 to 0.901) | 0.930 | 0.850 |
| 6.860 | 0.957 (0.874 to 0.989) | 0.818 (0.607 to 0.931) | 0.943 | 0.857 |
| 6.920 | 0.957 (0.874 to 0.989) | 0.864 (0.656 to 0.959) | 0.957 | 0.864 |
| 7.180 | 0.942 (0.855 to 0.981) | 0.864 (0.656 to 0.959) | 0.956 | 0.826 |
| 7.220 | 0.928 (0.837 to 0.972) | 0.864 (0.656 to 0.959) | 0.955 | 0.792 |
| 7.630 | 0.913 (0.819 to 0.962) | 0.864 (0.656 to 0.959) | 0.955 | 0.760 |
| 7.850 | 0.899 (0.801 to 0.952) | 0.909 (0.707 to 0.985) | 0.969 | 0.741 |
| 8.250 | 0.884 (0.784 to 0.942) | 0.909 (0.707 to 0.985) | 0.968 | 0.714 |
| 8.370 | 0.884 (0.784 to 0.942) | 0.955 (0.762 to 1.000) | 0.984 | 0.724 |
| 8.420 | 0.870 (0.767 to 0.931) | 0.955 (0.762 to 1.000) | 0.984 | 0.700 |
We performed further ROC analyses this time using the maximum possible over and estimates of wear from the wear calculations combined with the maximum and minimum reproducibility error from metal ion analysis. There were only marginal changes in the results. The full data can be seen in Table V.
Table V.
Sensitivities and specificities of various blood fractions after maximal measurement errors were taken into account (CI, confidence interval)
| Fraction | Value (µg/l) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| Abnormal wear defined as ≥ 2 mm3/year (maximum over measurement) | |||
| Blood cobalt | 4.50 | 0.904 (0.811 to 0.955) | 0.944 (0.720 to 1.000) |
| Blood chromium | 7.85 | 0.863 (0.763 to 0.925) | 0.944 (0.720 to 1.000) |
| Serum cobalt | 6.30 | 0.859 (0.751 to 0.926) | 0.929 (0.661 to 1.000) |
| Serum chromium | 7.30 | 0.891 (0.787 to 0.948) | 0.929 (0.661 to 1.000) |
| Abnormal wear defined as ≥ 2 mm3/year (maximum under measurement) | |||
| Blood cobalt | 4.50 | 0.929 (0.839 to 0.972) | 0.905 (0.696 to 0.984) |
| Blood chromium | 7.85 | 0.886 (0.787 to 0.943) | 0.905 (0.696 to 0.984) |
| Serum cobalt | 6.30 | 0.902 (0.797 to 0.957) | 0.941 (0.707 to 1.000) |
| Serum chromium | 7.30 | 0.902 (0.797 to 0.957) | 0.824 (0.580 to 0.944) |
| Abnormal wear defined as ≥ 3 mm3/year (maximum over measurement) | |||
| Blood cobalt | 5.04 | 0.928 (0.837 to 0.972) | 0.955 (0.762 to 1.000) |
| Blood chromium | 8.37 | 0.884 (0.784 to 0.942) | 0.955 (0.762 to 1.000) |
| Serum cobalt | 6.30 | 0.917 (0.814 to 0.967) | 0.944 (0.720 to 1.000) |
| Serum chromium | 7.70 | 0.917 (0.814 to 0.967) | 0.889 (0.657 to 0.979) |
| Abnormal wear defined as ≥ 3 mm3/year (maximum under measurement) | |||
| Blood cobalt | 5.04 | 0.941 (0.853 to 0.981) | 0.957 (0.770 to 1.000) |
| Blood chromium | 8.37 | 0.897 (0.799 to 0.951) | 0.957 (0.770 to 1.000) |
| Serum cobalt | 6.30 | 0.932 (0.832 to 0.977) | 0.947 (0.732 to 1.000) |
| Serum chromium | 7.70 | 0.932 (0.832 to 0.977) | 0.895 (0.671 to 0.981) |
As wear rates increased, there was a highly significant trend towards a disproportionate increase in serum Cr concentrations compared with the whole blood Cr concentration (Spearman Rank correlation = -0.646, p < 0.001).
The distribution of metal ions in whole blood and serum fractions: in vivo study
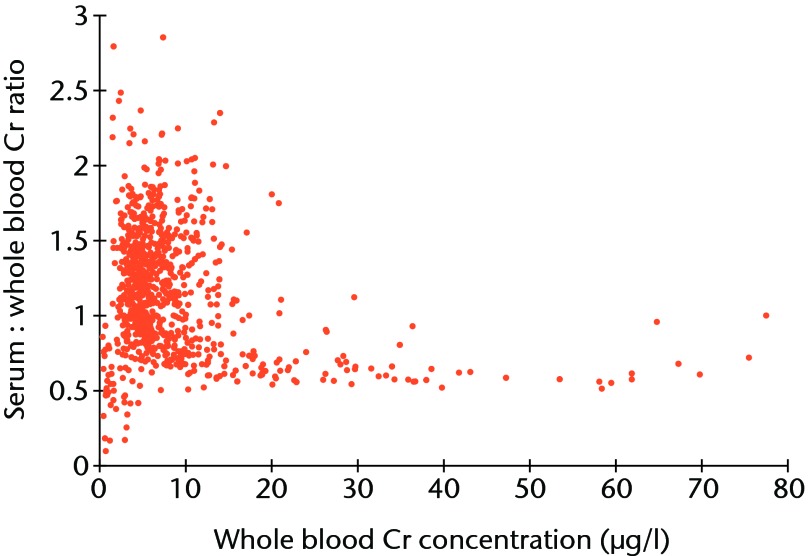
A total of 1048 patients gave blood samples. Patient demographics are included in Table VI. The Bland-Altman limits of agreements between serum and whole blood samples were -16.5 µg/l to 16.6 µg/l for Co, and -15.4 µg/l to 18.71 µg/l for Cr. The difference between Cr serum and whole blood became larger as Cr concentrations increased. The ratio of serum Cr to whole blood Cr stabilised at approximately 1.6 once an equivalent blood Co concentration of 20 µg/l was reached, the level above which gross metallosis (metal staining of peri-prosthetic tissues) is apparent (Fig. 6).2,17 This relationship was almost identical to that observed in the resurfacing patients, implying there was no difference in the underlying mechanisms of metal ion transport between hip resurfacing and THR patients (Spearman correlation = -0.656, p < 0.001).
Fig. 6.
Plot showing the relationship between the ratio of serum to whole blood chromium (Cr) plotted against whole blood Cr concentration. The ratio of serum to whole blood stabilises at approximately 1.6 as overall Cr concentration increases.
Table VI.
Patient demographics of those who gave matched samples for serum and whole blood metal ion analysis
| Implant* | |||
|---|---|---|---|
| Characteristic | ASR/ASR THR | BHR/BHR THR | Pinnacle |
| Patients (n) | 416 | 165 | 467 |
| Male:female | 245:171 | 97:68 | 127:340 |
| Mean time in situ (mths) (range) | 32 (1 to 82) | 55.4 (18 to 79.2) | 66 (12 to 109) |
| Mean age (yrs) (range) | 56 (25 to 83) | 51 (32 to 68) | 63 (21 to 83) |
| Median whole blood (µg/l) (range) | |||
| Cobalt | 2.53 (0.13 to 271) | 1.82 (0.63 to 147) | 2.13 (0.22 to 215) |
| Chromium | 5.82 (0.36 to 108) | 6.19 (0.78 to 53.5) | 5.30 (0.47 to 67.3) |
| Median serum (µg/l) (range) | |||
| Cobalt | 2.99 (0.20 to 228) | 2.29 (0.65 to 190) | 2.63 (0.37 to 204) |
| Chromium | 5.31 (0.42 to 115) | 5.48 (1.30 to 92.9) | 4.45 (0.62 to 108) |
* ASR, Articulating Surface Replacement; THR, total hip replacement; BHR, Birmingham Hip Resurfacing
The distribution of metal ions in whole blood and serum fractions: in vitro study
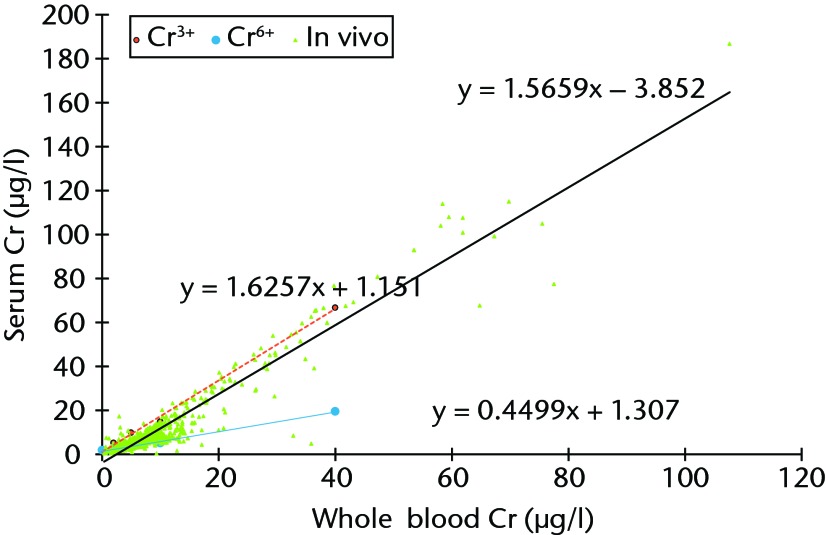
Blood samples spiked with Cr3+ showed a preferential increase in Cr concentration in the serum fractions. This was in contrast to blood samples spiked with Cr6+, where there was a preferential increase in Cr in the RBCs (Fig. 7). Blood samples spiked with Co2+ also showed preferential increases in Co concentrations in the serum fraction. The regression line equation for samples stored in EDTA tubes was calculated to be [Co2+]serum = [Co2 + 14] × (1.6301 + 0.2323), which gave an R2 value of 0.993.
Fig. 7.
Graph showing the plotted results for all patients in the study (n = 1048, green triangles), showing the relationship between whole blood and serum chromium (Cr) concentration. The best fit is represented by the solid black line (Spearman rank correlation r = 0.920, p < 0.001). The broken red line shows the relationship between serum and whole blood Cr values after blood was spiked with Cr3+ and centrifuged and analysed within 45 minutes (y = 1.6257 + 1.151), and the blue line shows that when blood was spiked with Cr6+ the hexavalent Cr ions became bound preferentially to RBCs (y = 0.4499x + 1.307).
The time from sampling to centrifugation had a very small effect on overall measured Cr concentrations and the distribution of the Cr species between the RBCs and serum, as did the type of tube used to collect the samples.
Discussion
Adverse reactions to metal debris (ARMD) are an increasingly recognised problem. There is a growing body of evidence to suggest that the vast majority of these reactions are seen in association with abnormally wearing MoM devices.2,5 There are currently no clear guidelines on how to interpret metal ion results, aside from preliminary recommendations issued by the Medicines and Healthcare Products Regulatory Agency (MHRA).18 A review on the current state of MoM hips also acknowledged that there is no current cut-off level at which poorly functioning hips can be identified.19
There are several reasons why it is desirable to identify abnormally wearing joints:
1. ARMD may be a silent (pain-free) process.20 It is therefore inappropriate to design studies and formulate guidance where joints are labeled ‘well-functioning’ simply if the patient has no pain. True ‘control’ patients should also have evidence that their prosthesis is functioning well in a tribological sense.
2. Our previous work,2 which included assessment of the largest cohort of patients who had experienced failure of their joints secondary to ARMD, suggested that accelerated wear leaves patients at greatly increased risk of subsequent development of a catastrophic immune cascade, which can culminate in extensive soft-tissue injury and/or osteolysis. The apparent tolerance of some patients to higher concentrations of metal debris may only be temporary. Notably, in our previous work where we had failed to identify any BHR patients with ARMD in a single-surgeon comparison series,21 four asymptomatic patients in this group with elevated blood metal ions went on to be revised for gross osteolysis within two years of the study.
3. Normal, expected wear of MoM bearing surfaces is thought to lead to the removal of surface imperfections, generally considered as a self-polishing process occurring in vivo.22 It is believed that the resulting smoother surfaces can harness a naturally occurring lubricating film, which leads to improved wear characteristics.23 In contrast, abnormal wear processes lead to roughening of the bearing surfaces and impairment of lubrication.23 In this way, increased wear leads to further wear. Increased wear leads to greater host exposure to elevated concentrations of metal debris, and the long term systemic sequelae are unknown.
This paper provides data to facilitate the development of guidelines for the interpretation of blood results in order to identify abnormally wearing implants in vivo. It includes comprehensive wear analysis of the largest collection of failed contemporary MoM bearings at an independent centre. This paper also includes analysis of the largest database of matched serum and whole blood samples in the published literature as well as the largest data set of Cr and Co blood concentrations in a healthy population.
Background blood metal ion concentrations
The background metal ion data shown in this report is included to provide context to clinicians. The data obtained from these healthy volunteers are not intended to be interpreted as a control group, as they were neither age- nor gender-matched and it was unknown whether these patients had metallic implants in their bodies. Despite this, the results show little variation in metal ion concentrations with an even smaller variation in Co compared with Cr concentrations. We speculate that this may be due to dietary variations24 or other factors such as smoking.25 Co has been shown to vary dynamically in response to exercise and it is therefore unsurprising that this element appears to be the more reliable indicator of wear.26,27 Analysis of Co is more easily conducted than Cr, and our own review of the literature from the last five years shows blood median ion levels of Co are well-matched irrespective of the laboratory or the country in which studies were performed. The same cannot be said of Cr (Table VII).28-31
Table VII.
Previously reported median values of blood chromium (Cr) and cobalt (Co) in cohorts of patients with a Birmingham Hip Resurfacing (BHR) measured using inductively coupled plasma mass spectrometry
| Authors | Patients (n) | Implant | Laboratory | Mean time to venesection (yrs) | Median Cr | Median Co |
|---|---|---|---|---|---|---|
| Daniel et al28 | 26 | BHR | Laboratory of Government Chemists Laboratories, Teddington, Middlesex, United Kingdom | 4 | 1.1 | 1.2 |
| Langton et al29 | 70 | BHR | Royal Surrey County Hospital, United Kingdom | 3.9 | 3.95 | 1.43 |
| Hart et al30 | 88 | 82 BHRs 6 Cormets (majority resurfacings) | Imperial Healthcare College, London, United Kingdom | 3.5 | 2.3 | 1.7 |
| Holland et al31 | 53 | BHR | Imperial Healthcare College, United Kingdom | 10 | 1.74 | 1.67 |
Use of metal ions to detect abnormal wear
A whole blood Co concentration ≥ 5.04 μg/l was found to be 93% sensitive and 96.0% specific for detecting abnormal wear (defined at the generous arbitrary value of 3 mm3/year). Serum Co concentrations ≥ 6.30 μg/l also showed high sensitivity and specificity for the detection of abnormal wear. We believe therefore that either serum or whole blood Co can be used for metal ion screening tests. However, the use of whole blood eliminates the need for the separation of serum, an extra step for a lab technician and one in which contaminants can be introduced, and also provides a truer representation of systemic exposure. As our results suggest, wear products appear to fill the serum compartment preferentially (see below). The measurement of whole blood metal ion concentrations therefore ‘dilutes’ the effect of transient increases in wear.
However, the major limitation in studies of this kind is that the data are drawn from are an inherently biased sample, in that all of the examined prostheses failed early. It would be ideal to examine and compare with a control group, such as those implants that have outlasted their recipient. This could only be done practically with the establishment of an autopsy bank.
Interchangeability of whole blood and serum
While serum and whole blood metal ion concentrations showed significant and strong correlation with each other, one fraction could not reliably predict the other; our results substantiate the conclusions of Daniel et al32 and Smolders et al33 who concluded that whole blood and serum concentrations cannot be used interchangeably due to the unacceptably wide limits of agreement. Compared with these two studies, our current study contains a wider range of ion data due to the poor performance of the ASR prosthesis. This allowed us to observe the trend of Cr preferentially storing in the serum compartment as ion concentrations increased in vivo (Fig. 6). This phenomenon prompted us to design an in vitro study to investigate the distribution of Co and Cr ions in serum and whole blood with increasing concentrations of metallic load.
The results of the in vitro study showed that Cr3+ (trivalent Cr) ions had a stronger affinity for serum while Cr6+ (hexavalent Cr) ions showed a preference for RBCs. When blood was spiked with trivalent Cr in vitro, the in vivo distribution of Cr was replicated. This phenomenon provides indirect evidence that it is Cr3+ that is primarily released from MoM implants. These findings are further supported by the in vitro studies by Ordóñez et al,34 who found Cr3+ to be associated with the serum protein transferrin in vitro, and by Merritt et al,35 who showed that Cr6+ ions generated from corrosion processes are preferentially taken into RBCs rather than serum. These results are also consistent with the in vivo findings of Walter et al.36 The results of the current study prompted us to examine the haematocrit values of the blood samples drawn from patients from whom the explants were retrieved. It was found that at lower levels of wear (< 5 mm3/year), haematocrit concentrations significantly affected the serum and whole blood Cr concentration ratio. A multiple regression model using whole blood Cr:serum Cr as the dependent variable and haematocrit and wear rate as the explanatory variables returned an R2 value of 0.345 (p = 0.002, with haematocrit coefficient = 0.480). This implied that at lower levels of wear, Cr concentrations were significantly affected by the pre-operative Cr concentration, which is likely to be stored preferentially in the RBCs.35 At higher levels of wear this relationship became progressively weaker, implying that the overall increase in Cr produced by the MoM device rendered the pre-operative Cr concentration insignificant. These factors warrant further discussion elsewhere but the findings reinforce the idea that serum and whole blood values are not interchangeable for clinical guidance, and this is particularly so for Cr concentrations. After a review of the literature and consideration of the results presented in this paper we propose a ‘cobalt ladder’ in order to guide the interpretation of blood Co results in mobile patients with a unilateral MoM resurfacing arthroplasty (Table VIII).2,17,37
Table VIII.
The ‘cobalt ladder’ for interpreting blood cobalt results in mobile patients with a unilateral metal-on-metal (MoM) resurfacing arthroplasty
| Whole blood cobalt concentration | Interpretation/assessment | |
|---|---|---|
| < 1 µg/l | 93% of the normal population are within this limit | |
| < 2 µg/l | Values are near to background. Expected wear of MoM resurfacings (see Table VII) | |
| 2 µg/l to 4.5 µg/l | Abnormal wear is possible. Consider device, bearing diameter, orientation of the acetabular component and patient activity levels | |
| 4.5 µg/l to 5.6 µg/l | High specificity for abnormal wear | |
| > 5.6 µg/l | 100% specific for abnormal wear (drawn at lower limit) | |
| > 10 µg/l | Abnormal wear unequivocal – high risk of early joint failure37 | |
| > 20 µg/l | Metal staining of the joint2,17 |
The Co values believed to be of concern as stated in this paper are similar to those proposed in the clinical study by Hart et al,30 in which a blood Co of 4.97 µg/l was shown to have a sensitivity of 63% and specificity of 86% for a failing MoM joint. How these values relate to the present study can be seen in Figure 5. With regard to serum values, Van Der Straeten et al38 found that an upper value of serum Co of 4.0 µg/l should be regarded as abnormal for a unilateral resurfacing. Like Hart et al,30 these authors also found that this value had a much greater specificity (96%) than sensitivity (22%). Again this is consistent with our data, which showed that this serum Co concentration was 88% specific for detecting abnormal wear.
Conclusions
Serum or whole blood Co and Cr concentrations are reliable indicators of the performance of a MoM bearing surface. There are significant differences between elements and blood fractions that must be taken into account during clinical assessment of patients. We recommend that whole blood Co should be the screening test of choice, and a level of 4.5 μg/l should be regarded as indicative of a poorly functioning joint. We emphasise that these results apply only to hip resurfacings. Total hip replacements have a further metallic interface between the head and the stem, which can lead to significant metal ion generation.39 The preferential concentration of Cr in the serum compartment likely indicates that trivalent Cr (Cr3+) is the predominant species released from MoM hip prostheses (resurfacings or THRs), rather than the hexavalent Cr species (Cr6+).
Funding Statement
This study was funded via a grant from the British Orthopaedic Association/Joint Action
Footnotes
Author contributions:R. P. Sidaginamale: Writing the paper, Data collection and analysis, Study design
T. J. Joyce: Data collection and analysis, Study design
J. K. Lord: Data collection and analysis, Study design
R. Jefferson: Data collection and analysis, Study design
P. G. Blain: Data collection and analysis, Study design
A. V. F. Nargol: Data collection and analysis, Study design
D. J. Langton: Writing the paper, Overall study concept, Data collection and analysis
ICMJE Conflict of Interest:None declared
References
- 1.Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg [Br] 2008;90-B:847–851 [DOI] [PubMed] [Google Scholar]
- 2.Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg [Br] 2011;93-B:164–171 [DOI] [PubMed] [Google Scholar]
- 3.Langton DJ, Jameson SS, Joyce TJ, et al. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg [Br] 2011;93-B:1011–1016 [DOI] [PubMed] [Google Scholar]
- 4.Canadian Hip Resurfacing Study Group. A survey on the prevalence of pseudotumors with metal-on-metal hip resurfacing in Canadian academic centers J Bone Joint Surg [Am] 2011;93-A(Suppl 2):118–121 [DOI] [PubMed] [Google Scholar]
- 5.Kwon YM, Glyn-Jones S, Simpson DJ, et al. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J Bone Joint Surg [Br] 2010;92-B:356–361 [DOI] [PubMed] [Google Scholar]
- 6.No authors listed. IARC monographs on the evaluation of carcinogenic risk to humans. 2004. http://monographs.iarc.fr/ (date last accessed 9 January 2013).
- 7.No authors listed. Office for National Statistics: 2001 Census. http://www.ons.gov.uk/ons/guide-method/census/census-2001/index.html (date last accessed 1 May 2013).
- 8.No authors listed. NHS Blood and Transplant. http://www.nhsbt.nhs.uk/ (date last accessed 1 May 2013).
- 9.EFORT. Consensus statement: current evidence on the management of metal-on-metal bearings. http://www.efort.org/communications/pdf/2012_05_10_MoM_Consensus_statement.pdf (date last accessed 9 January 2013). [DOI] [PubMed]
- 10.Harrington CF, Taylor A. Metal-on-metal hip implants: UK quality assurance of blood cobalt and chromium after hip implants. BMJ 2012;344:4017. [DOI] [PubMed] [Google Scholar]
- 11.No authors listed. Quebec multielement external quality assessment scheme (QMEQAS). http://www.inspq.qc.ca/ctq/paqe/qmeqas/default.asp?Page=2d&Lg=en (date last accessed 9 January 2013).
- 12.Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplasty 1998;13:890–895 [DOI] [PubMed] [Google Scholar]
- 13.Langton DJ, Joyce TJ, Mangat N, et al. Reducing metal ion release following hip resurfacing arthroplasty. Orthop Clin North Am 2011;42:169–180 [DOI] [PubMed] [Google Scholar]
- 14.Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis. J Bone Joint Surg [Am] 2008;90-A(Suppl 3):125–134 [DOI] [PubMed] [Google Scholar]
- 15.Sieber HP, Rieker CB, Köttig P. Analysis of 118 second-generation metal-on-metal retrieved hip implants. J Bone Joint Surg [Br] 1999;80-B:46–50 [DOI] [PubMed] [Google Scholar]
- 16.Schmalzried TP, Szuszczewicz ES, Northfield MR, et al. Quantitative assessment of walking activity after total hip or knee replacement. J Bone Joint Surg [Am] 1998;80-A:54–59 [PubMed] [Google Scholar]
- 17.De Smet K, De Haan R, Calistri C, et al. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg [Am] 2008;90-A:202–208 [DOI] [PubMed] [Google Scholar]
- 18.Medicines and Healthcare Products Regulatory Agency (MHRA). Medical Device Alert: all metal-on-metal (MoM) hip replacements (MDA/2012/036). http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON155761 (date last accessed 9 January 2013).
- 19.United States Food and Drug Administration. Information for orthopaedic surgeons about metal-on-metal hip implant surgery. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241667.htm (date last accessed 9 January 2013).
- 20.Wynn-Jones H, Macnair R, Wimhurst J, et al. Silent soft tissue pathology is common with a modern metal-on-metal hip arthroplasty. Acta Orthop 2011;82:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langton DJ, Jameson SS, Joyce TJ, et al. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg [Br] 2010;92-B:38–46 [DOI] [PubMed] [Google Scholar]
- 22.Joyce TJ, Grigg H, Langton DJ, Nargol AVF. Quantification of self-polishing in vivo from explanted metal-on-metal total hip replacements. Trib Int 2011;44:513–516 [Google Scholar]
- 23.Chan FW, Bobyn JD, Medley JB, Krygier JJ, Tanzer M. The Otto Aufranc Award: wear and lubrication of metal-on-metal hip implants. Clin Orthop Relat Res 1999;369:10–24 [DOI] [PubMed] [Google Scholar]
- 24.Arnich N, Sirot V, Rivière G, et al. Dietary exposure to trace elements and health risk assessment in the 2nd French Total Diet Study. Food Chem Toxicol 2012;50:2432–2449 [DOI] [PubMed] [Google Scholar]
- 25.Fresquez MR, Pappas RS, Watson CH. Establishment of toxic metal reference range in tobacco from US cigarettes. J Anal Toxicol 2013:Epub. [DOI] [PMC free article] [PubMed]
- 26.Khan M, Kuiper JH, Richardson JB. The exercise-related rise in plasma cobalt levels after metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg [Br] 2008;90-B:1152–1157 [DOI] [PubMed] [Google Scholar]
- 27.De Haan R, Campbell P, Reid S, Skipor AK, De Smet KA. Metal ion levels in a triathlete with a metal-on-metal resurfacing arthroplasty of the hip. J Bone Joint Surg [Br] 2007;89-B:538–541 [DOI] [PubMed] [Google Scholar]
- 28.Daniel J, Ziaee H, Pradhan C, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study. J Bone Joint Surg [Br] 2007;89-B:169–173 [DOI] [PubMed] [Google Scholar]
- 29.Langton DJ, Sprowson AP, Joyce TJ, et al. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of the Articular Surface Replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg [Br] 2009;91-B:1287–1295 [DOI] [PubMed] [Google Scholar]
- 30.Hart AJ, Sabah SA, Bandi AS, et al. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg [Br] 2011;93-B:1308–1313 [DOI] [PubMed] [Google Scholar]
- 31.Holland JP, Langton DJ, Hashmi M. Ten-year clinical, radiological and metal ion analysis of the Birmingham Hip Resurfacing: from a single, non-designer surgeon. J Bone Joint Surg [Br] 2012;94-B:471–476 [DOI] [PubMed] [Google Scholar]
- 32.Daniel J, Ziaee H, Pynsent PB, McMinn DJ. The validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg [Br] 2007;89-B:736–771 [DOI] [PubMed] [Google Scholar]
- 33.Smolders JM, Bisseling P, Hol A, et al. Metal ion interpretation in resurfacing versus conventional hip arthroplasty and in whole blood versus serum: how should we interpret metal ion data. Hip Int 2011;21:587–595 [DOI] [PubMed] [Google Scholar]
- 34.Ordóñez YM, Montes-Bayón M, Blanco-González E, et al. Metal release in patients with total hip arthroplasty by DF-ICP-MS and their association to serum proteins. J Anal At Spectrom 2009;24:1037–1043 [Google Scholar]
- 35.Merritt K, Brown SA. Release of hexavalent chromium from corrosion of stainless steel and cobalt-chromium alloys. J Biomed Mater Res 1995;29:627–633 [DOI] [PubMed] [Google Scholar]
- 36.Walter LR, Marel E, Harbury R, Wearne J. Distribution of chromium and cobalt ions in various blood fractions after resurfacing hip arthroplasty. J Arthroplasty 2008;6:814–821 [DOI] [PubMed] [Google Scholar]
- 37.Langton DJ, Gandhi JN, Sidaginamale RP, Takamura KM, Nargol AVF. The clinical implications of an elevated blood metal ion result post MoM hip resurfacing. Procs American Academy of Orthopaedic Surgeons Annual Meeting, 2012.
- 38.Van Der Straeten C, Grammatopoulos G, Gill HS, et al. The 2012 Otto Aufranc Award: the interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop Relat Res 2012:Epub. [DOI] [PMC free article] [PubMed]
- 39.Urban RM, Tomlinson MJ, Hall DJ, Jacobs JJ. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty. J Arthroplasty 2004;19(8 Suppl3):94–101 [DOI] [PubMed] [Google Scholar]