Abstract
Phosphorylcholine (PC) based phospholipid bilayers have proven useful as capillary coating materials due to their inherent resistance to non-specific protein adsorption. The primary limitation of this important class of capillary coatings remains the limited long-term chemical and physical stability of the coatings. Recently, a method for increasing phospholipid coating stability in fused silica capillaries via utilization of polymerized, synthetic phospholipids was reported. Here, we expand upon these studies by investigating polymerized lipid bilayer capillary coatings with respect to separation performance including run-to-run, day-to-day and column-to-column reproducibility and long term stability. In addition, the effects of pH and capillary inner diameter on polymerized phospholipid coated capillaries were investigated to identify optimized coating conditions. The coatings are stabilized for protein separations across a wide range of pH values (4.0-9.3), a unique property for capillary coating materials. Additionally, smaller inner diameter capillaries (≤ 50 μm) were found to yield marked enhancements in coating stability and reproducibility compared to wider bore capillaries, demonstrating the importance of capillary size for separations employing polymerized phospholipid coatings.
1 Introduction
Phospholipid bilayers (PLBs) are useful as surface coatings in capillary electrophoresis (CE) [1-3] due to the inherent biocompatibility of the hydrated phosphorylcholine headgroup, which is highly protein resistant [4-7]. Non-specific protein adsorption to capillary walls leads to a number of deleterious effects in CE and other techniques, including: irreproducible electroosmotic flow and migration time [8,9], reversed EOF [10], reduced detector response [11], skewed peak shapes, and decreased resolution and separation efficiency [11]. While fluid PLBs markedly reduce protein adsorption, they are by nature dynamic and inherently unstable structures, posing a significant obstacle to PLB utilization in many bioanalytical and biotechnological applications. Specifically, fluid PLBs lack the desired chemical, thermal, and mechanical stability to serve as long-term biocompatible coatings on silica supports. For example, PLB capillary coatings used for CE prepared using naturally occurring phospholipids require regeneration every 1-5 runs due to PLB degradation [1-3, 12]. Moreover, fluid PLBs are readily damaged by brief exposures to common chemical and physical insults that may be encountered in chemical separations, including air bubbles, exposure to organic solvents and surfactants [3-5, 12].
A number of strategies have been employed to increase the stability of PLBs [13-20], the most robust of which is direct polymerization of lipid monomers to form stabilized phospholipid bilayers (SPBs). Several synthetic lipids have been reported, many of which can be polymerized with > 95% efficiency [21-25]. The net result is a bilayer membrane, that while not directly covalently attached to the surface, forms a permanent coating via the formation of large polymer networks in the self-assembled membrane. Using such materials, SPBs have been prepared that are stable to surfactants, organics, dehydration and rehydration and long-term storage [4, 5, 26-27]. Moreover, these SPBs exhibit marked reductions in nonspecific protein adsorption and support incorporation of functional membrane proteins [3, 12, 28].
Formation of stable cross-linked SPBs on fused-silica capillary and spherical substrates using bis-SorbPC (1,2-bis[10-(2′,4′-hexadienoyloxy)decanoyl]-sn-glycero-2-phosphorylcholine), has shown that the resulting SPBs exhibit the same properties as planar PLBs and SPBs [3, 12, 27]. With respect to capillary coatings, bis-SorbPC was deposited onto fused silica capillary walls and silica particles via self-assembly and subsequently polymerized via radical initiation to form poly(bis-SorbPC) [3, 12]. The resulting films were highly stabilized to chemical insults and long-term storage, reduced non-specific protein adsorption and required no regeneration [3]. In this work, we extend these investigations to explore key factors affecting the utility and performance of poly(bis-SorbPC) coatings to better understand the practical limitations of poly(bis-SorbPC) coatings and identify optimal conditions for successful utilization of poly(bis-SorbPC) capillary coatings..
2 Experimental
2.1 Materials and Reagents
Fused silica capillaries were from InnovaQuartz (Phoenix, AZ). Coumarin 334 was from Eastman Kodak (Rochester, NY). NaHSO3 and K2S2O8 were from Sigma-Aldrich Inc. (St. Louis, MO). Bis-SorbPC (1,2-bis[10-(2′,4′-hexadienoyloxy)decanoyl]-sn-glycero-2-phosphorylcholine) was synthesized according to previously described methods [29]. Six-histidine-tagged enhanced green fluorescent protein (6xHis-EGFP) was prepared from transfected E. coli and purified using Ni2+-NTA metal affinity chromatography. R-phycoerythrin, biotin conjugate (RPE) was from Molecular Probes (Eugene, OR). All buffer solutions were prepared using deionized H2O obtained from Barnstead EasyPure UV/UF H2O purification system with a minimum conductivity of 18.0 MΩ and filtered with 0.2 μm pore size filters.
2.2 Capillary Preparation
Fused silica capillaries were rinsed with 0.1 M NaOH and then with deionized H2O. Stock solution of bis-SorbPC was dried of organic solvents using an Ar stream to yield a thin film on the interior of a glass vial. The lipid film was maintained under vacuum for at least ten hours to ensure complete solvent removal. The dried film was resuspended with H2O to a concentration of 1 mg/mL and sonicated to clarity to obtain small unilamellar vesicles (SUV). Capillaries were coated by introducing the lipid solution via gravity induced flow for 30 min. Polymerization was performed in situ by introducing the redox initiator (65 mM K2S2O8 and 20 mM NaHSO3 prepared in degassed H2O) into the coated capillary via gravity induced flow. The initiator was replaced with fresh initiator after the first 1.5 hours and allowed to polymerize for a minimum of six hours. Following polymerization, the capillaries were thoroughly washed with H2O and running buffer prior to use.
2.3 Capillary Preparation for Stability Studies
Fused silica capillaries (50 μm i.d.) were employed to study the PLB coating stability. EOF was determined for each capillary on the 1st, 5th, 10th, 30th and 45th days after polymerization using Coumarin 334 as a neutral marker. At the end of each day’s experiment, the capillaries were rinsed with deionized H2O and allowed to dry at room temperature until further use. Coatings were not regenerated nor refreshed during the time course of the experiments.
To investigate chemical stability of the poly(bis-SorbPC) coating, the capillary was treated in the following order after 45 days of storage/utilization prior to investigation: 30 min with running buffer, 30 min with Triton X-100, 60 min with H2O and finally 30 min with running buffer. EOF was then determined for six runs, as well as the separation of model proteins to determine migration time reproducibility before and after treatment with the surfactant.
2.4 Instrumentation
A capillary electrophoresis instrument with laser induced fluorescence detection (CE/LIF) assembled in-house was used in this study [30]. Separation capillaries were either unmodified bare fused silica capillaries or poly(bis-SorbPC) coated capillaries with varying inner diameters (i.d.), as indicated. The total length of the capillary was 42 cm and was 32 cm to the detector. Samples were injected hydrodynamically for 10 s. Separations were performed at +24 kV.
2.5 Data Analysis and Presentation
The migration times of proteins and of the EOF marker were determined using Cutter Version 5.0 software [31]. Data are reported as mean (%RSD) in which the number of samples (n) are indicated throughout. Investigation of pH and capillary i.d. effects on coating stability is presented such that the x-axis values represent cumulative separation time. The cumulative time reported in the figure axes is based on the total time than an individual capillary was used to perform separations, i.e. that the voltage was applied. Cumulative time is determined based on the migration time of the EOF marker in each CE run added to the combined total run time of the proceeding runs. EOF suppression in coated capillaries [EOF(coated)] was calculated with respect to EOF in bare fused silica capillaries [EOF(bare)] as follows:
3 Results and discussion
SPB coatings present a number of advantages with respect to long-term coating stability. In a previous report, the preparation of poly(bis-SorbPC) capillary coatings and characterization of the chemical nature and stability of the polymeric phospholipid film was described [3]. To better understand the poly(bis-SorbPC) coatings with respect to chemical separations and long-term utilization, we investigated a series of parameters that affect the overall stability and utility of the poly(bis-SorbPC) coatings.
3.1. Effect of pH on Coating Stability
The pH of the separation buffer plays a key role in modulating the EOF within a CE separation, as well as affecting the stability and chemical nature of dynamic and static capillary coatings [32-34]. This is particularly important in poly(bis-SorbPC) coatings where defects within the bilayer make the interstitial fluid layer between the bilayer and the capillary surface readily accessible, thus facilitating modulation of the silica surface charge state. The effect of pH on the stability and performance of poly(bis-SorbPC) coated capillaries was evaluated within the pH range from 4.0 - 9.3 over a 10 day period. This pH range represents the most commonly utilized range for CE separations of biomolecules. While a number of methods can be used to characterize lipid bilayers on planar surfaces, e.g. ellipsometry, atomic force microscopy and contact angle measurements [5], these techniques are not suitable for direct characterization of lipid coatings inside small i.d. fused silica capillaries. A good indicator of PLB coating stability is the magnitude and reproducibility of EOF within the capillary [1-3].
At pH 7.4 and 9.3, mean EOF values obtained are indistinguishable with a high degree of reproducibility (Table 1). Conversely, at pH 4.0, the EOF decays slightly over the first day (10 runs) of utilization and remains stable throughout the 5th day. On day 10, a slight, but reproducible decrease in mean EOF was observed. This initial decline followed by smaller, reproducible decreases in EOF may result from a slow exchange of the buffer in the interstitial water layer beneath the bilayer in the first ca. 50 minutes of separation which then equilibrates at the final value. Interestingly, degradation of unpolymerized phospholipid coatings leads to a substantial increase in EOF until the mean EOF reaches a value closely approximating that of a bare capillary. Further, previous investigations have shown a high degree of pH-sensitivity to the structure and stability of the PLB [33]. Though slight variations in the mean EOF were observed at differing pHs, the run to run reproducibility remains high (Table 1). In a bare capillary, the EOF magnitude increased substantially between pH 4.0 and 9.3, as predicted based on ionization of surface silanols. Interestingly, in poly(bis-SorbPC) coated capillaries, the mean EOF was increased at neutral and basic pH proportionally to EOF values observed in bare capillaries, whereas the EOF measured at pH 4.0 is greater than that observed in a bare capillary. These differences are likely attributable to the differences in lipid packing that are manifest as bilayer that is permeable to analytes less than ca. 2000 Da yet is resistant to non-specific absorption [35-36]. Thus, the partially permeable bilayer allows for alterations in the net surface charge and zeta potential of the capillary wall via permeation of low molecular weight buffer ions.
Table 1.
Reproducibility of EOF in (poly)bis-SorbPC coated capillaries as a function of pH.
| EOF × 10−4 (cm2/Vs)a) |
||||
|---|---|---|---|---|
| Buffer | Day 1 | Day 5 | Day 10 | Bare |
| Citrate pH 4.0 | 2.7 (4.4) | 2. 7 (1.0) | 2.5 (0.49) | 1.2 (6.62) |
| MOPS pH 7.4 | 3.5 (1.6) | 3.6 (1.0) | 3.5 (0.40) | 6.8 (0.89) |
| Borate pH 9.3 | 3.7 (0.30) | 3.6 (0.64) | 3.6 (0.42) | 7.1 (0.56) |
Conditions: Capillary: 42 cm × 50 μm i.d., 32 cm to detector; E = 240 V/cm. Coumarin 334 was used as EOF marker.
The EOF values on each day are the mean (%RSD) of 10 runs.
3.2. Effect of Capillary Inner Diameter (i.d.) on Coating Stability
In situ formation of coated capillaries relies on vesicle fusion, followed by self-assembly into the bilayer structure. Polymerization is achieved via the introduction of radical initiators in the absence of excess lipid vesicles in the solution volume. The capillary i.d., affects the surface area to volume ratio and the associated shear forces through the capillary and thereby may influence vesicle fusion and SPB formation, as well as the stability of the coating during the critical time window of the washing and polymerization processes. Degradation of the lipid coating prior to or during polymerization would markedly affect coating efficiency and coating stability in a manner that is dependent upon capillary i.d.
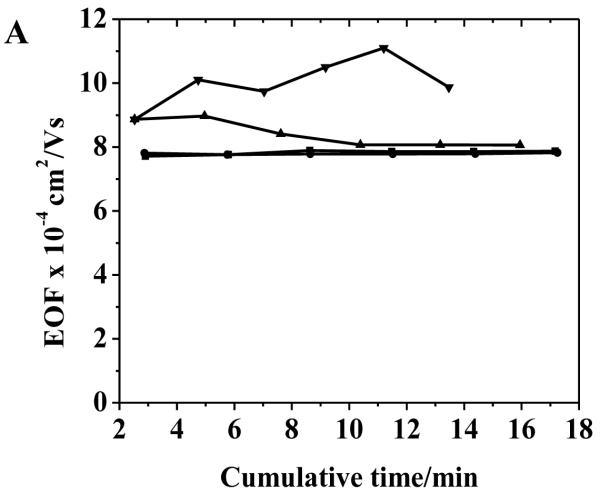
Capillaries ranging from 25 to 100 μm i.d. were evaluated with respect to stability and reproducibility of the poly(bis-SorbPC) coating compared to bare capillaries (Fig. 1 and Table 2). The suppression of EOF was found to be 54%, 50%, 46% and 35% in poly(bis-SorbPC) coated capillaries with 25 μm, 50 μm, 75 μm and 100 μm i.d., respectively. Higher EOF values were obtained with larger i.d. capillaries for both bare and poly(bis-SorbPC) coated capillaries, with significantly reduced reproducibility (Fig. 1). For capillaries with i.d. ≤ 50 μm, lower EOF values were obtained that were retained on day 15, whereas capillaries with i.d. larger than this value demonstrated varying degrees of stability and EOF uniformity. In addition to lower EOF magnitudes in both coated and bare capillaries, smaller i.d. capillaries provide marked enhancements in coating stability as indicated by EOF compared to larger i.d. capillaries with respect to column to column and day to day reproducibility. As seen in Fig. 1B, EOF measured in 75 μm i.d. capillaries was highly reproducible during the 1st day of utilization following polymerization, though the magnitude of EOF increases and reproducibility decreases significantly following dry storage. In contrast, the EOF measured in 100 μm i.d. capillaries was markedly less reproducible, even during the 1st day. The instability of EOF in the larger i.d. capillaries is an indication of reduced coating stability and likely results from either degradation of the SPB prior to or during the polymerization process or from reduced crosslink densities within the polymer structure with higher flow velocities.
Figure 1.
Effect of capillary inner diameter on EOF stability and reproducibility in (A) bare and (B) poly(bis-SorbPC) coated capillaries. The time (x-axis) represents the cumulative separation time within the individual capillary. Capillary i.d.: (■) 25 μm; (●) 50 μm; (▲) 75 μm, and (▼) 100 μm. Buffer: 20 mM borate at pH 9.3; E = 240 V cm−1.
Table 2.
Reproducibility of EOF in (poly)bis-SorbPC coated capillaries as a function of capillary inner diameter.
| Capillary i.d. | EOF × 10−4 (cm2/Vs)a) |
|||
|---|---|---|---|---|
| Day 1 | Day 15 | Meanb) | Barec) | |
| 25 μm | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 7.8 (0.3) |
| 50 μm | 3.9 (0.8) | 4.0 (1.8) | 4.0 (1.8) | 7.8 (0.9) |
| 75 μm | 4.6 (0.7) | 5.9 (13) | 5.3 (16.9) | 8.4 (5.0) |
| 100 μm | 6.5 (7.0) | 6.4 (25) | 6.6 (17) | 10.0 (8) |
Conditions: Capillary: 42 cm × 50 μm i.d., 32 cm to detector; E = 240 V/cm. Buffer, 20 mM borate, pH 9.3
The EOF values on each day are the mean (%RSD) of 10 runs.
The mean is calculate for 20 runs (n = 20).
The mean for bare capillary is calculated for 6 runs (n= 6).
The data obtained in 75 μm capillaries can be explained by a coating that is stable during the time course of polymerization, yet represents a lower cross link density, which in turn reduce the long term stability. Conversely, in 100 μm capillaries, the coating appears to degrade during the polymerization process resulting in substantially reduced coating uniformity and a subsequent reduction in both short and long term stability as indicated by capillary EOF. These data are in agreement with previous studies showing the effect of capillary i.d. on EOF stability in DDAB [32] coated capillaries where rapid decay of coating stability was observed with increasing capillary i.d. [37].
3.3. Time-based Stability of Coating
The long term stability of poly(bis-SorbPC) capillary coatings was evaluated over a period of 45 days after the initial polymerization. Table 3 provides the EOF measured in three capillary columns over the 45 days period. On each day, capillaries were used for 10 runs: one for the buffer, six for EOF marker and three for protein mixtures without regeneration or refreshing the capillary between runs. As seen in Table 3, the EOF slightly increases up to the 10th day for all three capillary columns and then remains relatively constant. The maximum increase in EOF on the 45th day after extended dry storage was 26% for capillary C, 16% for capillary A, and 11% for capillary B. Newly conditioned capillaries were used to determine the EOF for bare capillaries over this time frame. Additionally, though the magnitude of EOF increases slightly, the overall reproducibility in EOF within a series of experiments remains very high as is evidenced by the low %RSD (Table 3). These data suggest that the lipid coating remains mostly intact during the duration of storage. Furthermore, dry storage of lipid films is extremely harsh and can be readily avoided simply by storing the capillaries immersed in buffer.
Table 3.
Long term stability of polymer lipid coatings.
| Capillary | EOF × 10−4 (cm2/Vs)a) |
||||
|---|---|---|---|---|---|
| Day 1 | Day 5 | Day 10 | Day 30 | Day 45 | |
| Bare | 9.1 (0.88) | 9.5 (0.51) | 9.5 (1.70) | 9.9 (1.9) | 9.8 (0.78) |
| Non-polymerized | 2.3 (6.9) | 4.3 (3.0) | 6.7 (1.5) | N/A | N/A |
| Polymerized A | 4.8 (1.7) | 5.0 (0.65) | 5.4 (0.59) | 5.5 (1.2) | 5.5 (1.4) |
| Polymerized B | 5.0 (0.46) | 5.3 (0.39) | 5.4 (1.2) | 5.6 (1.1) | 5.8 (0.97) |
| Polymerized C | 4.6 (0.99) | 5.0 (1.5) | 5.4 (0.29) | 5.6 (0.31) | 5.7 (0.50) |
The EOF values on each day are the average of six runs. Buffer, 20 mM borate, pH 9.3, 42 cm × 50 μm i.d. capillary, 32 cm to detector; E = 240 V/cm. N/A indicates that the non-polymerized lipid coating was not stable at this time of the study.
To correlate these results with separation performance of the capillaries, we investigated the separation of two fluorescent proteins using poly(bis-SorbPC) coated capillaries as a function of capillary storage and utilization time. Figure 2 show a series of electropherograms obtained using three different poly(bis-SorbPC) coated capillaries, as well as a bare capillary for comparison. During these investigations, the migration time for each protein was highly reproducible among columns; with %RSD values < 1 % on day 10 and < 2 % on day 45 (Table 4). The high migration time reproducibility further confirms the long term stability of the poly(bis-SorbPC) coatings and supports the utilization of these materials for protein separations.
Figure 2.
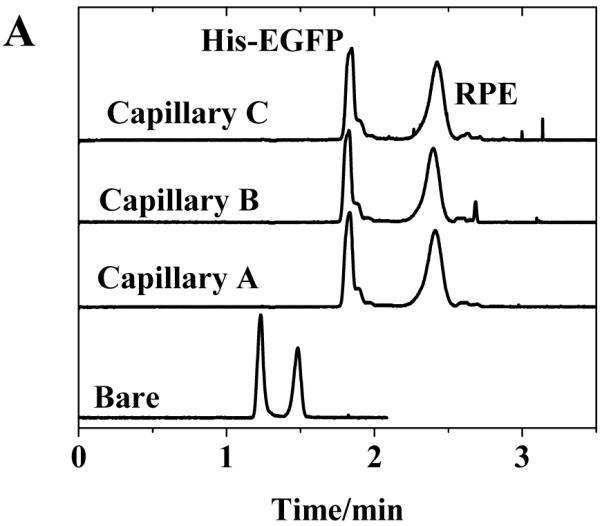
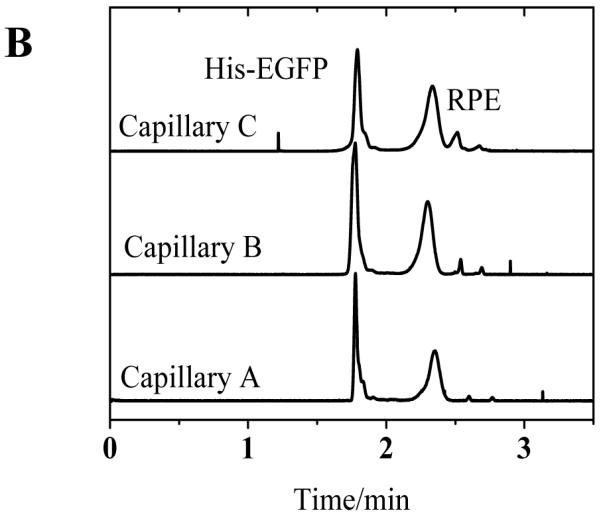
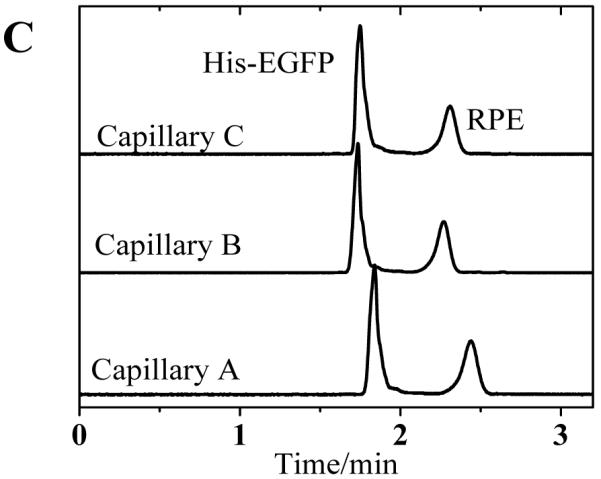
Separation of His-EGFP and RPE among three columns over an extended period of dry storage (A) 10 days (B) 30 days and (C) 45 days. Buffer, 20 mM borate, pH 9.3 E = 240 V cm−1.
Table 4.
Reproducibility of protein migration times as function of time.
| Mean migration time (min) (n =3) |
||||||
|---|---|---|---|---|---|---|
| Capillary | Day 10 | Day 30 | Day 45 | |||
| His-EGFP | RPE | His-EGFP | RPE | His-EGFP | RPE | |
| 1 | 1.8 (0.16) | 2.4 (0.08) | 1.8 (0.04) | 2.37 (0.23) | 1.8 (1.25) | 2.4 (1.80) |
| 2 | 1.8 (0.37) | 2.4 (0.26) | 1.8 (0.47) | 2.30 (0.42) | 1.7 (0.29) | 2.3 (0.13) |
| 3 | 1.8 (0.08) | 2.4 (0.09) | 1.8 (0.24) | 2.34 (0.26) | 1.7 (0.62) | 2.3 (0.69) |
Conditions: Capillaries, 42 cm × 50 μm i.d., 32 cm to detector; E = 240 V/cm; 20 mM borate buffer at pH 9.3. Coumarin 334 was used as EOF marker. The values in bracket are %RSD.
3.4 Chemical Stability of poly(bis-SorbPC) Coating
The utilization of organic solvents and micelle forming materials presents a number of advantages in CE. Among these include: (i) improved analyte solubility (ii) minimized joule heating and (iii) enhanced separation selectivity [38-41]. Though, a number of useful coating materials have been demonstrated, few are stable in buffers consisting of organic and/or surfactant additives [42-43]. It was previously shown that UV polymerized poly(bis-SorbPC) coatings are stable to exposure to surfactant, extensive H2O /buffer rinses, pH extremes and drying/rehydration [3,28], though the separation performance of the coatings was not demonstrated following these chemical and physical challenges. Fig. 3 shows representative electropherograms for protein separations obtained before and after exposure to 0.1% Triton X-100, a condition that readily disrupts unpolymerized PLB coatings. The migration time and electrophoretic mobility of both proteins exhibited high reproducibility before and after surfactant exposure, indicating that not only the coating structure, but also performance remains reproducible following exposure to these challenges (Table 4).
Figure 3.
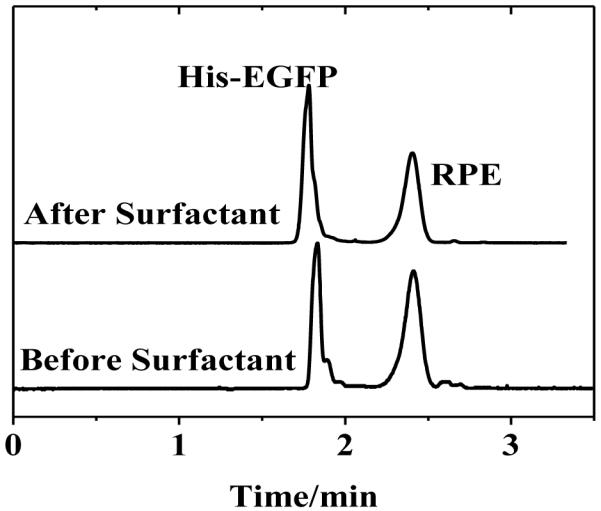
Separation of proteins in poly(bis-SorbPC) coated capillaries before and after treating the capillary with Triton X-100. Buffer, 20 mM borate, pH 9.3 E = 240 V cm−1.
4. Conclusions
Polymerized phospholipid membranes yield highly stable and highly reproducible capillary coatings for CE separations. Poly(bis-SorbPC) coatings were stable and uniform across a wide pH range and exhibited long term reproducibility when stored for up to 45 days. The separation performance was only marginally affected by dry storage and exposure to surfactants and organic challenges, conditions that completely disrupt PLBs prepared using natural lipids. Importantly, the stability and reproducibility of poly(bis-SorbPC) coatings was significantly dependent upon capillary i.d., with capillaries ≤ 50 μm providing optimal separation performance.
We investigated the stability of polymerized lipid bilayer capillary coatings.
Effects of pH and capillary inner diameter on stability of coating were studied.
Smaller inner diameter capillaries provide a very stable coating.
The polymerized lipid bilayer coatings are stable across a wide range of pH values.
The polymerized lipid bilayer coatings are stable for an extended period of time.
Acknowledgements
This study was supported by the National Institutes of Health under grant numbers GM095763 and EB007047.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cunliffe JM, Baryla NE, Lucy CA. Anal. Chem. 2002;74:776. doi: 10.1021/ac015627u. [DOI] [PubMed] [Google Scholar]
- [2].Wang C, Lucy CA. Anal. Chem. 2005;77:2015. doi: 10.1021/ac0489622. [DOI] [PubMed] [Google Scholar]
- [3].Mansfield E, Ross EE, Aspinwall CA. Anal. Chem. 2007;79:3135. doi: 10.1021/ac0618829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ross EE, Spratt T, Liu S, Rozanski LJ, O’Brien DF, Saavedra SS. Langmuir. 2003;19:1766. [Google Scholar]
- [5].Ross EE, Spratt T, Liu S, Rozanski LJ, O’Brien DF, Saavedra SS. Langmuir. 2003;19:1752. [Google Scholar]
- [6].Glasmästar K, Larsson C, Höök F, Kasemo B. J. Colloid Interface Sci. 2002;246:40. doi: 10.1006/jcis.2001.8060. [DOI] [PubMed] [Google Scholar]
- [7].Malmsten MJ. J. Colloid Interface Sci. 1995;172:106. [Google Scholar]
- [8].Hjertén S, Kubo K. Electrophoresis. 1993;14:390. doi: 10.1002/elps.1150140164. [DOI] [PubMed] [Google Scholar]
- [9].McCormick RM. Anal. Chem. 1988;60:2322. doi: 10.1021/ac00172a003. [DOI] [PubMed] [Google Scholar]
- [10].Righetti PR. Capillary Electrophoresis in Analytical Biotechnology. CRC Press; Boca Raton, Florida: 1996. [Google Scholar]
- [11].Tim W, Roberto R-D, Mingde Z. Capillary Electrophoresis of Proteins. Marcel Dekker, Inc.; New York: 1999. [Google Scholar]
- [12].Mansfield E, Ross EE, D’Ambruoso GD, Keogh JP, Huang Y, Aspinwall CA. Langmuir. 2007;23:11326. doi: 10.1021/la7008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raffy S, Teissie J. Biophys. J. 1999;76:2072. doi: 10.1016/S0006-3495(99)77363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Graff A, Winterhalter M, Meier W. Langmuir. 2001;17:919. [Google Scholar]
- [15].Hotz J, Meier W. Langmuir. 1998;14:1031. [Google Scholar]
- [16].Nikolelis DP, Drivelos DA, Simantiraki MG, Koinis S. Anal. Chem. 2004;76:2174. doi: 10.1021/ac0499470. [DOI] [PubMed] [Google Scholar]
- [17].Nikolelis DP, Mitrokosta M. Biosens. Bioelectron. 2002;17:565. doi: 10.1016/s0956-5663(02)00017-9. [DOI] [PubMed] [Google Scholar]
- [18].Holden MA, Jung S–Y, Yang T, Castellana ET, Cremer PS. J. Am. Chem. Soc. 2004;126:6512. doi: 10.1021/ja048504a. [DOI] [PubMed] [Google Scholar]
- [19].Lindén MV, Holopainen JM, Laukkanen A, Riekkola ML, Weidmer SK. Electrophoresis. 2006;27:3988. doi: 10.1002/elps.200600002. [DOI] [PubMed] [Google Scholar]
- [20].Varjo SJO, Hautala JT, Wiedmer SK, Riekkola M–L. J. Chromatogr. A. 2005;1081:92. doi: 10.1016/j.chroma.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [21].O’Brien DF, Armitage BA, Benedicto A, Bennett DE, Lamparski HG, Lee YS, Srisiri W, Sisson TM. Accounts of Chemical Research. 1998;31:861. [Google Scholar]
- [22].Mueller A, O’Brien DF. Chemical Reviews. 2002;102:727. doi: 10.1021/cr000071g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sisson TM, Lamparski HG, Kolchens S, Elayadi A, O’Brien DF. Macromolecules. 1996;29:8321. [Google Scholar]
- [24].Lawson GE, Lee Y, Singh A. Langmuir. 2003;19:6401. [Google Scholar]
- [25].Akama K, Awai K, Yano Y, Tokuyama S, Nakano Y. Polymers for Advanced Technologies. 2000;11:280. [Google Scholar]
- [26].Ross EE, Bondurant B, Spratt T, Conboy JC, O’Brien DF, Saavedra SS. Langmuir. 2001;17:2305. [Google Scholar]
- [27].Ross EE, Mansfield E, Huang Y, Aspinwall CA. J. Am. Chem. Soc. 2005;127:16756. doi: 10.1021/ja0539995. [DOI] [PubMed] [Google Scholar]
- [28].Subramaniam V, Alves ID, Salgado GFJ, Lau PW, Wysocki RJ, Salamon Z, Tollin G, Hruby VJ, Brown MF, Saavedra SS. J. Am. Chem. Soc. 2005;127:5320. doi: 10.1021/ja0423973. [DOI] [PubMed] [Google Scholar]
- [29].Lamparski H, Liman U, Barry JA, Frankel DA, Ramaswami V, Brown MF, O’Brien DF. Biochemistry. 1992;31:685. doi: 10.1021/bi00118a008. [DOI] [PubMed] [Google Scholar]
- [30].Hapuarachchi S, Janaway GA, Aspinwall CA. Electrophoresis. 2006;27:4052. doi: 10.1002/elps.200600232. [DOI] [PubMed] [Google Scholar]
- [31].Shackman JG, Watson CJ, Kennedy RT. J. Chromatogr. A. 2004;1040:273. doi: 10.1016/j.chroma.2004.04.004. [DOI] [PubMed] [Google Scholar]
- [32].Yassine MM, Lucy CA. Anal. Chem. 2004;76:2983. doi: 10.1021/ac035372f. [DOI] [PubMed] [Google Scholar]
- [33].Hautala JT, Wiedmer SK, Riekkola ML. Electrophoresis. 2005;26:176. doi: 10.1002/elps.200406143. [DOI] [PubMed] [Google Scholar]
- [34].Gulcev MD, Lucy CA. Anal. Chem. 2008;80:1806–1812. doi: 10.1021/ac702408u. [DOI] [PubMed] [Google Scholar]
- [35].Heitz BA, Xu J, Jones IW, Keogh JP, Comi TJ, Hall HK, Jr., Aspinwall CA, Saavedra SS. Langmuir. 2011;27:1882. doi: 10.1021/la1025944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Muhandiramlage TP, Cheng Z, Roberts DL, Keogh JP, Hall HK, Jr., Aspinwall CA. Anal. Chem. 2012;84:9754. doi: 10.1021/ac301510k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gulsev MD, McGinite TM, Bahnasy MF, Lucy CA. Analyst. 2010;135:2688. doi: 10.1039/c0an00279h. [DOI] [PubMed] [Google Scholar]
- [38].Sarmini K, Kenndler E. J. Chromatogr. A. 1988;811:201. [Google Scholar]
- [39].Geiser L, Cherkaoui S, Veuthey JL. J. Chromatogr. A. 2002;979:389. doi: 10.1016/s0021-9673(02)01254-2. [DOI] [PubMed] [Google Scholar]
- [40].Riekkola ML, Jussila M, Porras SP, Valko IE. J. Chromatogr. A. 2000;892:155. doi: 10.1016/s0021-9673(00)00108-4. [DOI] [PubMed] [Google Scholar]
- [41].Roy KI, Lucy CA. J. Chromatogr. A. 2002;964:213. doi: 10.1016/s0021-9673(02)00657-x. [DOI] [PubMed] [Google Scholar]
- [42].Belder D, Husmann HJ. Chromatogr. A. 2000;868:63. doi: 10.1016/s0021-9673(99)01165-6. [DOI] [PubMed] [Google Scholar]
- [43].Diress AG, Yassine MM, Lucy CA. Electrophoresis. 2007;28:1189. doi: 10.1002/elps.200600440. [DOI] [PubMed] [Google Scholar]