Abstract
Prenatal alcohol exposure is known to have many profound detrimental effects on human fetal development (fetal alcohol spectrum disorders), which may manifest as lifelong disabilities. However, how alcohol affects the auditory/vestibular system is still largely unknown. This is the first study to investigate morphological effects of alcohol on the developing octavolateral system (the inner ear and lateral line) using the zebrafish, Danio rerio. Zebrafish embryos of 2 hours post fertilization (hpf) were treated in 2% alcohol for 48 hours and screened at 72 hpf for morphological defects of the inner ear and lateral line. Octavolateral organs from both alcohol-treated and control zebrafish were examined using light, confocal, and scanning electron microscopy. We observed several otolith phenotypes for alcohol-treated zebrafish including zero, one, two abnormal, two normal, and multiple otoliths. Results of this study show that alcohol treatment during early development impairs the inner ear (smaller ear, abnormal otoliths, and fewer sensory hair cells) and the lateral line (smaller neuromasts, fewer neuromasts and hair cells per neuromast, and shorter kinocilia of hair cells). Early embryonic alcohol exposure may also result in defects in hearing, balance, and hydrodynamic function of zebrafish.
Introduction
Maternal alcohol consumption during gestation is one of the most preventable causes of abnormal development of human fetuses.1,2 A substantial range of adverse effects, including physical, behavioral, and cerebral anomalies, are known to arise when a fetus is exposed to alcohol. The congenital disease, fetal alcohol spectrum disorders (FASD), is comprised of particular conditions caused by mild to severe prenatal alcohol exposure such as fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD). Characteristics of these conditions range from growth regression, craniofacial deformities, cardiac defects, and central nervous system dysfunction.1,2 Despite the well-known effects of FASD, an estimated 1% of all births in the USA show signs of this completely preventable developmental disability.3,4 The alarming incidence of prenatal alcohol exposure has led to many studies examining the deleterious effects of developmental toxicity. Recent studies have found that early embryonic developmental exposure to alcohol affects many processes such as behavior, learning and memory, skeletal morphogensis, and vision.5–9 Impaired speech and learning abilities have also been found as a result of fetal alcohol exposure.10–12 Although hearing plays an integral role in communicative skills, only a few human case studies have focused on hearing and have found some hearing loss as a result of fetal alcohol exposure during development.13–17 Currently, little is known about how alcohol, a developmental teratogen, specifically affects the inner ear that is responsible for mechanosensation such as hearing and balance.
The zebrafish (Danio rerio) is a widely used model for the study of organogenesis in vertebrates because of its advantageous traits such as short spawning intervals, external fertilization, transparency from embryo through larval stages, and rapid development. These features facilitate in vivo examination of morphology of the octavolateral organs (the inner ear and lateral line) during the embryonic development. The zebrafish is not only an organism suitable for investigating gestational alcohol exposure to embryos,5,6,9,18 but also a well-established model for developmental studies of the inner ear.19–21 The zebrafish has been used to study autosomal recessive nonsyndromic hearing loss in humans such as Usher syndrome type 1B (MYO7A, DFNB2 and DFNA11), 1C (USH1C, DFNB18) and 1D (CDH23, DFNB12).22–24 More recently, the zebrafish has been successfully used to model sensorineural hearing loss, the loss of function of the OTOGL gene in humans.25 Previous studies have demonstrated the validity of the use of zebrafish to study human FAS because the characteristics of alcohol-induced defects in zebrafish are similar to those observed in humans.26–28 In this study, we focus on the morphological effects of embryonic alcohol exposure on the early development of the octavolateral organs in zebrafish.
To investigate the effects of embryonic alcohol exposure on hair cell systems, we examined some components of the octavolateral organs that include inner ear organs such as otolithic organs (utricle, saccule, and lagena), semicircular canals (anterior, horizontal, and posterior) with associated ampullae, and lateral line neuromasts such as anterior lateral line (ALL) neuromasts in the head and posterior lateral line (PLL) neuromasts in the trunk and tail. Like those of other fishes, sensory hair cells in the octavolateral organs of the zebrafish transduce mechanosensory stimulation into electrical signals.29 Hair cells associated with otolithic organs, semicircular canals, and lateral line neuromasts detect sound (or linear acceleration), angular acceleration, and hydrodynamic stimuli, respectively.30 The mechanosensory information from these octavolateral organs is conveyed to target nuclei in the medulla for central processing.30 Because hair cells are critical to mechanosensory transduction, this study sought to investigate whether early embryonic alcohol exposure causes any morphological defects of the inner ear and/or the lateral line system. Preliminary results of this study have been published in abstract form.31
Development of the zebrafish ear begins with the otic vesicle that forms at 16 hours post fertilization (hpf) and gives rise to the auditory and vestibular components of the inner ear.20 Both the utricle and saccule appear at approximately 19 hpf, but the lagena does not begin to form until 11 dpf. Inner ear hair cells begin to appear on maculae associated with otoliths between 24 and 30 hpf. Smaller patches, cristae, form in the anterior, posterior, and lateral regions of the ear in association with semicircular canals by 72 hpf. At this time, zebrafish larvae often have eighteen superficial ALL neuromasts and nine superficial PLL neuromasts on each side of the fish, appearing as early as 34 hpf.32,33 The neuromasts of larval zebrafish have been previously identified and named according to their stereotypical locations from the head to the tail.34,35 Each neuromast consists of a compilation of hair cells, supporting cells, mantle cells, and periderm cells.36 Lateral line hair cells are structurally and functionally similar to inner ear hair cells and are easily accessible for live imaging and experimental manipulation.
Methods
Animal model
Adult reproductive zebrafish (wild-type strains AB and WIK) were purchased from the Zebrafish International Resource Center, Eugene, Oregon. Transgenic zebrafish embryos (SqET4) that express green fluorescent protein (GFP) in sensory hair cells of the lateral line and inner ear were used to quantify the total number of hair cells of saccular epithelia.37 Fish care and egg production were conducted as described by Nüsslein-Volhard and Dahm and Westerfield.38,39 Briefly, they were raised in 3-L polycarbonate tanks on a stand-alone zebrafish rack system (Aquatic Habitat, Apopka, FL) with recirculating water maintained at 28.5±1.0°C and pH at 7.4±0.4. Fish rooms were kept on a cycle of 14 hours of light and 10 hours of dark. Daily monitoring of water temperature, conductivity, ammonium, and pH levels assured an ideal aqueous environment for zebrafish. Adult zebrafish were fed twice daily with live artemia (INVE Aquaculture, Salt Lake City, UT) and an artificial mixture of larval food supplement AP-150 (Aquatic Eco-Systems, Apopka, FL), spirulina flake (Aquatic Eco-Systems), and ArteMac-3 (Aquafauna Bio-Marine, Inc., Hawthorne, CA). Male and female zebrafish that were normally kept in separate tanks were placed together in 3-L tanks (6–10 zebrafish per tank) once every 2 weeks for breeding. To collect eggs, 50 mL Petri dishes with a mesh cover were placed into the tanks. Subsequently, the Petri dishes were taken out 2 h after the light cycle began in the morning. Fertilized eggs were immediately collected and rinsed in E3 embryo medium containing 5 mM NaCl, 0.017 mM KCl, 0.33 mM CaCl2·2H2O, 0.33 mM MgSO4·7H2O, and dH2O.38 Zebrafish embryos between 0 and 2 hpf (0–64 cell stages) with intact chorions were used for experiments. The animal use protocol for all procedures used in this study was approved by the University of Miami Animal Care and Use Committee and complies with the animal use guidelines of the National Institutes of Health.
Alcohol treatment
After collecting and rinsing, fertilized zebrafish embryos were immediately placed in 50 mL Petri dishes (maximum of 35 per dish) with a 2% alcohol solution (v/v) made from ethyl alcohol USP 200 proof (Sigma-Aldrich, St. Louis, MO) in E3. This alcohol concentration was used for all experiments examining morphological defects after alcohol exposure. Control embryos were also immediately placed in 50 mL Petri dishes with alcohol-free E3. The Petri dishes were covered and subsequently incubated at 28.5±1.0°C. After 24 h of incubation, solutions for both control and treated embryos were replaced with fresh solutions, and dead embryos were removed at this time. After 48 h of incubation, the 2% alcohol-treated group was rinsed 5 times in E3 for ∼30 sec each, placed in alcohol-free E3, and once again incubated at the same temperature until 72 hpf, when embryos normally hatch. Zebrafish at 72 hpf were used for all of the following experiments.
Measurement of embryonic alcohol concentration
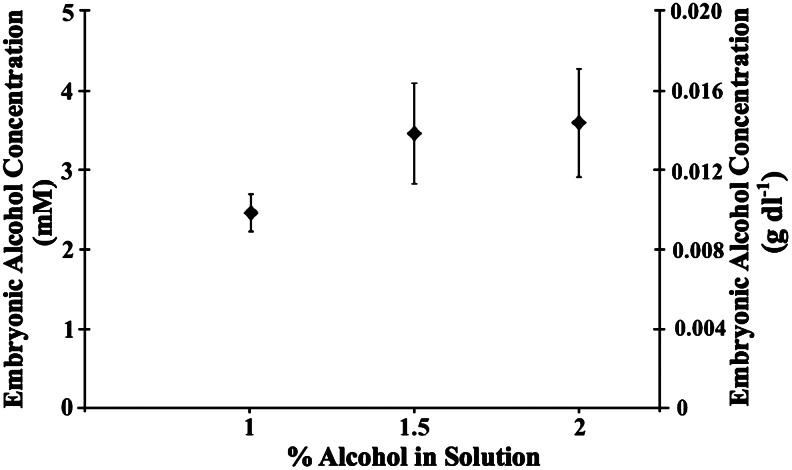
At 72 hpf the alcohol concentrations in zebrafish treated in 1%, 1.5%, and 2% alcohol solutions were measured with an alcohol assay that included a yeast alcohol dehydrogenase (ADH) and NAD reagent (Ethanol L3-K Assay, Genzyme Diagnostics, Framingham, MA).18 Three alcohol concentrations were used to show how the embryonic alcohol concentration changes with the concentration of alcohol solution (Fig. 1). To perform the assay, whole zebrafish embryos/larvae were separately rinsed 5 times for ∼30 sec with E3 to remove residual alcohol. Three groups of 45 control fish and 45 alcohol-treated fish per concentration were collected and transferred into separate 1.5 mL centrifuge tubes and placed on ice. Once embryo/larval movement ceased, excess solution was removed and the fish were homogenized with a pestle. The homogenate was then centrifuged at 12,000 rpm with a Centrifuge 5804R (Eppendorf North America, Hauppauge, NY) at 4°C for 10 min. Supernatant from each concentration (20 μL) was transferred into 12×75 mm disposable glass tubes. The assay was then performed according to package insert. A single cell module spectrophotometer (Beckman Du 530, UV/Vis, Beckman Coulter, Miami, FL) was used to measure the production of NADH, which was proportional to the amount of alcohol in the supernatant. Three readings were taken at 340 nm and 380 nm and then averaged for each concentration to obtain the embryo/larval alcohol concentration in millimoles per liter of tissue and grams per deciliter. An alcohol calibrator, included in the assay kit, was used as a standard.
FIG. 1.
Tissue alcohol concentrations in 72-hpf zebrafish embryos after 48 h exposure in alcohol solution concentrations of 1%, 1.5%, and 2%. Data are represented as means±1 SEM.
Classification and measurements of otoliths and otic vesicles
Chorions of unhatched embryos were manually removed with forceps. Both 2% alcohol-treated and control zebrafish were anesthetized in 0.02% buffered MS-222 (w/v, ethyl 3-aminobenzoate methanesulfonate salt, Sigma-Aldrich) until the movement of zebrafish ceased. Zebrafish were laterally positioned on a 35-mm MatTek dish and one ear of each fish was randomly selected and imaged using a SteREO Discovery V20 fluorescence microscope with an AxioCam MRm digital camera and AxioVision software 4.8 (Zeiss, Thornwood, NY). For classification of otolith phenotypes, we examined a total of 1111 zebrafish and classified them according to the number, location, and morphology of otoliths. Otic vesicles in 228 zebrafish were examined at 225X with the Zeiss stereomicroscope and measured using ImageJ (the National Institute of Mental Health, Bethesda, MD).
Neuromast and hair cell quantification
To visualize neuromasts, control and alcohol-treated wild-type zebrafish were stained with a 5 μM solution of vital fluorescent dye 4-[4-(diethylamino) styryl)-N-methylpyridinium iodide (4-Di-2-ASP, Invitrogen, Carlsbad, CA) for 10 min at room temperature in the dark.40 One side of each anesthetized fish was examined at 225X to determine the presence or absence of individual ALL and PLL neuromasts using the Zeiss SteREO Discovery V20 fluorescence microscope with a GFP filter set. The intensity of fluorescent light stimulating embryos was calibrated with an X-Cite optical power measurement device (XR2100 and XP750, EXFO America, Richardson, TX) to ensure constant light intensity used for both control and experimental groups.
For quantification of neuromast hair cells, control and alcohol-treated zebrafish were first anesthetized in 0.02% MS-222 and subsequently stained with 3 μM Yo-Pro-1 fluorescent vital dyes (Invitrogen) for ∼10 min to visualize the nuclei of hair cells in neuromasts.41 The number of hair cells present in each neuromast was determined by counting the number of nuclei stained. Some of these zebrafish were double stained with 3 μM FM 1-43X (n-(3-triethylammoniumpropyl)-4-(dibutylamino)-styryl) pyridinium dibromide (Invitrogen) for ∼10 sec to label the cell bodies of neuromast hair cells.41,42 To count hair cells in neuromasts, we focused on ALL neuromasts SO2, IO4, O1, O2, MI1, and D1 and PLL neuromasts P1, P2, P3, and P4 on one side of zebrafish. These neuromasts typically appear between 34 to 50 hpf.34,43
To quantify saccular hair cells, SqET4 transgenic zebrafish were fixed in 4% paraformaldehyde at 4°C for 2 h, then rinsed 3 times for 10 minutes in PBS. To visualize the entire saccular epithelium, the saccular otolith was dissolved in 3% Triton X-100 (Sigma-Aldrich) for 1 h at room temperature. All zebrafish were subsequently positioned laterally in Vectashield antifading solution (Vector Laboratories, Burlingame, CA) in 35-mm MatTek dishes. For image acquisition, a 35-mm MatTek dish was placed in a QE-1 HC 35-mm quick exchange heated/cooled platform attached to the confocal microscope stage, and the temperature of the platform was set at 28.5±1.0°C using CL-100 temperature controller and TCM-1 thermal cooling module (Werner Instruments, Hamden, CT). Images of hair cells in both the inner ear and neuromasts were taken using a Nikon C-1 confocal unit consisting of three laser lines (488, 543, and 632 nm) and a TE-2000 inverted microscope (Nikon, Melville, NY), or a Zeiss Axioskop 2 FS plus fluorescent light source with a 63X Plan Apochromat water immersion objective lens. After 3D reconstruction of hair cells was made with the Nikon EC-1 software, the area of saccular epithelium, number of hair cells in each epithelium, and areas of hair cell bodies were measured using the Nikon Elements software. To obtain hair cell density, hair cell count was divided by the area of saccular epithelium.
Scanning electron microscopy (SEM)
SEM procedures to prepare zebrafish samples were modified from Prince et al.44 Control and 2% alcohol-treated zebrafish at 72 hpf were overanesthetized with 0.1% MS-222 and subsequently fixed in 2.5% glutaraldehyde in Millonig's phosphate buffer (pH=7.3, Electron Microscopy Sciences, Hatfield, PA) for 2 h at 4°C. After three 5-min rinses in Millonig's buffer, the fish were post-fixed in 1% osmium tetroxide in Millonig's for 1.5 h. They were then rinsed three times for 5 min each and dehydrated gradually in an alcohol series (50%, 70%, and 95%) twice for 5 min each, and then in 100% four times for 10 min each. To eliminate the alcohol in the fish, they were then gradually immersed in acetone: 1:1 acetone to alcohol, then 2:1 for 10 min each, and then 3 times (once for 10 min and twice for 5 min) in 100% acetone. Following the acetone treatment, the fish were then critical point dried by gradually submerging them in serial dilutions of acetone and hexamethyldisilazane (HMDS, EMS): 2:1, 1:1, and 1:2 for 10 min each time and in 100% HMDS twice for 5 min each time. They were then laterally mounted onto aluminum stubs with double-sided carbon adhesive tape and coated with Au and Pd using a Hummer Sputter Coater to decrease electron charging of the samples while viewing and imaging with SEM.
Neuromasts and their hair cell ciliary bundles were visualized using SEM (JSM-5600LV, JOEL USA, Peabody, MA). Images of neuromasts from the top view were taken at 4500x and analyzed with JSM 5000 software. The apertures of individual neuromasts were manually traced with ImageJ to determine the neuromast area. For each neuromast, its longest kinocilium was traced and measured with ImageJ to represent the kinocilium length of neuromast hair cells.
Statistical analyses
The results were represented as means±1 SEM, and the overall significance level α was set at 0.05. Student's t-tests were used to assess significant differences between means of control and alcohol-treated zebrafish. In order to reduce type-I error, α was adjusted for each t-test according to the following equation: 1−(1−a) 1/(1+k−i), where k was the number of t-tests conducted and i was the ith individual t-test.45 If a P value was smaller than the corresponding adjusted α, we concluded a significant difference between control and alcohol-treated zebrafish. Significance is represented in Figures 2–5 with an asterisk (*) compared to no asterisk for insignificance.
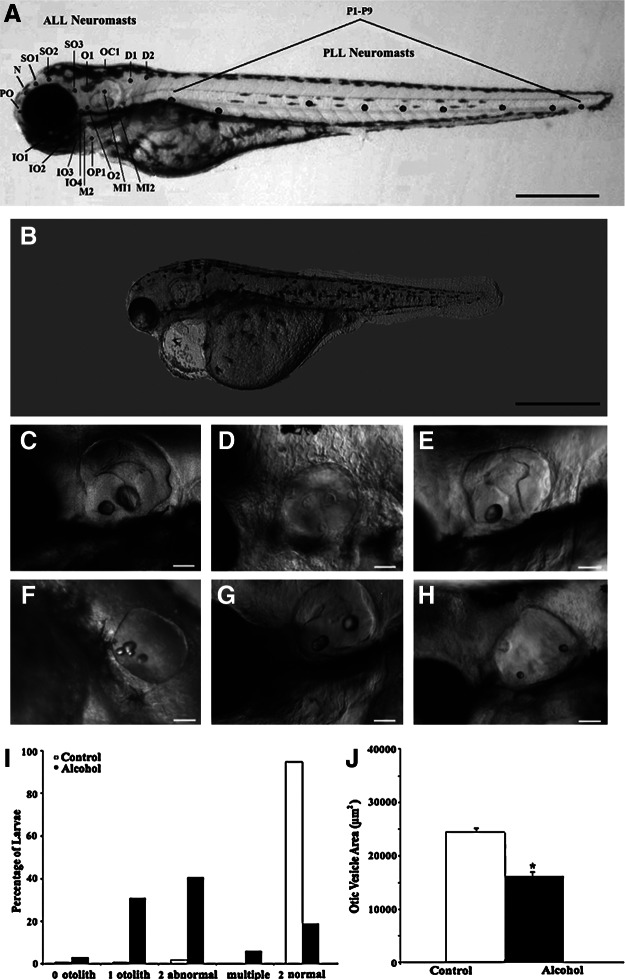
FIG. 2.
Gross phenotypic morphology of control (A) and 2% alcohol-treated (B) zebrafish. Otolith phenotypes of alcohol-treated zebrafish are 2 normal otoliths (C), 0 otoliths (D), 1 otolith (E), multiple otoliths (F), and 2 abnormal otoliths (G and H). The images in C–H are lateral views of the left otic vesicle. Scale bar=500 μm for A and B, 50 μm for C–H. Black dots in (A) are stereotypical loci of lateral line neuromasts in a normal zebrafish, showing ALL and PLL neuromasts. Nomenclature of ALL and PLL neuromasts was adapted from Metcalfe et al.33 and Raible and Kruse.34 (I) Distributions of percentages of otolith phenotypes observed in control and alcohol-treated zebrafish. (J) Comparison in average otic vesicle area between control and alcohol-treated zebrafish. Histograms are means+1 SEM.
FIG. 5.
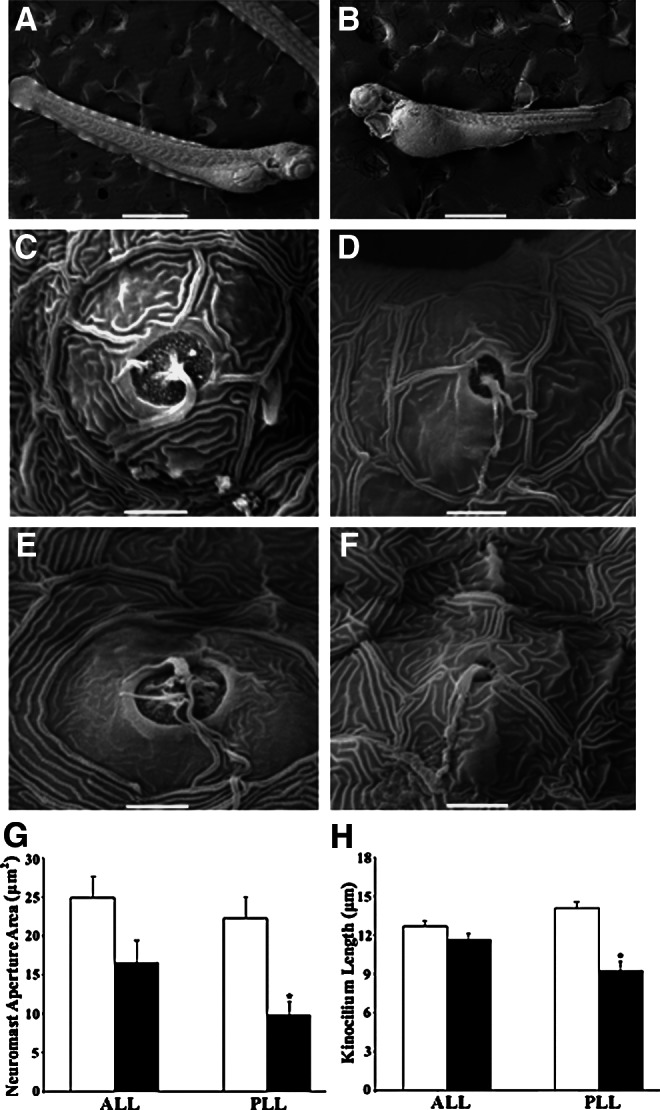
Scanning electron micrographs of control (A, C, and E) and alcohol-treated (B, D, and F) zebrafish. SEM images of apertures and ciliary bundles of hair cells in ALL neuromasts (C and D) and PLL neuromasts (E and F). Scale bars=500 μm in A and B, 5 μm in C–F. (G) Comparisons in average aperture area of ALL and PLL neuromasts between control and alcohol-treated larvae. (H) Comparisons in average kinocilium length of ALL and PLL hair cells between control and alcohol-treated zebrafish. White histograms are control, and black histograms are alcohol treated.
Results
Embryonic/larval alcohol concentration
The analysis of 72-hpf, alcohol-treated zebrafish revealed that the alcohol absorbed during treatment had not completely metabolized 24 h after rinsing and removing them from the alcohol solution. For zebrafish treated in 1.0% (167 mM), 1.5% (251 mM), and 2.0% (334 mM) alcohol solutions, the average amount of alcohol in zebrafish tissue was 2.4±0.2, 3.4±0.6, 3.6±0.7 mM or 0.011, 0.015, 0.016 g/dl, respectively (n=45 fish per group, three groups per concentration) (Fig. 1).
Otolith phenotypes and otic vesicle area
Aside from visual observation of gross morphological effects such as overall edema and reduced total body length of 72-hpf zebrafish treated in 2% alcohol concentration (Fig. 2A and B), early alcohol exposure also altered the otolith formation. After embryonic alcohol exposure, five distinct otolith phenotypes found in this study were classified as zero, one, two normal, two abnormal, and multiple otoliths (Fig. 2C–H). For alcohol-treated zebrafish with defective otoliths, most of them had 1 otolith or 2 abnormal otoliths. Two abnormal otoliths were categorized for otoliths that were visibly malformed in size and/or misplaced such as two smaller otoliths (Fig. 2G), ectopic otoliths (Fig. 2H), or fused otoliths (not shown). For zebrafish with 1 otolith, the otolith was often observed in the anterior portion of the otic vesicle (Fig. 2E), the position of the lapillus. Some zebrafish had only one otolith located between normal positions of the lapillus and sagitta, and a few had an otolith in the posterior portion of the otic vesicle (not shown). Otoliths of multiple-otolith zebrafish were usually small and misplaced in the otic vesicle (Fig. 2F). Percentages of otolith phenotypes observed in zebrafish (n=517 control and n=593 alcohol-treated zebrafish) are shown in Figure 2I. Otolith defects were found in most alcohol-treated zebrafish (∼81%) but only in a minority (<3%) of control zebrafish. The average otic vesicle areas for control and alcohol-treated zebrafish were 24,542.2±280.3 μm2 (n=127) and 16,577.5±552.3 μm2 (n=102), respectively. There was a significant difference in area of the otic vesicle between control and alcohol-treated zebrafish (Table 1 and Fig. 2J).
Table 1.
Statistical Test Results
| Experiments | P values | P value rank | Adjusted α | Significance |
|---|---|---|---|---|
| # of neuromasts (Fig. 4C) | 1.03×10−77 | 1 | 0.005 | Yes (*) |
| # of hair cells per neuromast (Fig. 4D) | 1.74×10−28 | 2 | 0.006 | Yes (*) |
| Otocyst area (Fig. 2B) | 2.25×10−26 | 3 | 0.006 | Yes (*) |
| # of saccular hair cells (Fig. 3B) | 2.80×10−7 | 4 | 0.007 | Yes (*) |
| PLL neuromast aperture area (Fig. 5G) | 5.12×10−5 | 5 | 0.009 | Yes (*) |
| PLL kinocilium length (Fig. 5H) | 7.97×10−5 | 6 | 0.010 | Yes (*) |
| ALL neuromast aperture area (Fig. 5C) | 0.019 | 7 | 0.013 | No |
| Saccular hair cell body area (Fig. 3C) | 0.027 | 8 | 0.017 | No |
| ALL kinocilum length (Fig. 5H) | 0.350 | 9 | 0.025 | No |
| Saccular hair cell density (Fig. 3D) | 0.490 | 10 | 0.050 | No |
Saccular hair cells
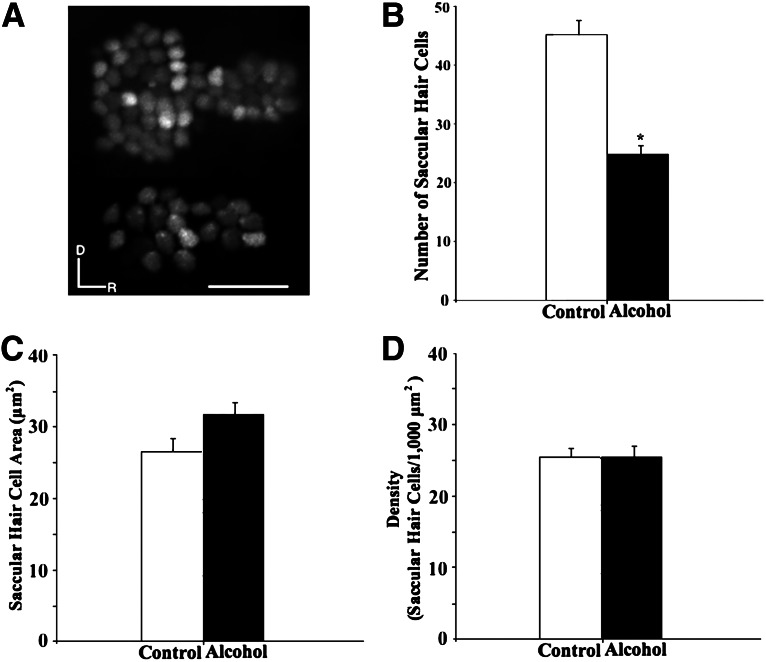
Examination of SqET4 zebrafish embryos exposed to alcohol during early development revealed some changes in hair cells in the saccular sensory epithelium. Alcohol-treated zebrafish larvae exhibited splayed and jagged saccular hair cells (Fig. 3A), and they had significantly fewer saccular hair cells than control zebrafish (24.5±1.4, n=10; 45.15±2.4, n=13; Table 1 and Fig. 3B). However, no significant difference was found in saccular hair cell body area between control and alcohol-treated zebrafish (26.6±1.5 μm2, n=7; 31.1±1.4 μm2, n=5; Table 1 and Fig. 3C), nor in comparison of saccular hair cell density between alcohol-treated and control zebrafish (25.3±2.0 cells/1,000 μm2, n=10; 25.2±1.5 cells/1,000 μm2, n=13; Table 1 and Fig. 3D).
FIG. 3.
(A) Images of saccular hair cells expressing GFP of control (upper) and alcohol-treated (lower) transgenic zebrafish. D, dorsal; R, rostral. Scale bar=25 μm. Comparison in saccular hair cell counts (B), saccular hair cell body area (C), and saccular hair cell density (D) between control and alcohol-treated zebrafish.
Number of neuromasts and number of hair cells per neuromast
The total number of unilateral neuromasts per zebrafish at 72 hpf was determined by counting the number of stained neuromasts in stereotypical loci as shown in Figure 2A. Neuromasts of control and alcohol-treated zebrafish were clearly identified and counted using a vital stain (Fig. 4A and B). Control zebrafish (23.0±0.2, n=112) had significantly more neuromasts than alcohol-exposed fish (8.0±0.3, n=87) (Table 1 and Fig. 4C). Hair cells in individual neuromasts of control zebrafish were typically organized in a rosette pattern as shown in the insets of Figure 4A. Embryonic alcohol exposure resulted in a reduced number of hair cells though the rosette pattern remained, as shown in the insets of Figure 4B. Alcohol-treated fish (3.9±0.1, n=87) had significantly fewer hair cells per neuromast than control fish (6.2±0.1, n=112) (Table 1 and Fig. 4D).
FIG. 4.
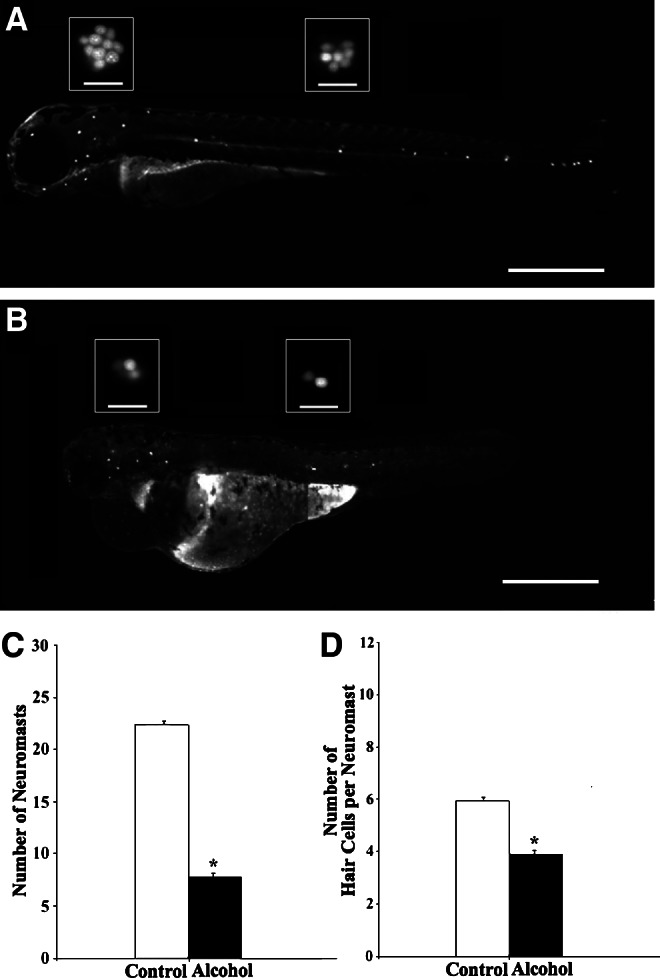
Lateral views of control (A) and alcohol-treated (B) zebrafish stained with 5 μM 4-Di-2-ASP, showing neuromasts in white dots. Scale bars=500 μm. The insets in A and B are Yo-Pro-1 labeled hair cell nuclei in one ALL neuromast (left) and one PLL neuromast (right). Scale bars in the insets are 25 μm. (C) Comparison in neuromast counts between control and alcohol-treated zebrafish. (D) Comparison in average number of hair cells per neuromast between control and alcohol-treated zebrafish.
Neuromast aperture area and kinocilium length
To have a better assessment of neuromast structure, we examined the neuromast aperture areas and ciliary bundle length using SEM. Figure 5 shows examples of whole fish bodies post fixation (A and B), ALL neuromasts (C and D), and PLL neuromasts (E and F) of 72-hpf control and alcohol-treated zebrafish. The area of neuromast apertures of control and alcohol-treated zebrafish ranged from 3.1 to 71.5 μm2 (25.8±2.5, n=15) and from 0.9 to 53.0 μm2 (11.8±2.0, n=11), respectively. The area of ALL neuromast apertures fell in a range from 5.9 to 71.3 μm2 (26.6±2.5 μm2, n=14) for control fish and from 4.0 to 53.0 μm2 (17.8±3.1 μm2, n=18) for alcohol-treated fish. Significant difference in ALL neuromast aperture area was not found between control and alcohol-treated fish (Table 1 and Fig. 5G). The area of PLL neuromast apertures ranged from 3.1 to 59.0 μm2 (25.4±2.8 μm2, n=64) for control fish and from 0.9 to 31.4 μm2 (9.4±2.0 μm2, n=40) for alcohol-treated fish. There was a significant difference in PLL neuromast aperture area between alcohol-treated and control fish (Table 1 and Fig. 5G).
The kinocilium length of neuromast hair cells of control zebrafish ranged from 7.4 to 18.1 μm (13.1±0.5 μm, n=12), whereas the kinocilium length of alcohol-treated fish distributed from 3.5 to 14.5 μm (9.8±0.2 μm, n=7). Kinocilia in ALL neuromasts were not significantly different in length between control and alcohol-treated groups (11.7±0.5 μm, n=8 and 11.4±0.4 μm, n=8, Table 1 and Fig. 5H). However, the average kinocilium length of PLL neuromasts was significantly shorter for alcohol-treated (9.1±0.7 μm, n=8) than control fish (13.6±0.4 μm, n=14) (Table 1 and Fig. 5H).
Discussion
Embryonic alcohol exposure
The dose of alcohol used in this study was based on our preliminary results, as well as studies done by other labs. This study and previous studies have demonstrated that the survival rate of 2% alcohol-exposed embryos was above 70%, which provided a sufficient number of fish to examine the effects of alcohol exposure.46,47 In addition to a higher mortality rate at higher concentrations (e.g., 2.5% and 3%), severe developmental defects were present, such as cyclopia and axial malformations.18,48,49 Lower doses of alcohol (1% and 1.5%) caused many fewer defects in the octavolateral organs than 2% alcohol. Therefore, 2% alcohol solution was chosen to investigate alcohol-induced morphological defects in the inner ear and lateral line in this study.
In addition, it is important to know the alcohol concentration in zebrafish embryos/larvae after alcohol exposure. Although some studies report tissue alcohol concentration,8,18,50 it is difficult to reliably compare among studies due to different alcohol concentrations, incubation durations, examination times, and methods of alcohol measurement. Nevertheless, it is worth noting that embryonic/larval alcohol concentrations measured in this study are similar to those reported by Li and others,8 for example, the embryonic/larval alcohol concentrations of 2.5 to 7.4 mM after zebrafish embryos were treated at 4.25 hpf for 6 h in the same alcohol solutions that we used to determine tissue alcohol concentration. In our study, we aimed to investigate alcohol exposure of long duration (e.g., 48 h during early development) due to potential implication relating to daily or binge drinking in humans, which is linked to more severe adverse effects in human fetal development when higher maternal alcohol consumption occurs over a longer period of time (e.g., 7 drinks per week all in one day versus 1 drink per day in 1 week).51 In Li's study, the embryonic alcohol concentration was higher than our measurement likely because theirs was done immediately following the alcohol treatment, without 24-hour incubation in E3. We measured tissue alcohol concentration 24 hours after rinsing and when normal hatching from the chorion typically occurs, which was also the time we examined larvae for octavolateral defects.
Furthermore, though a true comparison of tissue alcohol concentration levels in zebrafish and blood alcohol concentration levels in humans is difficult to assess, this study and others shows that a minimal amount of alcohol absorbance during zebrafish embryogenesis causes deleterious effects that could imply life-long damage to mechanosensory organs. Human consumption of as little as 1.5 social drinks is sufficient to acquire such minimal blood alcohol concentration that for some body types could meet the United States legal limit of alcohol intoxication, which is 17.4 millimoles per liter of blood (0.08%).
Inner ear damage
Like other vertebrates, the zebrafish's inner ear functions in hearing and balance. Morphological defects in otoliths and/or sensory epithelia affect auditory/vestibular function because mechanosensory transduction requires normal otoliths and receptor cells. In this study, we found defective otolith phenotypes with abnormalities in area, number, and location (see Fig. 2). For most zebrafish, we examined only the area and number of otoliths in one inner ear. However, alcohol could have different defective effects on both inner ears in the same individual fish. For example, in some cases we observed no otoliths in one ear and one otolith in the other ear, which likely causes differential mechanosensory defects between two inner ears. Clearly, zebrafish without otoliths in one or both ears might have a complete unilateral or bilateral loss of auditory and vestibular functions.
Additionally, we observed a reduced number of hair cells in saccular maculae of alcohol-treated zebrafish (Fig. 3). Fewer inner ear hair cells most likely hinder mechanosensory transduction, resulting in auditory and vestibular deficits in the zebrafish. Although the number of saccular hair cells in zebrafish is reduced after alcohol exposure, saccular hair cell area and density remain unchanged.
It has been reported that alcohol has broad effects on the development in mice and zebrafish, including defects in the brain, heart, eye, and inner ear due to alteration of expression of many genes involved in neuronal specification, neural growth factors, cell growth, and hematopoiesis.28,52 In addition, experimental evidence has shown that embryonic alcohol exposure altered expression of genes that are specifically involved in the induction and development of the otic vesicle in zebrafish. For example, acute embryonic alcohol exposure reduces expression of the raldh2 gene that is required to activate retinoic acid signalling at the beginning of gastrulation.53,54 Retinoic acid deficiency affects induction of the otic vesicle of zebrafish,55 and retinoic acid supplement can partially rescue alcohol-induced morphological defects of the otic vesicle.26
Previous zebrafish studies have revealed many genes that are responsible for morphogenesis of key structures/organs of the octavolateral system, particularly formation of the otic vesicle and otoliths.56–59 Mechanisms underlying morphological defects of the zebrafish ear after embryonic alcohol exposure are not fully understood. We propose that alcohol likely disrupts the biological processes that are critical for normal otolith formation. Otolith development is initiated with aggregation of seeding particles in the otic vesicle beginning at around 18–19 hpf, and their growth continues until 22 hpf. As described by Yu et al.,60 the incessant beating of small cilia in the otic vesicle moves nascent otolith particles to their normal anterior and posterior positions. Biogenesis of these cilia requires a gene known as iguana (igu; also known as dzip1).60–64 Mutations of iguana may cause defective cilia or absence of cilia, likely resulting in not only abnormal otolith deposition, but also malformed otoliths as shown by Yu et al.,60 which resemble otolith phenotypes of alcohol-treated larvae observed in this study.
The knockdown techniques used to target specific genes responsible for the development of the inner ear in previous studies resulted in phenotypes similar to those that we observed after exposing zebrafish embryos to alcohol. For example, otoc1 morphants had all the aberrant otolith phenotypes like zero, one, two abnormal, and multiple otoliths that we found in alcohol-treated zebrafish.65 In addition, various mutants reported by Malicki et al.,66 Whitfield et al.,67 and Schibler and Malicki68 with no otoliths and very small otoliths (such as empty ear, little richard, golas, half stoned, what's up, spock, no content, teeny rocks, pebbles, and condensed) resemble our alcohol-treated zebrafish without otoliths or with two abnormal otoliths.
Lateral line defects
The results of this study show that alcohol appears to have different defective effects on ALL and PLL neuromasts. Alcohol had more adverse effects on the development of PLL neuromasts than ALL neuromasts, particularly those in the tail. The last several PLL neuromasts (e.g., P5 to P9) were not present in most alcohol-treated fish. The absence of these PLL neuromasts might partially be a result of the shortened body of alcohol-treated zebrafish. Indeed, alcohol impairs the overall development of the zebrafish's trunk and tail (see Figs. 2B and 4B). We also believe that alcohol might impair the primordium migrating from head to tail.33,36 Many alcohol-treated zebrafish still had tails, though they were short. However, neuromasts were never formed to the end of the tail, in contrast to the formation of P5 to P9 shown in control zebrafish at 3 dpf. For the trunk neuromasts such as P1 to P4 that survived embryonic alcohol exposure, neuromast area, number of hair cells within a neuromast, and kinocilium length decreased as the position of trunk/tail neuromasts increased. For ALL neuromasts that survived alcohol insults, the neuromast aperture area and the kinocilium length did not change.
It is known that both ALL and PLL neuromasts are formed in a timely fashion.33,34,36 Head neuromasts such as O1, O2, and MI1 appear between 34 and 41 hpf, whereas SO2 and IO4 appear at 50 hpf. In this study, we observed that the ALL neuromasts (e.g., O1, O2, and MI1) that formed while zebrafish embryos were in alcohol solution were accounted for at 72 hpf, whereas most neuromasts that are known to form between 50 and 72 hpf (after the alcohol treatment) were not present in many zebrafish examined. Furthermore, mutations of genes such as dog are known to result in fewer lateral line neuromasts,67 as we also found after embryonic alcohol exposure. The decrease in number of lateral line neuromasts that we observed could imply disruption in expression of dog as a result of alcohol treatment during embryo development. In general, alcohol could affect the expression of multiple genes that play a role in normal development of the inner ear as well as the lateral line.
In addition to a reduced number of neuromasts in alcohol-treated zebrafish, we found that neuromasts present after alcohol exposure were morphologically impaired. Neuromasts that were present after embryonic alcohol exposure had a reduced number of hair cells per individual neuromast, which implies that these neuromasts had not fully developed or that hair cells were ablated, further leading to a possible reduction in proper function. We also found much shorter kinocilia of hair cells in PLL neuromasts of alcohol-treated fish than those of control fish. Because kinocilia play a vital role in mechanosensitivity of developing hair cells in the lateral line neuromasts in zebrafish,69 shortened kinocilia due to alcohol treatment likely affect normal function of nascent neuromast hair cells. Functional assessment of hair cells is underway to determine to what extent morphological damage from alcohol affects hair cell function.
Conclusions
This is the first systematic study of embryonic alcohol effects on the inner ear and the lateral line system in the zebrafish model. Our results reveal that embryonic alcohol exposure from 2 to 50 hpf causes explicit early developmental deformities in zebrafish's octavolateral organs. At 72 hpf, alcohol-treated zebrafish showed significant morphological defects, including impairment of otolith formation; reduced ear size; decreased number of inner ear hair cells, neuromasts, and hair cells per neuromast; and decreased area of neuromasts and length of kinocilia of hair cells in the trunk and tail. Structural defects found as a result of early embryonic alcohol exposure suggest possible functional deficits of mechanosensation such as hearing, balance, and water flow detection by zebrafish. Alcohol-induced defects in the mechanosensory system observed in this study could also be present in the human's auditory-vestibular system affected by fetal alcohol exposure.
Overall, it is important to elucidate the dangers of embryonic alcohol exposure at precise gestational time points, such as first, second, and third trimester, to clarify the stages of human fetal development during which alcohol exposure may be more likely to cause possible auditory and vestibular deficits. Development of the human's ear begins as early as the fourth week of gestation and by about the twenty-second week the inner ear is fully developed.70 Results of this study serve to further educate pregnant women and the general population by disseminating knowledge about the dangers of minimal alcohol consumption at any time point during gestation. Continued investigation of embryonic alcohol exposure on auditory and vestibular systems will essentially contribute to the long list of entirely preventable fetal alcohol spectrum disorders.
Acknowledgments
We would like to thank Alexandra A. DeSmidt, Phillip Davis, and Jon Rey for their assistance with this work; Ricardo Cepeda and Yolcar Chamorro for excellent fish care; and Drs. Phillip M. McCabe, Jeffrey S. Prince, and Lisa R. Ganser for their helpful comments on the manuscript. We are grateful to Dr. Tatjana Piotrowski's lab for providing SqET4 transgenic zebrafish that were generated by Dr. Vladamir Korzh's lab. Special thanks to Dr. Jeffrey S. Prince for his guidance in SEM and Dr. Richard Tokarz for his help on statistical analyses. This work was supported by R21DC009879 from the National Institutes of Health, the University of Miami Provost Research Award, and the College of Arts and Sciences Gabelli Fellowship.
Disclosure Statement
No competing financial interests exist.
References
- 1.Sokol RJ. Delaney-Black V. Nordstrom B. Fetal alcohol spectrum disorder. J Am Med Assoc. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary C. Fetal alcohol syndrome: Diagnosis, epidemiology, and developmental outcomes. J Pediatrics and Child Health. 2004;40:2–7. doi: 10.1111/j.1440-1754.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 3.Sampson PD. Striessguth AP. Bookstien FL. Little RE. Clarren SK. Dehaene P. Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratol. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.May PA. Gossage JP. Kalberg WO, et al. Prevalence and epidemiological characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Dis Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- 5.Carvan MJ., III Loucks E. Weber DN. Williams FE. Ethanol effects on the developing zebrafish: Neurobehavior and skeletal morphogenesis. Neurotoxicol Teratol. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Arenzan FJ. Carvan MJ., III Aijón J. Sánchez-González R. Arévalo R. Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol Teratol. 2006;28:342–348. doi: 10.1016/j.ntt.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Dlugos CA. Rabin RA. Ocular deficits associated with alcohol exposure during zebrafish development. J Comp Neurol. 2007;502:497–506. doi: 10.1002/cne.21320. [DOI] [PubMed] [Google Scholar]
- 8.Li XY. Yang HT. Zdanowicz M. Sicklick JK. Qi Y. Camp TJ. Diehl AM. Fetal alcohol exposure impairs hedgehog cholesterol modification and signaling. Lab Invest. 2007;87:231–240. doi: 10.1038/labinvest.3700516. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes Y. Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res. 2009;33:601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker M. Warr-Leeper GA. Leeper HAJ. Fetal alcohol syndrome: A description of oral motor, articulatory, short-term memory, grammatical and semantic abilities. J Comm Dis. 1990;23:97–124. doi: 10.1016/0021-9924(90)90016-r. [DOI] [PubMed] [Google Scholar]
- 11.Mattson SN. Riley EP. Delis DC. Stern C. Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- 12.Church MW. Kaltenbach JA. Hearing, speech, language, and vestibular disorders in the fetal alcohol syndrome: A literature review. Alcohol Clin Exp Res. 1997;21:495–512. doi: 10.1111/j.1530-0277.1997.tb03796.x. [DOI] [PubMed] [Google Scholar]
- 13.Pettigrew AG. Hutchinson I. Effects of alcohol on functional development of the auditory pathway in the brainstem of infants and chick embryos. Ciba Found Symp. 1984;105:26–46. doi: 10.1002/9780470720868.ch3. [DOI] [PubMed] [Google Scholar]
- 14.Church MW. Chronic in utero alcohol exposure affects auditory function in rats and in humans. Alcohol. 1987;4:231–239. doi: 10.1016/0741-8329(87)90017-6. [DOI] [PubMed] [Google Scholar]
- 15.Church MW. Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: Findings from case reports. Pediatrics. 1988;82:147–154. [PubMed] [Google Scholar]
- 16.Rintelmann WF. Church MW. Simpson TH. Root LE. Effects of maternal alcohol and/or cocaine on neonatal ABRs. Am Aud Soc Bull. 1995;20:14. [Google Scholar]
- 17.Cohen-Kerem R. Bar-Oz B. Nulman I. Papaioannou VA. Koren G. Hearing in children with fetal alcohol spectrum disorder (FASD) Can J Clin Pharmacol. 2007;14:307–312. [PubMed] [Google Scholar]
- 18.Reimers MJ. Flockton AR. Tanguay RL. Ethanol-and acetaldehyde-mediated developmental toxicity in zebrafish. Neurotoxicol Teratol. 2004;26:769–781. doi: 10.1016/j.ntt.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Riley BB. Genes controlling the development of the zebrafish inner ear and hair cells. Curr Top Dev Biol. 2003;57:357–388. doi: 10.1016/s0070-2153(03)57012-0. [DOI] [PubMed] [Google Scholar]
- 20.Haddon C. Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Millimaki BB. Sweet EM. Dhason MS. Riley BB Zebrafish atoh1 genes. Classic proneural activity in the inner ear and regulation by Fgf and Notch. Dev. 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- 22.Ernest S. Rauch GJ. Haffter P. Geisler R. Petit C. Nicolson T. Mariner is defective in myosin VIIA: A zebrafish model for human hereditary deafness. Human Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- 23.Söllner C. Rauch G-J. Siemens J. Geisler R. Schuster SC bengen Screen Consortium. Müller U. Nicolson T. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2000;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- 24.Phillips JB. Blanco-Sanchez B. Lentz JJ, et al. Harmonin (Ush1c) is required in zebrafish Müller glial cells for photoreceptor synaptic development and function. Dis Mod Mech. 2011;4:786–800. doi: 10.1242/dmm.006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yariz K. Duman D. Seco CZ, et al. Mutations in OTOGL, encoding otogelin-like, cause moderate sensorineural hearing loss. Am J Human Genet. 2012;91:872–882. doi: 10.1016/j.ajhg.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marrs JA. Clendenon SG. Ratcliffe DR. Fielding SM. Liu Q. Bosron WF. Zebrafish fetal alcohol syndrome model: Effects of ethanol are rescued by retinoic acid supplement. Alcohol. 2010;44:707–715. doi: 10.1016/j.alcohol.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali S. Champagne DL. Alia A. Richardson MK. Large-scale analysis of acute ethanol exposure in zebrafish development: A critical time window and resilience. PLoS ONE. 2011;6:e20037. doi: 10.1371/journal.pone.0020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loucks E. Ahlgren S. Assessing teratogenic changes in a zebrafish model of fetal alcohol exposure. J Vis Exp. 2012;61:e3704. doi: 10.3791/3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Z. Physiology of the ear and brain—How fishes hear. In: Farrell AP, editor. Encyclopedia of Fish Physiology: From Genome to Environment. Academic Press; 2011. pp. 292–297. [Google Scholar]
- 30.Tomchik SM. Lu Z. Octavolateral projections and organization in the medulla of a teleost fish, the sleeper goby (Dormitator latifrons) J Comp Neurol. 2005;481:96–117. doi: 10.1002/cne.20363. [DOI] [PubMed] [Google Scholar]
- 31.Zamora LY. Lu Z. Alcohol effects on the octavolateral system in developing zebrafish. Soc Neurosci Abstract. 2009:157. [Google Scholar]
- 32.Ghyssen A. Dambly-Chaudière C The lateral line microcosmos. 2007;21:2118–2130. doi: 10.1101/gad.1568407. [DOI] [PubMed] [Google Scholar]
- 33.Metcalfe WK. Sensory neuron growth cones comigrate with posterior lateral line primordial cells in zebrafish. J Comp Neurol. 1985;238:218–224. doi: 10.1002/cne.902380208. [DOI] [PubMed] [Google Scholar]
- 34.Raible DW. Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. [PubMed] [Google Scholar]
- 35.Metcalfe WK. Kimmel CB. Schabtach E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol. 1985;233:377–389. doi: 10.1002/cne.902330307. [DOI] [PubMed] [Google Scholar]
- 36.Ghysen A. Dambly-Chaudière C. Development of the zebrafish lateral line. Curr Opin Neurobiol. 2004;14:67–73. doi: 10.1016/j.conb.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Parinov S. Kondrichin I. Korzh V. Emelyanov A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn. 2004;231:449–459. doi: 10.1002/dvdy.20157. [DOI] [PubMed] [Google Scholar]
- 38.Nüsslein-Volhard C. Dahm R. Zebrafish: A Practical Approach. Oxford University Press; Oxford, England: 2002. [Google Scholar]
- 39.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) University of Oregon Press; Eugene, Oregon: 2007. [Google Scholar]
- 40.Asai Y. Chan DK. Starr CJ. Kappler JA. Kollma R. Hudspeth AJ. Mutation of the atrophin2 gene in the zebrafish disrupts signaling by fibroblast growth factor during development of the inner ear. Proc Natl Acad Sci USA. 2006;103:9069–9074. doi: 10.1073/pnas.0603453103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos F. MacDonald G. Rubel EW. Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Seiler C. Nicolson T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J Neurobiol. 1999;41:424–434. [PubMed] [Google Scholar]
- 43.Harris JA. Cheng AG. Cunnigham LL. MacDonald G. Raible DW. Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prince JS. Lynn MJ. Blackwelder PL. White vesicles in the skin of Aplysia californica cooper: A proposed excretory function. J Molluscan Studies. 2006;72:405–412. [Google Scholar]
- 45.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 46.Bilotta J. Barnett JA. Hancock L. Saszik S. Ethanol exposure alters zebrafish development: A novel model of fetal alcohol syndrome. Neurotoxicol Teratol. 2004;26:737–743. doi: 10.1016/j.ntt.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Sylvain NJ. Brewster DL. Ali DW. Zebrafish embryos exposed to alcohol undergo abnormal development of motor neurons and muscle fibers. Neurotoxicol Teratol. 2010;32:472–480. doi: 10.1016/j.ntt.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Baumann M. Sander K. Bipartite axiation follows incomplete epiboly in zebrafish embryos treated with chemical teratogens. J Exp Zool. 1984;230:363–376. doi: 10.1002/jez.1402300305. [DOI] [PubMed] [Google Scholar]
- 49.Blader P. Strähle U. Ethanol impairs migration of the prechordal plate in the zebrafish embryo. Dev Biol. 1998;20:85–201. doi: 10.1006/dbio.1998.8995. [DOI] [PubMed] [Google Scholar]
- 50.Bradfield JY. West JR. Maier SE. Uptake and elimination of ethanol by young zebrafish embryos. Neurotoxicol Teratol. 2006;28:629–633. doi: 10.1016/j.ntt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Maier SE. West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou FC. Zhao Q. Liu Y. Goodlett CR. Liang T. McClintick JN. Edenberg HJ. Li L. Alteration of gene expression by alcohol exposure at early neurulation. BMC Genom. 2011;12:124. doi: 10.1186/1471-2164-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kot-Leibovich H. Fainsod A. Ethanol induces embryonic malformations by competing for retinaldehyde dehydrogenase activity during vertebrate gastrulation. Dis Mod Mech. 2009;2:295–305. doi: 10.1242/dmm.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yelin R. Schyr RB. Kot H. Zins S. Frumkin A. Pillemer G. Fainsod A. Ethanol exposure affects gene expression in the embryonic organizer and reduces retinoic acid levels. Dev Biol. 2005;279:193–204. doi: 10.1016/j.ydbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Hans S. Westerfield M. Changes in retinoic acid signaling alter otic patterning. Dev. 2007;134:2449–2458. doi: 10.1242/dev.000448. [DOI] [PubMed] [Google Scholar]
- 56.Riley BB. Genes controlling the development of the zebrafish inner ear and hair cells. Curr Top Dev Biol. 2003;57:357–388. doi: 10.1016/s0070-2153(03)57012-0. [DOI] [PubMed] [Google Scholar]
- 57.Söllner C. Schwarz H. Geisler R. Nicolson T. Mutated otopetrin 1 affects the genesis of otoliths and the localization of starmaker in zebrafish. Dev Genes Evol. 2004;214:582–590. doi: 10.1007/s00427-004-0440-2. [DOI] [PubMed] [Google Scholar]
- 58.Murayama E. Herbomel P. Kawakami A. Takeda H. Nagasawa H. Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae. Mech Dev. 2005;122:791–803. doi: 10.1016/j.mod.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- 60.Yu X. Lau D. Ng CP. Roy S. Cilia-driven fluid flow as an epigenetic cue for otolith biomineralization on sensory hair cells of the ear. Dev. 2011;138:487–494. doi: 10.1242/dev.057752. [DOI] [PubMed] [Google Scholar]
- 61.Glazer A. Wilkinson A. Backer CB, et al. The Zn finger protein iguana impacts hedgehog signaling by promoting ciliogenesis. Dev Biol. 2010;337:148–156. doi: 10.1016/j.ydbio.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HR. Richardson J. Van Eeden F. Ingham PW. Gli2a protein localization reveals a role for Iguana/DZIP1 in primary ciliogenesis and a dependence of hedgehog signal transduction on primary cilia in the zebrafish. BMC Biol. 2010;8:65. doi: 10.1186/1741-7007-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rink JC. Gurley KA. Elliott SA. Sanchez AA. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326:1406–1410. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tay SY. Yu X. Wong KN. Panse P. Ng CP. Roy S. The iguana/DZIP1 protein is a novel component of the ciliogenic pathway essential for axonemal biogenesis. Dev Dyn. 2010;239:527–534. doi: 10.1002/dvdy.22199. [DOI] [PubMed] [Google Scholar]
- 65.Petko JA. Millimaki BB. Canfield VA. Riley BB. Levenson R. Otoc1: A novel otoconin-90 ortholog required for otolith mineralization in zebrafish. Dev Neurobiol. 2008;68:209–222. doi: 10.1002/dneu.20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malicki J. Schiler AF. Sonica-Krezel L, et al. Mutations affecting development of the zebrafish ear. Dev. 1996;123:275–283. doi: 10.1242/dev.123.1.275. [DOI] [PubMed] [Google Scholar]
- 67.Whitfield TT. Granato M. van Eeden FJ, et al. Mutations affecting development of the zebrafish inner ear and lateral line. Dev. 1996;123:241–254. doi: 10.1242/dev.123.1.241. [DOI] [PubMed] [Google Scholar]
- 68.Schibler A. Malicki J. A screen for genetic defects of the zebrafish ear. Mech Dev. 2007;124:592–604. doi: 10.1016/j.mod.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Kindt KS. Finch G. Nicolson T. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev Cell. 2012;23:329–341. doi: 10.1016/j.devcel.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore KL. Persaud TVN. Before We Are Born: Essentials of Embryology and Birth Defects. 7th. Saunders: Elsevier; 2008. pp. 287–288. [Google Scholar]